Key Points
ADCT-402 is a CD19-targeted ADC delivering SG3199, a cytotoxic DNA minor groove interstrand crosslinking PDB dimer warhead.
ADCT-402 has potent and selective antitumor activity against CD19-expressing hematological malignancies warranting clinical development.
Abstract
Human CD19 antigen is a 95-kDa type I membrane glycoprotein in the immunoglobulin superfamily whose expression is limited to the various stages of B-cell development and differentiation and is maintained in the majority of B-cell malignancies, including leukemias and non-Hodgkin lymphomas of B-cell origin. Coupled with its differential and favorable expression profile, CD19 has rapid internalization kinetics and is not shed into the circulation, making it an ideal target for the development of antibody-drug conjugates (ADCs) to treat B-cell malignancies. ADCT-402 (loncastuximab tesirine) is a novel CD19-targeted ADC delivering SG3199, a highly cytotoxic DNA minor groove interstrand crosslinking pyrrolobenzodiazepine (PDB) dimer warhead. It showed potent and highly targeted in vitro cytotoxicity in CD19-expressing human cell lines. ADCT-402 was specifically bound, internalized, and trafficked to lysosomes in CD19-expressing cells and, following release of the PBD warhead, resulted in formation of DNA crosslinks that persisted for 36 hours. Bystander killing of CD19− cells by ADCT-402 was also observed. In vivo, single doses of ADCT-402 resulted in highly potent, dose-dependent antitumor activity in several subcutaneous and disseminated human tumor models with marked superiority to comparator ADCs delivering tubulin inhibitors. Dose-dependent DNA crosslinks and γ-H2AX DNA damage response were measured in tumors by 24 hours after single dose administration, whereas matched peripheral blood mononuclear cells showed no evidence of DNA damage. Pharmacokinetic analysis in rat and cynomolgus monkey showed excellent stability and tolerability of ADCT-402 in vivo. Together, these impressive data were used to support the clinical testing of this novel ADC in patients with CD19-expressing B-cell malignancies.
Introduction
Human CD19 antigen is a 95-kDa type I membrane glycoprotein belonging to the immunoglobulin superfamily.1 In normal human tissue, CD19 expression is restricted to the various stages of B-cell development and differentiation (except hematological stem and plasma cells),2,3 and its expression is maintained in the majority of B-cell malignancies, including leukemias and non-Hodgkin lymphomas (NHLs) of B-cell origin.4
Because of its widespread expression, CD19 is an attractive therapeutic target, and several antibody-based therapies are in clinical development.5 A bispecific scFv anti-CD19/anti-CD3 T-cell engager blinatumomab (Blincyto) is approved for the treatment of second-line Philadelphia chromosome-negative relapsed or refractory (R/R) acute lymphoblastic leukemia (ALL).6 CD19 also remains the most investigated target for chimeric antigen receptor T-cell therapy.7
CD19 has rapid internalization kinetics8,9 and is not shed into the circulation.10 These features, coupled with its differential and favorable expression profile, make CD19 an ideal target for the development of antibody-drug conjugates (ADCs) to treat B-cell malignancies. Indeed, anti-CD19 auristatin-containing ADC denintuzumab mafodotin (SGN-CD19A)11 and maytansinoid-containing coltuximab ravtansine (SAR3419)12 have been investigated in the clinic.
As an alternative to the delivery of tubulin binding-based warheads, novel ADCs delivering highly cytotoxic pyrrolobenzodiazepine (PBD) dimers have been developed.13-15 PBD dimers are a class of exquisitely potent DNA minor groove interstrand crosslinking agents16 ; one of which, SG2000 (SJG-136) has shown activity against both solid tumor and hematological malignancies.17,18 Advantages over ADCs using other warheads including tubulin inhibitors (eg, auristatins, maytansinoids), DNA cleavage agents (eg, calicheamicin), and classical chemotherapeutics include the ability to target low copy number antigens and tumor-initiating cells14 and to exploit low drug–antibody ratios (DARs). Because of their novel mechanism of action, PBD-containing ADCs are active in tumors inherently resistant to other warhead types and against multidrug-resistant cancers.13
PBD dimer SG3199 (J.A.H., Michael J. Flynn, John P. Bingham, S. Corbett, Halla Reinert, A.T., Luke A. Masterson, Dyeison Antonow, L.A., Sajidah Chowdhury, D.G.W., Shenlan Mao, Jay Harper, C.E.G.H., F.Z., S. Chivers, P.H.v.B., and P.W.H., manuscript in preparation) is the released warhead component of the ADC payload tesirine (SG3249),19 currently being evaluated clinically in several ADCs including ADCT-301, ADCT-502, MEDI3726, and rovalpituzumab tesirine (Rova-T) for which phase 1 data in small cell lung cancer have been reported.20 It is currently in several phase 2 and 3 studies.
Here, we report the preclinical evaluation of the novel anti-CD19 ADC ADCT-402 (loncastuximab tesirine) containing the PBD dimer payload tesirine. On the basis of these impressive preclinical data, this agent is currently evaluated in phase 1 studies of both R/R NHL and R/R B-ALL.
Methods
Synthesis of ADC
RB4v1.2 antibody was conjugated to tesirine essentially as previously described.15 The ADC was formulated in 30 mM histidine, 200 mM sorbitol, 0.02% Tween-20, pH 6.0. The solution was filtered (0.22 μm) and stored at −70°C.
ADCs RB4v1.2-DM4 and hBU12-mc-MMAF were generated by Concortis (San Diego, CA) as described in Al-Katib et al21 and patent US 8 242 252 B2, respectively.
Characterization of ADCT-402 by SEC, HIC, and RPLC
Characterization of synthesized ADCT-402 was performed by size exclusion chromatography (SEC), hydrophobic interaction chromatography (HIC), and reduced reverse phase liquid chromatography (RPLC), as previously described.15
Human cell lines
The source of cell lines used in this study along with cell growth media are shown in supplemental Table 1, available on the Blood Web site.
CD19 cell surface density
Cell surface CD19 density was determined using Bangs Laboratory Quantum Simply Cellular Anti-human immunoglobulin G (IgG) beads according to the manufacturer’s instructions.
In vitro cell killing and bystander assay
Cell lines were incubated in growth medium with serial dilutions of ADCT-402, the isotype control ADC, or the free warhead SG3199 for 5 days at 37°C in a 5% CO2-gassed, humidified incubator. Cell viability was measured with CellTiter 96 AQueous One Solution Cell Proliferation (Promega) according to manufacturer’s instructions. The data were normalized to vehicle-treated cells. The 50% inhibitory (IC50) values were determined by using GraphPad software (GraphPad, La Jolla, CA). The mean and standard errors of the means of 3 independent IC50 values were determined.
For the bystander assay, Ramos or NCI-N87 cells were incubated for the indicated time points with ADCT-402. Conditioned medium was transferred to Karpas-299 cells, incubated for 4 days, and cell viability measured.
Internalization studies
Cells were exposed to ADCT-402 for 1 hour at 4°C and then incubated at 37°C where appropriate. Following permeabilization of cells using Tween-20 (0.1% volume-to-volume ratio in phosphate-buffered saline) for 15 minutes, samples were washed with phosphate-buffered saline and centrifuged at 4000 rpm (4°C). Removal of the supernatant was followed by addition of rabbit monoclonal antibody LAMP-1 (1:400; Cell Signaling) for 1 hour on ice. A further wash step was followed by adding both Alexa Fluor 488 Goat Anti-Human (1:200; Thermo Fisher Scientific) for detecting the ADC, and Alexa Fluor 568 Goat Anti-Rabbit (1:200; Thermo Fisher Scientific). Both secondary antibody incubations were performed on ice for 60 minutes. Nuclei were counterstained with Hoescht 33342 (Thermo Fisher Scientific), and then cytospins of cell samples were prepared and samples mounted in ProLong Gold Antifade (Life Tech) and coverslipped.
In vitro and in vivo crosslinking determination
Assessment of ADCT-402 efficacy in in vivo models
All animal studies were performed in facilities that are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, which assures compliance with accepted standards for the care and use of laboratory animals. Ramos, Daudi, and WSU-DLCL2 xenografts were established in 8- to 10-week-old female Fox Chase severe combined immunodeficiency (SCID; Charles River) by implanting 107 cells subcutaneously (SC) into their flanks. When group mean tumor volumes reached approximately 116 to 132 mm3, mice were randomly allocated into groups to receive test agent or vehicle. Each animal was euthanized when its tumor reached the end point volume or at study end. The time to end point (TTE) for analysis was calculated for each mouse by the following equation:
*Indicates the line obtained by linear regression of a log-transformed tumor growth data set.
The log-rank test was used to analyze the significance of differences between the TTE values of 2 groups. For the disseminated xenografts model, 5 × 106 Ramos cells and 1 × 107 NALM-6 cells were injected IV into the lateral tail vein of 5- to 7-week-old female Fox Chase SCID and NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac (Taconic) mice, respectively. Treatment with test compounds was initiated 7 and 3 days after tumor inoculation in Ramos and NALM-6 models, respectively. The day of euthanasia represented the TTE. Animals that did not reach the end point were euthanized at the end of the study and assigned a TTE value equal to the last study day. The log-rank test was used to determine the significance of the difference between the overall survival experiences (survival curves) of 2 groups based on their TTE values.
Immunohistochemistry of tumor xenografts and clinical samples sections
Detection of phospho-histone H2A.X (2577; Cell Signaling) and anti-PBD (mouse monoclonal 14B3-B7; Spirogen) in murine formalin fixed paraffin embedded (FFPE) tissue sections was performed using the Leica Bond Max automated staining platform. In brief, both primary antibodies were applied at 1:50 dilution for 30 minutes following antigen retrieval of sections using Epitope retrieval solution 2 (Leica Biosystems). Peroxidase block (3% to 4% [volume-to-volume ratio] H2O2) followed primary antibody application; antibody detection was performed using the Bond Polymer refine detection system (DS9800; Leica Biosystems). Detection of human CD19 (NCL CD19-163; Leica Biosystems) in FFPE tissue sections of murine xenograft and human clinical samples was performed using the Roche BenchMark Ultra staining system. In brief, CD19 primary antibody was applied for 30 minutes at 1:50 dilution following antigen retrieval of sections using CC1 solution (Ventana) for 60 minutes and a peroxidase blocking step. Detection of anti-CD19 antibody was performed using the Optiview DAB Detection Kit (760-700).
Human lymphoma and leukemia cases enrolled in this study (Table 1) were diagnosed according to the criteria of the World Health Organization classification for hematological malignancies23 by an expert hematopathologist (T.M.) at University College London Hospital. The immunohistochemical investigation was conducted according to the principles of the Helsinki declaration after approval of the internal review board, the National Research Ethics Service, Research Ethics Committee (REC) 4 (09/H0715/64).
CD19 expression in matched primary and relapsed clinical samples
| Clinical subtype . | Case no., P/R . | Biopsy type . | Time between biopsy (mo) . | CD19 intensity*† . |
|---|---|---|---|---|
| DBLCL | 1P | BM | 8 | 2 |
| 1R | BM | 3 | ||
| 2P | LN | 20 | 3 | |
| 2R | Mesenteric mass | 3 | ||
| 3P | Testis | 12 | 3 | |
| 3R | Penile mass | 3 | ||
| 4P | LN | 14 | 3 | |
| 4R | LN | 3 | ||
| 5P | BM | 8 | 2 | |
| 5R | LN | 3 | ||
| MCL | 6P | Salivary gland | 20 | 3 |
| 6R | Submandibular | 3 | ||
| 7P | BM | 11 | 3 | |
| 7R | LN | 3 | ||
| 8P | LN | 15 | 3 | |
| 8R | LN | 3 | ||
| 9P | Gastrointestinal | 8 | 3 | |
| 9R | Duodenal bulb | 3 | ||
| FL | 10P | LN | 27 | 3 |
| 10R | LN | 3 | ||
| 11P | LN | 26 | 3 | |
| 11R | LN | 3 | ||
| Small/chronic LL | 12P | BM | 20 | 3 |
| 12R | BM | 2 |
| Clinical subtype . | Case no., P/R . | Biopsy type . | Time between biopsy (mo) . | CD19 intensity*† . |
|---|---|---|---|---|
| DBLCL | 1P | BM | 8 | 2 |
| 1R | BM | 3 | ||
| 2P | LN | 20 | 3 | |
| 2R | Mesenteric mass | 3 | ||
| 3P | Testis | 12 | 3 | |
| 3R | Penile mass | 3 | ||
| 4P | LN | 14 | 3 | |
| 4R | LN | 3 | ||
| 5P | BM | 8 | 2 | |
| 5R | LN | 3 | ||
| MCL | 6P | Salivary gland | 20 | 3 |
| 6R | Submandibular | 3 | ||
| 7P | BM | 11 | 3 | |
| 7R | LN | 3 | ||
| 8P | LN | 15 | 3 | |
| 8R | LN | 3 | ||
| 9P | Gastrointestinal | 8 | 3 | |
| 9R | Duodenal bulb | 3 | ||
| FL | 10P | LN | 27 | 3 |
| 10R | LN | 3 | ||
| 11P | LN | 26 | 3 | |
| 11R | LN | 3 | ||
| Small/chronic LL | 12P | BM | 20 | 3 |
| 12R | BM | 2 |
BM, bone marrow; DBLCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; LL, lymphocytic leukemia; LN, lymph node; MCL, mantle cell lymphoma; P, primary; R, relapsed.
0 = negative, 1 = weak, 2 = moderate, 3 = strong staining as determined by pathologist review.
In all samples analyzed, >80% tumor cells were CD19+.
Assessment of ADCT-402 pharmacokinetics (PK)
All animal studies were performed at research facilities Association for Assessment and Accreditation of Laboratory Animal Care accredited.
Male Crl:CD(SD) rats (Charles River Laboratories, Edinburgh, United Kingdom) were dosed IV with ADCT-402. Blood was collected from tail veins at specified time points; serum was isolated and stored at −80°C.
Male and female purpose-bred Cynomolgus monkeys (Macaca fascicularis) were dosed IV with ADCT-402. Blood was collected from vena cephalica antebrachii or vena saphena at specific time points; serum was isolated and stored at −80°C.
In rat and monkey serum, quantification of total antibody and ADC (DAR ≥1) were determined using optimized electrogenerated chemiluminescence immunoassay homogenous formats. Calibration curves, quality controls, and study samples were diluted and mixed with a biotinylated anti-idiotypic antibody for both the total antibody and ADC measurements (biotinylated anti-PBD antibody for ADC in the monkey) and a Sulfo-tag labeled anti-idiotypic antibody for total antibody and a Sulfo-tag labeled anti-PBD antibody for the ADC (Sulfo-tag labeled anti-idiotypic antibody for ADC in the monkey) to allow complex formation. The complex was added to streptavidin-coated MSD plates and, following the addition of read buffer, the plate was read on the MSD QuickPlex Plate Reader (6000 Sector Imager [MSD] for the monkey).
The determination of free SG3199 in cynomolgus monkey serum was performed by liquid chromatography tandem mass spectometry and used deuterium-labeled SG3199 (SG3199-d10) as internal standard. Isolation of SG3199 (and spiked SG3199-d10) was performed by reduction with cyanoborohydride overnight and followed by off-line solid phase extraction using Oasis HLB 96-well µElution Plate, 30 µm particle size. The purified samples were analyzed using an AB Sciex Qtrap 5500 Liquid Chromatography Tandem Mass Spectometry System with a Zorbax SB-AQ Rapid Resolution HT (2.1 × 50 mm; 1.8 µm) column. The PK analysis was performed using Phoenix WinNonlin, version 6.2, with noncompartmental analysis.
Results
Properties of ADCT-402
ADCT-402 is composed of the humanized IgG1 antibody RB4v1.224 directed against human CD19 and conjugated at interchain cysteines to tesirine using maleimide chemistry. Tesirine consists of a cathepsin-cleavable valine-alanine linker that releases the PBD dimer warhead SG319919 (Figure 1A). ADCT-402 is 96% monodisperse as determined by SEC (Figure 1B). DAR was determined to be 2.3 by RPLC (Figure 1C) and HIC (Figure 1D).
Characterization of ADCT-402. (A) Structure of ADCT-402. (B) ADCT-402 characterized by size exclusion chromatography. (C) Reduced reverse phase liquid chromatography depicting reduced heavy and light chains of ADCT-402. (D) Hydrophobic interaction chromatography depicting drug–antibody ratio forms of ADCT-402.
Characterization of ADCT-402. (A) Structure of ADCT-402. (B) ADCT-402 characterized by size exclusion chromatography. (C) Reduced reverse phase liquid chromatography depicting reduced heavy and light chains of ADCT-402. (D) Hydrophobic interaction chromatography depicting drug–antibody ratio forms of ADCT-402.
Selective cytotoxicity of ADCT-402 in vitro
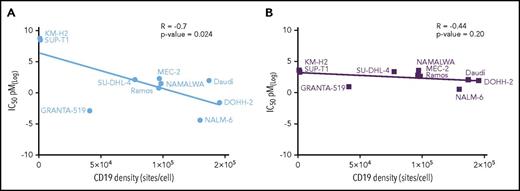
The in vitro cytotoxicity of ADCT-402 was determined in a panel of 8 CD19-expressing and 2 nonexpressing cell lines. The IC50 values were plotted against the cell surface CD19 density for each cell line (Figure 2A), in which a weak, yet significant negative correlation was observed (r = −0.7, P = .024). In contrast, SG3199, the PBD dimer warhead component of ADCT-402 did not show any relationship between IC50 value and cell surface CD19 (r = −0.44, P = .2), consistent with its nontarget nature. Interestingly, the Granta-519 and NALM-6 cell lines, which were the most sensitive to SG3199, also showed the greatest sensitivity to the ADC.
Correlation between in vitro cytotoxicity and cell surface CD19 density. (A) IC50 values (mean of 3 independent determinations) of ADCT-402 against the named cell lines are plotted against the measured cell surface CD19 levels expressed as sites/cell. The line is the regression through all data points. (B) The equivalent analysis performed for the naked PBD dimer warhead SG3199. Linear relationship between indicated variables was calculated by Pearson correlation coefficient r test.
Correlation between in vitro cytotoxicity and cell surface CD19 density. (A) IC50 values (mean of 3 independent determinations) of ADCT-402 against the named cell lines are plotted against the measured cell surface CD19 levels expressed as sites/cell. The line is the regression through all data points. (B) The equivalent analysis performed for the naked PBD dimer warhead SG3199. Linear relationship between indicated variables was calculated by Pearson correlation coefficient r test.
ADCT-402 mechanism of action
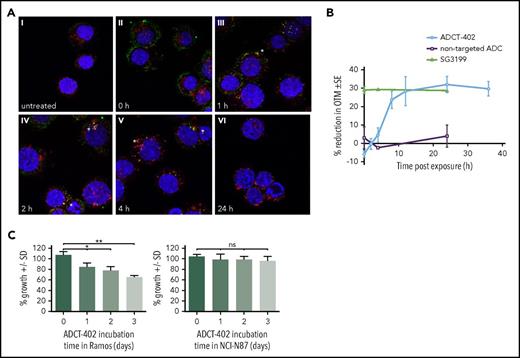
CD19-expressing Ramos cells exposed to ADCT-402 (1 hour at 4°C) showed prominent cell surface binding (T = 0, Figure 3A). On incubation at 37°C, ADCT-402 showed internalization with some cell membrane labeling still evident at 1 hour, but also some colocalization with lysosomes. This colocalization increased at 2 and 4 hours and no evidence of residual ADC staining was evident at 24 hours, suggesting complete lysosomal degradation.
Mechanism of action of ADCT-402. (A) Merged immunofluorescent images of CD19+, human Burkitt lymphoma-derived Ramos cells treated with 2 μg/mL ADCT-402 for 1 hour; washed and fixed after T = 0, 1, 2, 4, or 24 hours in medium at 37°C; and stained with labeled antihuman IgG (green), anti-LAMP-1 (red), and Hoechst 33342 (blue) nuclear stain. Yellow indicates colocalization, with specific sites of colocalization indicated by stars. Original magnification ×63. (B) Time course of DNA interstrand crosslinking measured as the % reduction on Olive Tail Moment in Ramos cells treated for 2 hours with ADCT-402 (40 pM), SG3199 (10 pM), or a nontargeted ADC (40 pM) followed by postincubation in drug-free medium for the indicated time. Results are the mean ± standard deviation from at least 3 independent experiments. (C) Percentage viability of CD19− Karpas-299 cells after 96 hours of exposure to media transferred from ADCT-402–treated CD19+ Ramos cells or CD19− NCI-N87 cells for 1, 2, or 3 days. Statistical analysis was done with unpaired t test with Welch’s correction (not assuming equal standard deviations). P values are 2-tailed. *P ≤ .05, **P ≤ .01. ns, not significant.
Mechanism of action of ADCT-402. (A) Merged immunofluorescent images of CD19+, human Burkitt lymphoma-derived Ramos cells treated with 2 μg/mL ADCT-402 for 1 hour; washed and fixed after T = 0, 1, 2, 4, or 24 hours in medium at 37°C; and stained with labeled antihuman IgG (green), anti-LAMP-1 (red), and Hoechst 33342 (blue) nuclear stain. Yellow indicates colocalization, with specific sites of colocalization indicated by stars. Original magnification ×63. (B) Time course of DNA interstrand crosslinking measured as the % reduction on Olive Tail Moment in Ramos cells treated for 2 hours with ADCT-402 (40 pM), SG3199 (10 pM), or a nontargeted ADC (40 pM) followed by postincubation in drug-free medium for the indicated time. Results are the mean ± standard deviation from at least 3 independent experiments. (C) Percentage viability of CD19− Karpas-299 cells after 96 hours of exposure to media transferred from ADCT-402–treated CD19+ Ramos cells or CD19− NCI-N87 cells for 1, 2, or 3 days. Statistical analysis was done with unpaired t test with Welch’s correction (not assuming equal standard deviations). P values are 2-tailed. *P ≤ .05, **P ≤ .01. ns, not significant.
DNA interstrand crosslinking was measured using a modification of the single cell gel electrophoresis (comet) assay. Crosslinking was expressed as the percentage decrease in Olive Tail Moment (OTM) compared with control irradiated cells. Following a 2-hour exposure of Ramos cells to ADCT-402, crosslink formation occurred (Figure 3B). Crosslinks form after an initial delay, reaching a peak at around 12 hours and persisting up to 36 hours. In contrast, the free warhead SG3199 reached the peak of crosslinking during the initial 2-hour exposure. An equivalent concentration of a nonbinding ADC did not show the formation of any crosslinks.
Ramos cells were treated with ADCT-402 for 1, 2, or 3 days before transferring the media onto CD19− Karpas-299 cells and incubating for 96 hours. ADCT-402 conditioned medium elicited a bystander effect after 1 day of pretreatment, as shown by a decrease in percentage survival in the conditioned medium-treated Karpas-299 cells compared with the nonconditioned medium (100% to 86%; Figure 3C, left). The effect was even greater after 2 or 3 days of preincubation in Ramos cells, as shown by a further decrease in percentage cell survival compared with the nonconditioned medium (80% and 65%, P = .001). Conversely, conditioned medium from ADCT-402–treated, CD19− NCI-N87 cells did not elicit any bystander effect when transferred onto Karpas-299 cells, regardless of preincubation time (Figure 3C, right).
ADCT-402 in vivo efficacy
ADCT-402 demonstrated dose-dependent antitumor activity against both SC and disseminated tumor models in vivo. In the SC Ramos xenograft, a single dose of ADCT-402 at 0.33, 0.66, and 1 mg/kg induced dose-dependent antitumor activity and resulted in 5/10 and 10/10 tumor-free survivors (TFSs) at the 0.66 and 1 mg/kg doses, respectively, on day 60 (Figure 4A). The resulting Kaplan-Meier survival curves show the dose-dependent extension of survival (log-rank test, P ≤ .004 for each comparison; supplemental Figure 1A).
Comparative antitumor activity of ADCT-402 in the human CD19-expressing Burkitt lymphoma-derived Ramos model. (A) In vivo antitumor activity of ADCT-402 in SC implanted Ramos xenograft model. ADCT-402 was administered IV at a group mean tumor volume of 120 mm3 as a single dose at 0.33, 0.66, or 1 mg/kg. (B) In vivo antitumor activity of ADCT-402 in SC implanted Ramos xenograft model in comparison with ADC RB4v1.2-DM4 and hBU12-mc-MMAF. All 3 ADCs were compared at a single dose of 1 mg/kg; in addition, RB4v1.2-DM4 and hBU12-mc-MMAF were tested at 3.3 mg/kg every 4 days × 2 and 3 mg/kg every 4 days × 4, respectively. (C) In vivo antitumor activity of ADCT-402 in SC implanted Ramos xenograft model where ADCT-402 was administered at a single dose of 1 mg/kg or fractionated dosing of 0.33 mg/kg given either every week × 3 or every 4 days × 3. (A-C) Data are shown as mean ± SEM from animal group sizes of 10 mice. (D) In vivo antitumor activity of ADCT-402 in a disseminated Ramos model. Kaplan-Meier survival plots show percentage animal survival over 91 days in an experiment in which ADCT-402 was administered at a single dose of 0.33 mg/kg or 1 mg/kg in comparison with vehicle or nontargeting ADC administered as a single dose of 1 mg/kg (each group, n = 10). PBS, phosphate-buffered saline; SEM, standard error of the mean.
Comparative antitumor activity of ADCT-402 in the human CD19-expressing Burkitt lymphoma-derived Ramos model. (A) In vivo antitumor activity of ADCT-402 in SC implanted Ramos xenograft model. ADCT-402 was administered IV at a group mean tumor volume of 120 mm3 as a single dose at 0.33, 0.66, or 1 mg/kg. (B) In vivo antitumor activity of ADCT-402 in SC implanted Ramos xenograft model in comparison with ADC RB4v1.2-DM4 and hBU12-mc-MMAF. All 3 ADCs were compared at a single dose of 1 mg/kg; in addition, RB4v1.2-DM4 and hBU12-mc-MMAF were tested at 3.3 mg/kg every 4 days × 2 and 3 mg/kg every 4 days × 4, respectively. (C) In vivo antitumor activity of ADCT-402 in SC implanted Ramos xenograft model where ADCT-402 was administered at a single dose of 1 mg/kg or fractionated dosing of 0.33 mg/kg given either every week × 3 or every 4 days × 3. (A-C) Data are shown as mean ± SEM from animal group sizes of 10 mice. (D) In vivo antitumor activity of ADCT-402 in a disseminated Ramos model. Kaplan-Meier survival plots show percentage animal survival over 91 days in an experiment in which ADCT-402 was administered at a single dose of 0.33 mg/kg or 1 mg/kg in comparison with vehicle or nontargeting ADC administered as a single dose of 1 mg/kg (each group, n = 10). PBS, phosphate-buffered saline; SEM, standard error of the mean.
In the same Ramos model, ADCT-402 was tested together with the CD19-targeted ADC delivering maytansinoid (RB4v1.2-DM4, DAR = 3.3) or auristatin (hBU12-mc-MMAF, DAR = 4.2) warheads. ADCT-402 at a single dose of 1 mg/kg was remarkably superior to both the ADCs delivering tubulin binders at an equivalent single dose (Figure 4B). This superiority was maintained for ADCT-402 at 1 mg/kg even when the comparator ADCs were administered at higher doses with multiple administrations: 3.3 mg/kg every 4 days × 2 for RB4v1.2-DM4 and 3 mg/kg every 4 days × 4 for hBU12-mc-MMAF, respectively (Figure 4B). The equivalent warhead amounts administered are given in supplemental Table 2 and Kaplan-Meier survival curves are shown in supplemental Figure 1B. ADCT-402 administered at 1 mg/kg induced a significant increase in survival compared with both comparator ADC irrespective of dose level and frequency of administration (log-rank test, P ≤ .001 for each comparison). In this same model, although a single dose of ADCT-402 at 1 mg/kg gave 10/10 TFS (Figure 4C), fractionating the dose to 0.33 mg/kg given weekly × 3, or every 4 days × 3, did not achieve the same level of efficacy (Figure 4C).
In a disseminated Ramos model, single-dose ADCT-402 also showed significant extension of survival in comparison with controls (log-rank test, P ≤ .001; Figure 4D) with 9/10 and 10/10 animals surviving at day 91 for the 0.33 and 1 mg/kg groups, respectively, compared with no survival at day 19 for the vehicle and 1 mg/kg nontarget ADC groups.
ADCT-402 also showed dose-dependent activity in the CD19-expressing Daudi xenograft model (Figure 5A). At a single dose of 0.3 mg/kg, all of the animals achieved complete regression with 7/10 classified as TFS at the end of the study (day 73). Kaplan-Meier survival curves show the dose-dependent extension of survival (log-rank test, P ≤ .001 for each comparison; supplemental Figure 2A).
Antitumor activity of ADCT-402 in a range of tumor models. (A) In vivo antitumor activity of ADCT-402 in SC implanted CD19-expressing Burkitt lymphoma-derived Daudi xenograft model. ADCT-402 was administered IV at a group mean tumor volume of 130 mm3 as a single dose at 0.1 or 0.3 mg/kg. (B) In vivo antitumor activity of ADCT-402 in SC implanted CD19-expressing diffuse large B-cell lymphoma-derived WSU-DLCL2 xenograft model. ADCT-402 was administered IV at a group mean tumor volume of 120 mm3 as a single dose at 0.3 or 1 mg/kg. (A-B) Mean ± SEM from animal group sizes of 10 mice. (C) In vivo antitumor activity of ADCT-402 in a disseminated CD19-expressing ALL-derived NALM-6 model. Kaplan-Meier survival plots show percentage of animal survival over 90 days in an experiment in which ADCT-402 was administered at a single dose of 0.33 or 1 mg/kg in comparison with vehicle or nontargeting ADC administered as a single dose of 1 mg/kg (each group, n = 10).
Antitumor activity of ADCT-402 in a range of tumor models. (A) In vivo antitumor activity of ADCT-402 in SC implanted CD19-expressing Burkitt lymphoma-derived Daudi xenograft model. ADCT-402 was administered IV at a group mean tumor volume of 130 mm3 as a single dose at 0.1 or 0.3 mg/kg. (B) In vivo antitumor activity of ADCT-402 in SC implanted CD19-expressing diffuse large B-cell lymphoma-derived WSU-DLCL2 xenograft model. ADCT-402 was administered IV at a group mean tumor volume of 120 mm3 as a single dose at 0.3 or 1 mg/kg. (A-B) Mean ± SEM from animal group sizes of 10 mice. (C) In vivo antitumor activity of ADCT-402 in a disseminated CD19-expressing ALL-derived NALM-6 model. Kaplan-Meier survival plots show percentage of animal survival over 90 days in an experiment in which ADCT-402 was administered at a single dose of 0.33 or 1 mg/kg in comparison with vehicle or nontargeting ADC administered as a single dose of 1 mg/kg (each group, n = 10).
ADCT-402 was less effective at treating the WSU-DLCL2 xenograft model (Figure 5B). Although a clear dose-response was observed, the highest single dose used (1 mg/kg) produced a 30-day tumor growth delay only. Yet, ADCT-402 resulted in significant, dose-dependent extension of survival (log-rank test, P ≤ .002 for each comparison; supplemental Figure 2B).
In a second disseminated model (NALM-6), ADCT-402 at 0.33 and 1 mg/kg single dose produced a significant increase in survival compared with control ADC (log-rank test, P ≤ .001; Figure 5C). At a dose of 1 mg/kg, 10/10 animals survived 90 days compared with only 21 days for the vehicle-treated group.
ADCT-402 pharmacodynamics
In mice with Ramos SC tumors, a single dose of ADCT-402 was administered at 0.3 or 1 mg/kg (supplemental Figure 3). Twenty-four hours after treatment, excised tumors showed a dose-related increase in staining intensity by an anti-PBD drug-linker antibody and a γ-H2AX antibody (Figure 6A). In contrast, tumors excised from mice treated with a nontargeted ADC, containing the same PBD dimer warhead, at 1 mg/kg did not show increased staining compared with vehicle control animals. CD19 staining remained high and homogeneous in all treatment groups (Figure 6A).
Pharmacodynamic studies of ADCT-402. (A) Representative scans of FFPE Ramos xenograft tumor sections, obtained from vehicle-treated control mice or mice treated with 0.3 and 1 mg/kg ADCT-402 or 1 mg/kg nontargeting ADC and stained with an anti-CD19 antibody (top), anti-PBD linker antibody (middle), or an anti-γ-H2AX antibody (bottom). (B) Histogram depicting % cells with γ-H2AX in tumor cell suspensions taken from Ramos xenograft tumors 24 hours after injection with vehicle or 0.3 or 1 mg/kg ADCT-402 or 1 mg/kg nontargeted ADC. Data represent the mean and SD from 3 individual mice per data point. **P ≤ .01, ***P ≤ .001. (C) Histogram depicting mean OTM in irradiated (I) or unirradiated (UI) tumor cell suspensions taken from Ramos xenograft tumors 24 hours after injection with vehicle or 0.3 mg/kg or 1 mg/kg ADCT-402 or 1 mg/kg nontargeted ADC. Data represent the mean and SD from 3 individual mice per data point. Statistical analysis was done with unpaired t test with Welch correction (not assuming equal SDs). P values are 2-tailed; *P ≤ .05, **P ≤ .01. SD, standard deviation.
Pharmacodynamic studies of ADCT-402. (A) Representative scans of FFPE Ramos xenograft tumor sections, obtained from vehicle-treated control mice or mice treated with 0.3 and 1 mg/kg ADCT-402 or 1 mg/kg nontargeting ADC and stained with an anti-CD19 antibody (top), anti-PBD linker antibody (middle), or an anti-γ-H2AX antibody (bottom). (B) Histogram depicting % cells with γ-H2AX in tumor cell suspensions taken from Ramos xenograft tumors 24 hours after injection with vehicle or 0.3 or 1 mg/kg ADCT-402 or 1 mg/kg nontargeted ADC. Data represent the mean and SD from 3 individual mice per data point. **P ≤ .01, ***P ≤ .001. (C) Histogram depicting mean OTM in irradiated (I) or unirradiated (UI) tumor cell suspensions taken from Ramos xenograft tumors 24 hours after injection with vehicle or 0.3 mg/kg or 1 mg/kg ADCT-402 or 1 mg/kg nontargeted ADC. Data represent the mean and SD from 3 individual mice per data point. Statistical analysis was done with unpaired t test with Welch correction (not assuming equal SDs). P values are 2-tailed; *P ≤ .05, **P ≤ .01. SD, standard deviation.
Tumor samples were analyzed for formation of DNA interstrand crosslinking and γ-H2AX foci. A significant increase in the level of γ-H2AX foci was observed in tumor cells at both ADCT-402 dose levels (Figure 6B), with no significant increase seen with the nontargeted ADC. DNA interstrand crosslinking in tumor cells, measured as the reduction in OTM, was also significant at both dose levels of ADCT-402 compared with vehicle or nontargeted ADC (Figure 6C). In contrast, no crosslinking was observed in peripheral blood mononuclear cell samples taken from the same mice (supplemental Figure 4). The selective targeting of ADCT-402 to human CD19-expressing tumor cells, resulting in the formation of DNA interstrand crosslinks and an associated DNA damage response, is therefore confirmed in vivo.
ADCT-402 PK in rat and cynomolgus monkey
Quantitation of total or PBD-conjugated antibody was determined in rat serum following a single administration of 1.5 mg/kg to nontumor-bearing rats (Figure 7A). The half-life for total and PBD-conjugated antibody was 9.9 and 10.4 days, respectively (ADCT-402 does not cross-react with rat CD19) (supplemental Table 3), indicating typical IgG1 kinetics and excellent stability in vivo.
Pharmacokinetics of ADCT-402 in rat and cynomolgus monkey. (A) Quantitation of total antibody and PBD-conjugated ADCT-402 in rat serum from 3 individual Crl:CD (SD) rats treated with a single IV dose of 1.5 mg/kg. (B) Quantitation of total antibody, PBD-conjugated antibody, and free PBD dimer warhead SG3199 in cynomolgus monkey serum after IV administration of 0.6 mg/kg ADCT-402 on days 1 and 22. The results are mean ± SEM (n = 2/5 male and 2/5 female).
Pharmacokinetics of ADCT-402 in rat and cynomolgus monkey. (A) Quantitation of total antibody and PBD-conjugated ADCT-402 in rat serum from 3 individual Crl:CD (SD) rats treated with a single IV dose of 1.5 mg/kg. (B) Quantitation of total antibody, PBD-conjugated antibody, and free PBD dimer warhead SG3199 in cynomolgus monkey serum after IV administration of 0.6 mg/kg ADCT-402 on days 1 and 22. The results are mean ± SEM (n = 2/5 male and 2/5 female).
Quantitation of total antibody, PBD-conjugated antibody, and free SG3199 was also determined following administration of 0.6 mg/kg ADCT-402 on days 1 and 22 to cynomolgus monkeys (Figure 7B). The exposure profile of ADCT-402 indicates typical IgG1 kinetics, a 14-day half-life (ADCT-402 does not cross-react with cynomolgus monkey CD19) (supplemental Table 3), and excellent stability in vivo.
ADCT-402 was well tolerated in the rat and monkey studies with an acceptable safety profile.
Discussion
PBD-based ADCs are becoming established as important next-generation agents in the ADC arena. They exploit a completely different cellular mechanism to the first-generation auristatin and maytansinoid tubulin inhibitors and a different mode of DNA interaction to warheads such as calicheamicin.25 Several PBD-containing ADCs have entered early-phase clinical trials, falling into 2 distinct classes based on linker site attachment to the PBD dimer: C2 and N10. ADCT-402 is an example of the PBD warhead SG3199 connected via its N10 position to a maleimidocaproyl valine-alanine dipeptide linker. This drug linker, tesirine, is the same as that in the clinical-stage agents rovalpituzumab tesirine, ADCT-301, ADCT-502, and MEDI3726. Because the N10-position imine is involved in covalent binding to DNA, linker attachment at this position produces a prodrug, adding a further level of safety over C2-linked agents. Consequently, attachment at the N10-imine requires a self-immolative linker that becomes completely traceless following cleavage, as found in tesirine. A CD19-directed ADC using a first-generation PBD dimer with linker site attachment at the C2 position has recently been described.26
ADCT-402 cytotoxicity showed a weak, yet significant negative linear correlation with cell surface CD19 target expression in a panel of 10 hematological cancer cell lines (eight CD19+ and 2 CD19−). This is in contrast to previously reported data for ADCT-301 and SGN-CD19B, which failed to show a clear relationship between sensitivity and antigen expression level.15,26 In the case of ADCT-301, the limited number of CD25-positive cell lines available all had high CD25 expression, which may have been above a threshold for accurately determining cytotoxic sensitivity. In this study, all cell lines, including those negative for CD19 expression, were sensitive to the free warhead SG3199, consistent with the nontargeted delivery of the warhead alone. Some differential sensitivity, unrelated to CD19 expression, was observed for SG3199. Notably, the mantle cell lymphoma cell line Granta-519 and ALL cell line Nalm-6 were the most sensitive to both the ADC and the free warhead. Hematological cancer cell lines have previously been shown to be more sensitive, in general, to solid tumor cell lines.27 In addition, certain hematological tumor types show exquisite sensitivity to PBD dimers, which may predict target ADC cytotoxicity independently of CD19 expression above a threshold expression level. It has previously been shown that, for example, defects in homologous recombination repair or DNA repair protein ERCC1 confer increased sensitivity to PBD dimers,28 suggesting a possible widening of therapeutic index in patients with tumors harboring these defects, and indicates potential biomarkers of response.
Evidence of rapid internalization of ADCT-402 is provided by the reduction in intensity of membrane immunofluorescence staining on CD19-expressing Ramos cells. Colocalization images suggest that processing of ADCT-402 is, at least in part, lysosomal. The time lag observed between the peak of DNA interstrand crosslink formation by ADCT-402 and by the naked warhead SG3199 reflects the time taken for internalization and cellular processing of the ADC compared with the readily diffusible PBD dimer. An important feature of the highly cytotoxic crosslinks produced is the minimal distortion of DNA, which contributes to the lack of repair and consequent persistence over an observed 36-hour period.
If ADCT-402 is to be efficacious in lymphomas with heterogeneous CD19 expression, the existence of bystander toxicity of target-negative tumor cells is important. PBD warhead released from the target-positive cell would be expected to be diffusible in contrast to less permeable payloads such as MMAF. In medium transfer experiments, CD19− Karpas-299 cells were killed by a soluble factor released from ADCT-402–treated Ramos cells into the medium. This soluble factor is assumed to be SG3199 warhead, released by lysosomal cleavage of the Val-Ala dipeptide linker in ADCT-402 and the self-immolative cleavage of the residual linker stub on the PBD N10 imine because we have shown that any transfer of intact ADC would be inactive against the CD19− cells.
In vivo efficacy experiments show remarkable dose-related improvements in survival in both SC and disseminated models in 3 different tumor types (Burkitt lymphoma, ALL, and diffuse large B-cell lymphoma). Clear superiority is observed compared with comparator ADCs delivering tubulin inhibitor drugs, reflecting the increased potency of the PBD dimer warhead and the inherent sensitivity of hematological malignancies to critical DNA damage. It is clear that a single dose of ADCT-402 can selectively deliver sufficient cytotoxic agent, not only to induce growth delay, but also sustained tumor regression and in many cases tumor eradication. Interestingly, in the Ramos model, peak drug concentration appears to be necessary for complete response because administering the same total dose fractionated into 3 administrations (over 2 weeks or 8 days) was significantly inferior, producing only tumor growth delay. This is in contrast to a recent report that observed similar antitumor activity against solid tumor xenografts in mice treated with single or fractionated dosing.29
Anti-PBD payload antibody and anti-γ-H2AX staining confirmed the dose-dependent delivery of ADCT-402 to the target tumor xenograft. In addition, quantitative pharmacodynamic assessment of DNA crosslinking and γ-H2AX foci containing cells was achieved. γ-H2AX has previously been shown to be a highly sensitive DNA damage response marker to DNA crosslinking agents including PBD dimers,30,31 and both γ-H2AX immunofluorescence and measurement of DNA interstrand crosslinks by comet assay have been used as pharmacodynamic assays in clinical trials of PBD dimer SG2000.17,31 Extrapolating from the in vivo data suggests that a threshold level of DNA interstrand cross-linking is required for tumor regression and eradication. This is consistent with previous data with CD25-targeting ADCT-30115 and with the inferior fractionated dosing observed in this study. The absence of DNA crosslinking in mouse peripheral blood mononuclear cells supports the specificity of ADCT-402 in vivo.
No change in expression of CD19 was observed in tumors 24 hours after ADCT-402 administration in vivo. An important consideration in the design of early-phase clinical studies of ADCT-402 is the level and heterogeneity of target CD19 expression in patient tumors in the relevant R/R setting. To address this, we examined CD19 expression in a panel of matched (initial diagnosis and R/R) clinical samples (Table 1). High and homogeneous CD19 expression was observed across the whole panel of matched samples, confirming maintenance of target antigen expression from initial diagnosis to relapse and confirming the excellent widespread nature of CD19 as an ideal target for clinical ADC development.
ADCT-402 was found to have a favorable safety and PK profile with excellent stability in both rat and cynomolgus monkey. Taken together, these impressive preclinical data were used to support the rapid clinical testing of ADCT-402 in patients with CD19-expressing B-cell malignancies. Phase 1 studies in both R/R NHL and R/R B-ALL are under way. Emerging clinical data confirm the potential of this therapy in targeting CD19-expressing B-cell malignancies.32-34
Partially presented in poster form at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2015, and at the American Association for Cancer Research Annual Meeting 2017, Washington, DC, 2 April 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Charles River Laboratory Services, Epo-GmbH, and Covance for conducting the in vivo studies, and PRA Health Services for the pharmacodynamics measurements. Animal studies conducted at EPO GmbH were approved by LaGeSo (A0452/8).
This work was supported by grants from the University College London Hospital/University College London, National Institute for Health Research Comprehensive Biomedical Research Centre (T.M.), and the Cancer Research UK (grant C2559A/A16569) (J.A.H.).
Authorship
Contribution: F.Z. designed experiments, interpreted data, prepared figures, and wrote the paper; S. Corbett, L.A., P.C.T., K.K., N.J., C.E.B., and F.D. designed and performed experiments; T.M., C.E.G.H., S. Chivers, and D.G.W. designed experiments and interpreted data; A.T. and P.W.H. provided guidance on pyrrolobenzodiazepine chemistry; J.A.H. designed experiments, interpreted data, and wrote the paper; and P.H.v.B. interpreted data, provided guidance, and intellectual input.
Conflict-of-interest disclosure: F.Z., C.E.B., C.E.G.H., S.C., and P.H.v.B. are employees of ADC Therapeutics and P.H.v.B., J.A.H., and P.W.H. are also major shareholders. L.A., P.C.T., D.G.W., A.T., P.W.H., and J.A.H. are employees of Spirogen/Medimmune limited. The remaining authors declare no competing financial interests.
The current affiliation of F.D. is Novasep, Le Mans, France.
Correspondence: Francesca Zammarchi, ADC Therapeutics (UK) Limited, QMB Innovation Centre, 42 New Rd, London E1 2AX, United Kingdom; e-mail: francesca.zammarchi@adctherapeutics.com.