In this issue of Blood, Gupta et al show that collagen-mediated signaling pathways within new platelets are significantly reduced and that collagen signaling is an acquired property of circulating platelets.1
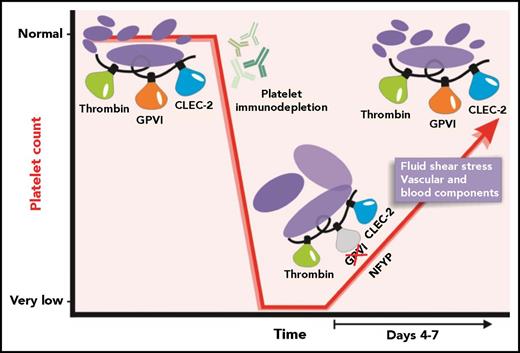
Under normal physiological conditions, platelet production maintains a platelet count at an appropriate level. Treatment of mice with anti-platelet antibodies results in a rapid specific immunodepletion of platelets. After the anti-platelet antibodies are cleared, recovery of the platelet count ensues. NFYPs are produced, and, during the recovery phase, a transient loss of GPVI-specific function can be detected. Time-dependent exposure to vascular and blood-specific components as the NFYPs circulate may subsequently enable GPVI function as a normal platelet count is restored.
Under normal physiological conditions, platelet production maintains a platelet count at an appropriate level. Treatment of mice with anti-platelet antibodies results in a rapid specific immunodepletion of platelets. After the anti-platelet antibodies are cleared, recovery of the platelet count ensues. NFYPs are produced, and, during the recovery phase, a transient loss of GPVI-specific function can be detected. Time-dependent exposure to vascular and blood-specific components as the NFYPs circulate may subsequently enable GPVI function as a normal platelet count is restored.
Circulating platelets are produced from megakaryocytes (MKs) primarily in the bone marrow (BM). Active MKs reside near marrow sinusoids and release proplatelets from their tips into the blood circulation.2 Newly formed young platelets (NFYPs) emerge from proplatelets with a full repertoire of surface proteins and receptors designed to bind extracellular matrix and plasma proteins including von Willebrand factor (VWF), fibrinogen, and collagens.
In a healthy organism, platelet production is a continuous process and it is a major challenge to capture and analyze platelet function in NFYPs, which represent <10% of a circulating platelet population. To circumvent these issues, Gupta and colleagues synchronized the age of a circulating platelet population using a murine model of platelet recovery after immunodepletion with antibodies targeting either glycoprotein Ibα (GPIbα) or integrin αIIbβ3 (Fc-independent and -dependent mechanisms, respectively). Control samples were generated from vehicle- or isotype-matched antibody-treated mice. As the platelet supply was renewed, the researchers carried out a temporal analysis of the function of the circulating platelet population that was enriched with NFYPs released by MKs.
As expected, NFYPs were larger and had surface levels of important activation receptors including integrin αIIbβ3, GPVI, GPIbα, and a second ITAM-signaling receptor, C-type lectin-like receptor 2 (CLEC-2), that were either within normal ranges or slightly elevated. However, the authors identified a pronounced reduction in response to the GPVI-specific agonist collagen-related peptide (CRP), as well as to collagen and convulxin in light transmission aggregometry. This loss of response to CRP was confirmed in flow cytometric assays measuring surface P-selectin and active αIIbβ3, in platelet adhesion after whole blood perfusion over collagen, and in a mechanical aorta injury model where functional GPVI is required for robust thrombus formation. This was a GPVI-specific effect as NFYP responses to thrombin and the CLEC-2–specific agonist rhodocytin were similar to those of platelets from control mice. Western blot analysis detected normal amounts of immunoreceptor tyrosine-based activation motif (ITAM)-related signaling proteins spleen tyrosine kinase (Syk), linker for activation of T cells (LATs), and phospholipase Cγ2 (PLCγ2) in NFYP but significantly reduced phosphorylation in response to CRP exposure. This effect was transient as full GPVI-mediated degranulation and αIIbβ3 activation was restored once the circulating platelet count was normalized (see figure). The authors propose that downregulation of GPVI-ITAM signaling in NFYPs serves to protect them from excessive activation while traversing the collagen-rich endothelium during their release from MKs.
Platelets have an array of collagen-binding receptors including GPV, GPVI, CD36, and the integrin α2β1. Engagement of any of these receptors by collagen triggers immediate and robust activation of platelet pathways and/or adhesion. GPVI orchestrates the major platelet signaling response to collagen via engagement of an ITAM-coupled signaling pathway,3 which triggers phosphorylation of Src family kinases, as well as Syk, LAT, and PLCγ2.
NFYPs are likely to be equivalent to reticulated platelets, which are larger, contain femtogram amounts of ribonucleic acid,4 and exhibit a significant rate of degranulation across the first day in circulation. Interestingly and perhaps out of step with the current study, there are a number of reports showing that young platelets have greater functional capacity than those that have been in circulation for at least 1 day.5,6
The nature of the molecular switch that triggers new platelets to eventually develop functional GPVI signaling is a key question arising from this study. Possible triggers that could conceivably facilitate development of fully functional GPVI signaling may include exposure to fluid shear rates ranging from 100 to 10 000 s−1 across the vasculature7 and/or removal of an inhibitory signal(s) present in the BM compartment or onset of molecular signals generated by new platelets engaging a blood element. The fact that CLEC-2 responses were intact and only GPVI-mediated responses appear to be modulated in new platelets implies that the new platelet effect may be somehow linked to GPVI receptor structure. GPVI is a 60-kDa member of the immunoglobulin-like superfamily of receptors and has 2 immunoglobulin-like domains connected to an O-glycosylated region, a transmembrane domain, and a cytoplasmic tail. Although the GPVI monomer is functional, at least a portion of GPVI is present in a dimeric form8 that binds collagen with high affinity. Like CLEC-2, ligand-induced signaling by GPVI is strongest when the receptor dimerizes, however, unlike CLEC-2, which has ITAM sequences within its cytoplasmic tail, GPVI uses ITAM sequences with the Fc receptor γ (FcRγ) chain by forming a salt bridge with FcRγ.3 It will be of interest to evaluate any differences in receptor dimerization capacity and quantify signaling proteins directly associated with the cytoplasmic regions of GPVI/FcRγ along with presence and function of inhibitory molecules in new platelets.
Finally, numerous studies have shown degrees of hyporesponsiveness in neonatal platelets to standard platelet agonists, but most dramatically collagen.9 This is reflected in decreased granule secretion and impaired fibrinogen binding and platelet aggregation, which persists for several weeks after birth, although notably normal hemostasis is not impaired, possibly because of elevated red cell parameters and increased amounts of a higher-molecular-weight VWF. The mechanism underlying transient hyporesponsiveness to CRP and other agonists in neonatal platelets remains largely unknown10 ; however, when considering the work from Gupta and colleagues, it is intriguing to speculate that the rapid generation of platelets at times of need (ie, in development or following immune depletion) could be facilitated by ablation of GPVI signaling.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal