Key Points
Newly formed young platelets produced by MKs in response to acute thrombocytopenia display a selective GPVI-ITAM signaling defect.
This signaling defect protects mice from occlusive arterial thrombus formation.
Abstract
At sites of vascular injury, exposed subendothelial collagens trigger platelet activation and thrombus formation by interacting with the immunoreceptor tyrosine-based activation motif (ITAM)–coupled glycoprotein VI (GPVI) on the platelet surface. Platelets are derived from the cytoplasm of megakaryocytes (MKs), which extend large proplatelets into bone marrow (BM) sinusoids that are then released into the bloodstream, where final platelet sizing and maturation occurs. The mechanisms that prevent activation of MKs and forming proplatelets in the collagen-rich BM environment remain largely elusive. Here, we demonstrate that newly formed young platelets (NFYPs) released after antibody-mediated thrombocytopenia in mice display a severe and highly selective signaling defect downstream of GPVI resulting in impaired collagen-dependent activation and thrombus formation in vitro and in vivo. The diminished GPVI signaling in NFYPs is linked to reduced phosphorylation of key downstream signaling proteins, including Syk, LAT, and phospholipase Cγ2, whereas the G protein–coupled receptor and C-type lectin-like receptor 2 signaling pathways remained unaffected. This GPVI signaling defect was overcome once the platelet counts were restored to normal in the circulation. Overall, these results indicate that the GPVI-ITAM signaling machinery in NFYPs after antibody-mediated thrombocytopenia only becomes fully functional in the blood circulation.
Introduction
Platelets are circulating anucleate cell fragments originating from mature megakaryocytes (MKs)1 contributing to hemostasis, thrombosis, and inflammatory processes. The complex microenvironment of the bone marrow (BM) comprising extracellular matrix components surrounding cells and growth factors controls MK maturation and platelet production.2,3 Adhesion molecules in the BM niche like fibronectin, fibrinogen, von Willebrand factor, and collagens regulate proplatelet formation and final platelet release into the bloodstream.3-5 Collagen I and IV are present within the marrow cavity and the vascular niche, with collagen IV enriched at the sinusoids.6 Remarkably, collagens are also powerful platelet activators, acting on the glycoprotein VI (GPVI)/Fc receptor γ–chain complex triggering a tyrosine phosphorylation cascade and ultimately resulting in the activation of phospholipase Cγ2 (PLCγ2).7 GPVI signaling has been well studied in platelets and to some extent in MKs. However, to date, the mechanisms preventing MKs and forming proplatelets from getting activated in a collagen-rich BM environment are largely unknown. Here, we show that GPVI/immunoreceptor tyrosine-based activation motif (ITAM) signaling is partially inactive in newly formed young platelets (NFYPs) generated in response to acute thrombocytopenia and becomes fully functional only when platelets are in the circulation. This downregulation of GPVI signaling might serve to protect MKs and proplatelets from premature activation in the collagen-rich BM microenvironment.
Study design
Detailed methodology is provided in supplemental Methods (available on the Blood Web site).
Mice and antibody treatment for platelet depletion
Animal studies were approved by the district government of Lower Franconia. 4- to 6-week-old NMRI male mice from Janvier Labs (Le Genest-Saint-Isle, France) were used for experiments. Polyclonal rat anti-mouse GPIbα antibody (4 µg/g bodyweight; Emfret Analytics, Eibelstadt, Germany) was IV injected to induce platelet depletion. In other experiments, 100 µg anti-αIIbβ3 monoclonal antibody (MWReg30) was injected IV together with the platelet-activating factor inhibitor CV-6209 (3 µg/g bodyweight) to prevent acute systemic reactions.8
Platelet functional assays
Data analysis
Results from at least 3 experiments per group are presented as mean ± standard deviation (SD). Differences between 2 groups were assessed by the Student t test. Significance values are noted in the figure legends.
Results and discussion
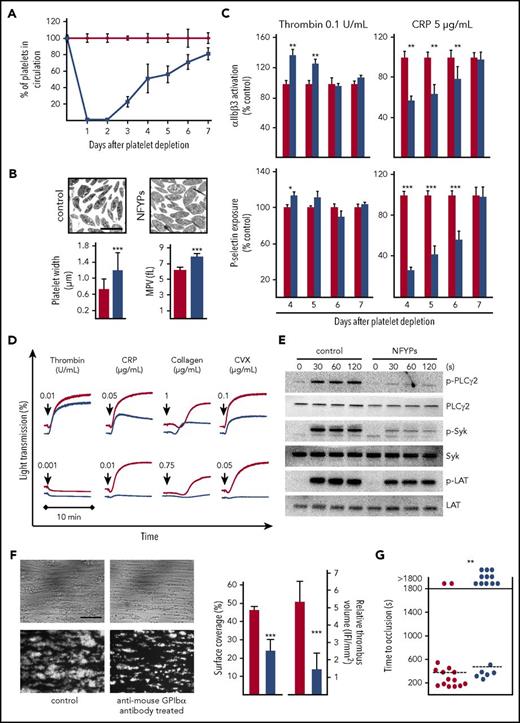
IV injection of mice with anti-GPIbα antibody, which depletes circulating platelets by Fc-independent mechanisms,8 resulted in immediate thrombocytopenia with >97% reduction of circulating platelet numbers for the first 2 days. By days 4 and 5, platelet counts recovered to 40%-60% and reached nearly normal levels after 7 days (Figure 1A). The newly generated circulating platelets on days 4 and 5 after depletion showed increased size (Figure 1B) and are further referred to as NFYPs. The surface expression of major receptors was largely unaltered on NFYPs, with an increase in integrin αIIbβ3 and C-type lectin-like receptor 2 (CLEC-2) but normal GPVI and GPIb levels (supplemental Table 1).
NFYPs after anti-mouse GPIbα antibody–induced thrombocytopenia display impaired GPVI signaling. (A) Male NMRI mice were injected with 4 µg/g of anti-mouse GPIbα antibodies, platelet count was determined by flow cytometry. Data points are represented as percent values ± SD compared with vehicle-treated controls (phosphate-buffered saline injected) (100%) at the indicated time points after depletion. The red line represents vehicle-treated mice, and the blue line represents anti-mouse GPIbα antibody–treated mice. (B) Representative transmission electron microscopy images of resting control platelets and NFYPs. Scale bar, 2.5 μm. Platelet width was determined by analyzing electron microscopy images. Mean platelet volume (MPV) on day 5 after platelet depletion was measured with an automated blood cell analyzer (Sysmex). The red bar represents vehicle-treated control mice, and the blue bar represents anti-mouse GPIbα antibody–treated mice. (C) Integrin αIIbβ3 activation and P-selectin exposure as measure of α-granule release of control platelets (red bar) and NFYPs (blue bar) in response to thrombin (0.1 U/mL) and CRP (5 µg/mL) on the indicated days after platelet depletion by anti-mouse GPIbα antibodies. Results are represented as percent activation with respect to control set at 100%. Results are depicted as mean fluorescence intensities ± SD of 5 mice per group and are representative of 4 individual experiments. *P < .05, **P < .01, and ***P < .001. (D) Platelets isolated from vehicle-treated controls (red line) or anti-GPIbα-antibody–treated mice (blue line) on day 5 after depletion were stimulated with the indicated agonists, and light transmission was recorded on a Fibrintimer 4-channel aggregometer. (E) Determination of whole-cell tyrosine phosphorylation pattern. Platelets were isolated on day 5 after depletion and stimulated with 0.1 μg/mL CRP under stirring conditions at 37°C, and aliquots were taken at the indicated time points. Samples were probed with anti-phospho-specific antibodies against PLCγ2 (Y759), Syk (Y519/520), and LAT (Y191). The membranes were stripped and reprobed for the total protein expression. (F) Heparinized whole blood from vehicle-treated mice (red) or anti-GPIbα antibody–treated mice (blue) on day 5 after depletion was perfused over immobilized collagen (0.2 mg/mL) at a shear rate of 1000 s−1. Blood from antibody-treated mice was reconstituted with isolated blood cells from other antibody-treated mice to replenish normal platelet counts. Representative phase contrast and fluorescent images (anti-GPIX-DyLight488) are shown. Data represent mean surface coverage and relative thrombus volume ± SD (n = 8 per group; scale bar, 50 μm). ***P < .001. (G) The abdominal aorta of vehicle-treated (red) and anti-GPIbα antibody–treated (blue) mice day 5 after depletion was injured by tight compression with a forceps, and blood flow was monitored for 30 minutes with an ultrasonic flow probe. The time to stable vessel occlusion is shown. Each symbol represents 1 individual mouse. **P < .01.
NFYPs after anti-mouse GPIbα antibody–induced thrombocytopenia display impaired GPVI signaling. (A) Male NMRI mice were injected with 4 µg/g of anti-mouse GPIbα antibodies, platelet count was determined by flow cytometry. Data points are represented as percent values ± SD compared with vehicle-treated controls (phosphate-buffered saline injected) (100%) at the indicated time points after depletion. The red line represents vehicle-treated mice, and the blue line represents anti-mouse GPIbα antibody–treated mice. (B) Representative transmission electron microscopy images of resting control platelets and NFYPs. Scale bar, 2.5 μm. Platelet width was determined by analyzing electron microscopy images. Mean platelet volume (MPV) on day 5 after platelet depletion was measured with an automated blood cell analyzer (Sysmex). The red bar represents vehicle-treated control mice, and the blue bar represents anti-mouse GPIbα antibody–treated mice. (C) Integrin αIIbβ3 activation and P-selectin exposure as measure of α-granule release of control platelets (red bar) and NFYPs (blue bar) in response to thrombin (0.1 U/mL) and CRP (5 µg/mL) on the indicated days after platelet depletion by anti-mouse GPIbα antibodies. Results are represented as percent activation with respect to control set at 100%. Results are depicted as mean fluorescence intensities ± SD of 5 mice per group and are representative of 4 individual experiments. *P < .05, **P < .01, and ***P < .001. (D) Platelets isolated from vehicle-treated controls (red line) or anti-GPIbα-antibody–treated mice (blue line) on day 5 after depletion were stimulated with the indicated agonists, and light transmission was recorded on a Fibrintimer 4-channel aggregometer. (E) Determination of whole-cell tyrosine phosphorylation pattern. Platelets were isolated on day 5 after depletion and stimulated with 0.1 μg/mL CRP under stirring conditions at 37°C, and aliquots were taken at the indicated time points. Samples were probed with anti-phospho-specific antibodies against PLCγ2 (Y759), Syk (Y519/520), and LAT (Y191). The membranes were stripped and reprobed for the total protein expression. (F) Heparinized whole blood from vehicle-treated mice (red) or anti-GPIbα antibody–treated mice (blue) on day 5 after depletion was perfused over immobilized collagen (0.2 mg/mL) at a shear rate of 1000 s−1. Blood from antibody-treated mice was reconstituted with isolated blood cells from other antibody-treated mice to replenish normal platelet counts. Representative phase contrast and fluorescent images (anti-GPIX-DyLight488) are shown. Data represent mean surface coverage and relative thrombus volume ± SD (n = 8 per group; scale bar, 50 μm). ***P < .001. (G) The abdominal aorta of vehicle-treated (red) and anti-GPIbα antibody–treated (blue) mice day 5 after depletion was injured by tight compression with a forceps, and blood flow was monitored for 30 minutes with an ultrasonic flow probe. The time to stable vessel occlusion is shown. Each symbol represents 1 individual mouse. **P < .01.
NFYPs showed significantly stronger αIIbβ3 integrin activation and marginally increased α-granule release upon thrombin stimulation (0.1 U/mL), which could have been caused by an increase in integrin β3 expression (supplemental Table 1). In stark contrast, NFYPs displayed a pronounced activation defect in response to the GPVI agonist collagen-related peptide (CRP) (Figure 1C). Likewise, GPVI-dependent platelet aggregation was markedly reduced in NFYPs (Figure 1D), whereas activation responses to G protein–coupled receptor agonists and CLEC-2 stimulation were unaffected (Figure 2D-E; supplemental Figure 1A). NFYPs revealed decreased tyrosine phosphorylation of Syk, LAT, and PLCγ2 on GPVI stimulation (Figure 1E), further emphasizing compromised GPVI signaling in these cells. Of note, the expression levels of Syk and PLCγ2 remained unaltered in platelets on days 4 to 7 (supplemental Figure 1B).
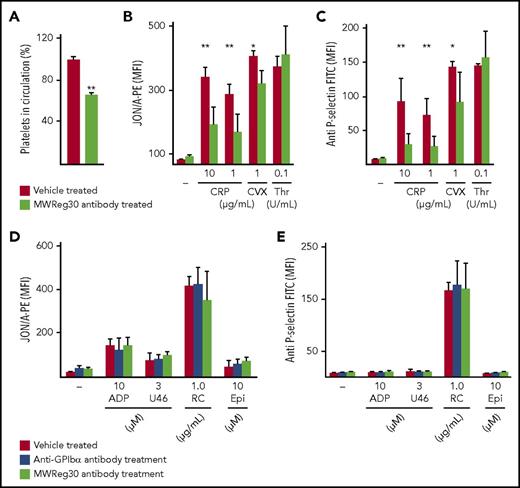
NFYPs after MWReg30-induced thrombocytopenia display impaired GPVI signaling. (A) Platelet count in circulation on day 5 after MWReg30 treatment was determined by flow cytometry. Data bars are represented as percent values ± SD compared with vehicle-treated controls (100%) on day 5 after depletion by gating on anti-GPIb/phycoerythrin and anti-GPV/fluorescein isothiocyanate antibody. **P<.001. Flow cytometric analysis of (B) platelet integrin αIIbβ3 activation and (C) P-selectin exposure of control and MWReg30-treated mice on day 5 (n = 4). CVX, convulxin. *P < .05, **P < .01. (D) Integrin αIIbβ3 activation (binding of JON/A-phycoerythrin) and (E) P-selectin exposure on day 5 after anti-GPIbα antibody or MWReg30 (anti-αIIbβ3) treatment in response to indicated agonists (n = 5). Epi, epinephrine; FITC, fluorescein isothiocyanate; MFI, mean fluorescence intensity; RC, rhodocytin; U46, U46619.
NFYPs after MWReg30-induced thrombocytopenia display impaired GPVI signaling. (A) Platelet count in circulation on day 5 after MWReg30 treatment was determined by flow cytometry. Data bars are represented as percent values ± SD compared with vehicle-treated controls (100%) on day 5 after depletion by gating on anti-GPIb/phycoerythrin and anti-GPV/fluorescein isothiocyanate antibody. **P<.001. Flow cytometric analysis of (B) platelet integrin αIIbβ3 activation and (C) P-selectin exposure of control and MWReg30-treated mice on day 5 (n = 4). CVX, convulxin. *P < .05, **P < .01. (D) Integrin αIIbβ3 activation (binding of JON/A-phycoerythrin) and (E) P-selectin exposure on day 5 after anti-GPIbα antibody or MWReg30 (anti-αIIbβ3) treatment in response to indicated agonists (n = 5). Epi, epinephrine; FITC, fluorescein isothiocyanate; MFI, mean fluorescence intensity; RC, rhodocytin; U46, U46619.
Ex vivo whole-blood perfusion experiments demonstrated significantly reduced adhesion and aggregate formation of NFYPs on collagen (surface coverage control: 46.17 ± 2.0%; NFYPs: 24.03 ± 6.2%, P < .001; Figure 1F). In a model of mechanical aorta injury, where occlusive thrombus formation occurs through GPVI-ITAM-PLCγ2–dependent mechanisms,14,15 87% of control mice formed stable occlusions within 6 to 8 minutes, whereas only 35% of the vessels occluded in mice with NFYPs (Figure 1G). Altogether, these results demonstrated that newly produced platelets in early phase of their life in the circulation are hyporesponsive to GPVI stimulation.
To exclude that the observed GPVI signaling defect in NFYPs was induced by side effects of the anti-GPIbα platelet-depleting antibody, we studied NFYPs in mice treated with MWReg30 (anti-integrin αIIbβ3 antibody), which depletes platelets through an Fc-dependent mechanism.8 MWReg30 was injected together with the platelet-activating factor inhibitor CV-620916 to circumvent acute systemic reaction mediated by Fc receptor–bearing cells.8 Similar to the anti-GPIbα antibody, MWReg30 induced severe thrombocytopenia and the platelet count recovered to ∼65 ± 2.8% on day 5 (Figure 2A). NFYPs showed an increased size (mean forward scatter ± SD vehicle treated: 378 ± 38; MWReg30 antibody treated; 642 ± 122) and normal GPVI surface expression (supplemental Table 2). NFYPs displayed impaired activation responses to GPVI agonists (Figure 2B-C) but unaltered G protein–coupled receptor and CLEC-2 signaling (Figure 2D-E; supplemental Figure 1A). Importantly, this GPVI-ITAM signaling defect was not observed in aged platelets in the circulation (supplemental Figure 2A), and there was no difference in activation between vehicle- and nonimmune immunoglobulin G–treated mice (supplemental Figure 1C-D), further excluding antibody-mediated side effects. Together, these results corroborate the observation that GPVI signaling is diminished in newly produced platelets independently of the exact mechanism underlying the preceding antibody-induced thrombocytopenia.
In the final stages of platelet production, proplatelets release fragments into the blood circulation that are larger than normal platelets. These “preplatelets” have a diameter of 2 to 10 μm and have been shown to reversibly convert into barbell-shaped proplatelets, which further mature into platelets.17 Proplatelets and preplatelets are found at very low numbers in healthy humans and mice.17,18 These structures significantly increase after acute antibody-induced thrombocytopenia in mice and acquire normal platelet size as counts recover.19 In our study, newly produced platelets after antibody-induced thrombocytopenia were also significantly enlarged in size, which decreased over time to normal platelets as the counts were restored in the circulation. In line with this, we found that reticulated platelets in steady state and NFYPs were similarly increased in size compared with nonreticulated platelets (supplemental Figure 2B). Morowski et al used low doses of the anti-GPIbα antibody to reduce the platelet counts to defined ranges without altering platelet activation responses.9 We administered a higher dose of antibody (100 µg) to induce acute platelet loss and thereby trigger MKs to rapidly release NFYPs and preplatelet-like fragments into the circulation. Notably, the antibody treatment did not affect MK ultrastructure, maturation, ploidy levels, surface expression levels of GPVI and αIIbβ3, or proplatelet formation in a BM explant model (supplemental Figure 3).
Together, our data suggest that newly formed platelets may only assemble or activate their GPVI-ITAM signaling machinery once they enter the circulation. We identified this mechanism using a model of platelet depletion/recovery in which the number of newly formed platelets is very high in a certain time window after depletion. However, we speculate that this mechanism is also operative under steady state but is difficult to assess, as the percentage of NFYPs is much lower and gain of GPVI-ITAM functionality may occur with different kinetics compared with platelet recovery after depletion. Based on the specific hypophosphorylation of the GPVI signaling pathway in NFYPs, it is tempting to speculate that molecular switches exist that interfere with proximal GPVI signaling in MKs and proplatelets to prevent their premature activation. The exact underlying mechanism was not identified here, but it might be based on inhibitory signals generated by components of the BM microenvironment that keep the GPVI-ITAM machinery in an “off-state,” with G6b-B20 as one possible receptor to generate such signals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stefanie Hartmann, Jonas Müller, Sabine Schübert, and Melanie Hüttenrauch for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 688 and grants Ni556/9-2 [B.N.] and CH1734/1-1 [D.C.]) and the Rudolf Virchow Center. S.G. was supported by a grant from the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg, and National Institutes of Health, National Heart, Lung, and Blood Institute grant PO1-HL40387. M.B. is supported by the Emmy Noether (grant BE5084/3-1).
Authorship
Contribution: S.G., D.C., and M.B. performed experiments, analyzed data, and wrote the paper; M.M. and M.S. performed experiments and analyzed data; and B.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, Institute of Experimental Biomedicine I, University Hospital Würzburg and Rudolf Virchow Center, University of Würzburg, Josef-Schneider-Str 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal