CD38 is highly and uniformly expressed on multiple myeloma (MM) cells, and at relatively low levels on normal lymphoid and myeloid cells, and in some tissues of nonhematopoietic origin. CD38 is a transmembrane glycoprotein with ectoenzymatic activity, and also functions as a receptor and adhesion molecule. Altogether, this has triggered the development of several CD38 antibodies including daratumumab (fully human), isatuximab (chimeric), and MOR202 (fully human). CD38 antibodies have pleiotropic mechanisms of action including Fc-dependent immune-effector mechanisms, direct apoptotic activity, and immunomodulatory effects by the elimination of CD38+ immune-suppressor cells. CD38-targeting antibodies are generally well tolerated and induce partial response or better in ∼30% of heavily pretreated MM patients as monotherapy. Based on their distinct mechanisms of action, favorable toxicity profile, and single-agent activity, CD38 antibodies are attractive partners in combination regimens. Indeed, deep responses and prolonged progression-free survival can be achieved in relapsed/refractory MM patients when CD38 antibodies are combined with immunomodulatory agents or proteasome inhibitors. Infusion-related reactions, which typically occur during the first infusion, are the most frequent adverse events. Attention should also be paid to the interference of CD38 antibodies with certain laboratory assays, which may complicate response evaluation and blood compatibility testing. Several studies are currently examining the role of CD38-based therapies in newly diagnosed and high-risk smoldering MM. Furthermore, CD38 antibodies are currently also under investigation in other hematologic malignancies, including acute lymphoblastic leukemia, natural killer/T-cell lymphoma, and acute myeloid leukemia, as well as in solid tumors.
Introduction
CD38 was identified in 1980 by E. L. Reinherz, S. Schlossman, and colleagues during their pioneering analysis of the human lymphocyte surface using monoclonal antibodies in search of the T-cell receptor.1 ,2 One of the molecules identified, T10 (now CD38), initially served as marker for the study of thymocytes and activated T cells.3 More than 35 years later, CD38-targeting antibodies are transforming multiple myeloma (MM) treatment because of their marked activity as single agents and in combinations, as well as their manageable toxicity profile. In this review, we will focus on CD38 function and tissue distribution, as well as on modes of action and mechanisms of resistance of CD38 antibodies. We will discuss the efficacy of CD38 antibodies both as single agents and in combination. The management of clinically relevant aspects of CD38 antibody therapy, including interference in laboratory assays, will also be discussed.
CD38 tissue distribution
CD38 is highly expressed not only on plasma cells, but also on other lymphoid and myeloid cells, as well as on red blood cells and platelets.4 A comparison of CD38 expression levels across these cellular populations shows that, after plasma cells, natural killer (NK) cells express the highest levels of CD38, followed by subpopulations of B and T cells.5 The protein is also expressed in a subset of hematological tumors, and shows especially broad and high expression levels in MM. CD38 is also expressed in tissues of nonhematopoietic origin including prostatic epithelial cells,6 pancreatic islet cells, as well as in the perikarya and dendrites of some neurons. Other CD38+ cells include airway-striated muscle cells,7 renal tubules, retinal gangliar cells, and corneal cells.8
Control of CD38 expression
The promoter of CD38 is complex. The 5′-flanking promoter region of the gene contains cytosine-phosphate-guanine dinucleotide (CpG) islands and a binding site for transcription factor Sp1, but lacks canonical TATA and CAAT boxes. Upstream of these CpG islands are potential binding sites for various immunological transcription factors such as T-cell–specific transcription factor-1α (TCF-1α), nuclear factor for interleukin-6 (NF–IL-6), and interferon-responsive factor-1 (IRF-1). A further level of control lies in the 5′ end of intron 1, which contains a retinoic acid–responsive element (RARE) and a binding site for peroxisome proliferator–activated receptor γ (PPARγ).9
CD38 function
CD38 as a receptor
CD38 was inferred to be a receptor after observations of signals induced by agonistic antibodies.10 These signals were accessory cell- and IL-2–dependent and additive with both CD3 and CD2. The CD38 molecule was later reported to be involved in triggering activation and proliferation signals that are lineage-unrestricted.11 Since the identification of CD31 as a specific ligand,12 CD38 has been generally recognized as a receptor, despite its intrinsic inability to act in this function, because of a very short cytoplasmic domain. Indeed, to act as a receptor, CD38 needs to be redirected to specialized microdomains of the cell membrane, in close proximity to professional receptors. This point has been confirmed by visually monitoring the formation of the immunological synapse in T lymphocytes.13
CD38 as an ectoenzyme
CD38 is a multifunctional ectoenzyme involved in the catabolism of nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP), the 2 main substrates of the molecule identified so far.14,15 This reaction leads to the generation of potent intracellular Ca2+-mobilizing compounds (cyclic adenosine diphosphate [ADP] ribose, ADP ribose, and nicotinic acid adenine dinucleotide phosphate). The balance between CD38 and its extracellular substrate determines cytoplasmic levels of NAD+. Consequently, inhibition of CD38 activity is followed by increased intracellular NAD+ levels, independently of the actions of the other NAD+-consuming enzymes (CD157, poly ADP ribose polymerase, and sirtuins).16 Recent studies suggest that CD38 is also involved in the production of adenosine, which has immunosuppressive effects.17
Mechanism of action of CD38 antibodies
Fc-dependent immune effector mechanisms
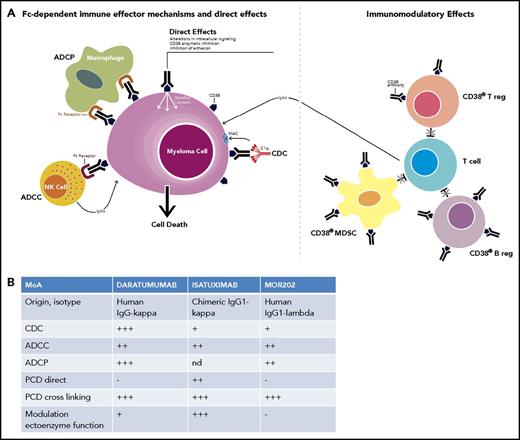
The relatively high expression of CD38 on MM cells, in combination with its role as receptor and ectoenzyme, suggested CD38 as a potential therapeutic antibody target for the treatment of MM. More than 25 years ago, several CD38-targeting antibodies were generated with activity against MM cells,18,19 but these chimeric and humanized antibodies were not evaluated clinically (Figure 1). In 2008, the fully human immunoglobulin G1-κ (IgG1-κ) antibody daratumumab, was the first CD38 antibody that was administered in MM. Daratumumab was selected from a panel of 42 antibodies based on its unique ability to induce complement-dependent cytotoxicity (CDC) (Figure 2).20 Daratumumab also kills MM cells via antibody-dependent cellular cytoxicity (ADCC)20 and antibody-dependent cellular phagocytosis (ADCP)21 via antibody binding to activating Fcγ receptors (FcγRs) on immune effector cells. Furthermore, FcγR-mediated crosslinking of tumor-bound daratumumab induces programmed cell death.22 Next to these Fc-dependent immune effector mechanisms, direct effects such as modulation of CD38 enzymatic function may also contribute to the antitumor activity of daratumumab.23
History of CD38 antibodies. The timeline describes the discovery of CD38 protein, and the chronological introduction of CD38 antibodies in human trials. The timeline also shows the approved treatment options with CD38-targeted therapy in MM. DPd, daratumumab plus pomalidomide-dexamethasone; DRd, daratumumab plus lenalidomide-dexamethasone; DVd, daratumumab plus bortezomib-dexamethasone; EMA, European Medicines Agency; FDA, US Food and Drug Administration; IMiD, immunomodulatory drug; Pd, pomalidomide-dexamethasone; PI, proteasome inhibitor; Rd, lenalidomide-dexamethasone; Vd, bortezomib-dexamethasone.
History of CD38 antibodies. The timeline describes the discovery of CD38 protein, and the chronological introduction of CD38 antibodies in human trials. The timeline also shows the approved treatment options with CD38-targeted therapy in MM. DPd, daratumumab plus pomalidomide-dexamethasone; DRd, daratumumab plus lenalidomide-dexamethasone; DVd, daratumumab plus bortezomib-dexamethasone; EMA, European Medicines Agency; FDA, US Food and Drug Administration; IMiD, immunomodulatory drug; Pd, pomalidomide-dexamethasone; PI, proteasome inhibitor; Rd, lenalidomide-dexamethasone; Vd, bortezomib-dexamethasone.
Mechanism of action of CD38 antibodies. (A) CD38-targeting antibodies have pleiotropic mechanisms of action, which can be subdivided into (i) Fc-dependent immune-effector mechanisms; (ii) direct effects; and (iii) immunomodulatory effects. The Fc-dependent immune-effector mechanisms include antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC). The process of ADCC is achieved through the activation of FcRs on NK cells and myeloid cells by tumor-cell attached CD38 antibodies. Subsequent release of perforin and granzymes from effector cells as well as interactions with death ligands FasL and tumor necrosis factor–related apoptosis-inducing ligand lead to MM cell death. In ADCP phagocytosis is mediated by monocytes, macrophages, neutrophils, and dendritic cells following interaction of the Fc tail of the therapeutic antibody with FcRs on these effector cells. CDC is initiated following the interaction of the antibody Fc domains with the classic complement-activating protein C1q, which leads to activation of downstream complement proteins, resulting in assembly of the membrane attack complex (MAC), which punches holes in MM tumor cells. The chemotactic complement molecules, C3a and C5a, are also produced during this process. These molecules can recruit and activate immune-effector cells. Direct effects include induction of apoptosis, as well as inhibition of CD38 ectoenzyme function, which may lead to reduced adenosine levels in the BM myeloma niche. Adenosine is an immunosuppressor that helps the tumor to evade the host immune response by promoting regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), and depressing NK- and T-cell effectors. CD38 antibodies have immunomodulatory effects via the eradication of CD38+ Tregs, regulatory B cells (Bregs), and MDSCs, which result in CD4+ and CD8+ T-cell expansion, and potentially a better host-antitumor immune response. Adapted from van de Donk et al78 with permission. (B) The relative contribution of these different mechanisms of action to MM cell killing differs among daratumumab, isatuximab, and MOR202. The efficacy of these 3 CD38 antibodies was directly compared in preclinical studies in terms of direct induction of programmed cell death (PCD), induction of PCD after crosslinking, inhibition of CD38 ectoenzyme activity, and the induction of ADCC, ADCP, and CDC. The immunomodulatory effects of the CD38 antibodies were not compared in a head-to-head analysis, and therefore were not included in panel B. MoA, mechanism of action; nd, not determined.
Mechanism of action of CD38 antibodies. (A) CD38-targeting antibodies have pleiotropic mechanisms of action, which can be subdivided into (i) Fc-dependent immune-effector mechanisms; (ii) direct effects; and (iii) immunomodulatory effects. The Fc-dependent immune-effector mechanisms include antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC). The process of ADCC is achieved through the activation of FcRs on NK cells and myeloid cells by tumor-cell attached CD38 antibodies. Subsequent release of perforin and granzymes from effector cells as well as interactions with death ligands FasL and tumor necrosis factor–related apoptosis-inducing ligand lead to MM cell death. In ADCP phagocytosis is mediated by monocytes, macrophages, neutrophils, and dendritic cells following interaction of the Fc tail of the therapeutic antibody with FcRs on these effector cells. CDC is initiated following the interaction of the antibody Fc domains with the classic complement-activating protein C1q, which leads to activation of downstream complement proteins, resulting in assembly of the membrane attack complex (MAC), which punches holes in MM tumor cells. The chemotactic complement molecules, C3a and C5a, are also produced during this process. These molecules can recruit and activate immune-effector cells. Direct effects include induction of apoptosis, as well as inhibition of CD38 ectoenzyme function, which may lead to reduced adenosine levels in the BM myeloma niche. Adenosine is an immunosuppressor that helps the tumor to evade the host immune response by promoting regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), and depressing NK- and T-cell effectors. CD38 antibodies have immunomodulatory effects via the eradication of CD38+ Tregs, regulatory B cells (Bregs), and MDSCs, which result in CD4+ and CD8+ T-cell expansion, and potentially a better host-antitumor immune response. Adapted from van de Donk et al78 with permission. (B) The relative contribution of these different mechanisms of action to MM cell killing differs among daratumumab, isatuximab, and MOR202. The efficacy of these 3 CD38 antibodies was directly compared in preclinical studies in terms of direct induction of programmed cell death (PCD), induction of PCD after crosslinking, inhibition of CD38 ectoenzyme activity, and the induction of ADCC, ADCP, and CDC. The immunomodulatory effects of the CD38 antibodies were not compared in a head-to-head analysis, and therefore were not included in panel B. MoA, mechanism of action; nd, not determined.
Similarly, isatuximab (IgG1-κ, chimeric) and MOR202 (IgG1-λ, fully human) have multiple mechanisms of action including CDC, ADCC, and ADCP.23,24 Furthermore, isatuximab has strong proapoptotic activity independent of cross-linking and inhibits CD38 ectoenzyme function.23,-25 The relative contribution of these different mechanisms of action to MM cell killing differs among the 3 CD38 antibodies,23,-25 which can be explained by targeting different epitopes on the CD38 molecule (Figure 2).20,24 It is currently unknown whether these functional differences observed between different CD38 antibodies affect their therapeutic utility.
Immunomodulatory effects
Interestingly, daratumumab also has immunomodulatory effects through the eradication of CD38-expressing regulatory T, regulatory B, and myeloid-derived suppressor cells (Figure 2).5 This shift away from an immunosuppressive microenvironment results in CD4+ and CD8+ T-cell expansion, elevated antiviral and alloreactive functional T-cell responses, increased T-cell clonality, and, potentially, a better host-antitumor immune response.5 Indeed, responders had higher expression levels of activation markers along with increased granzyme B production in CD8+ T cells following daratumumab treatment.26
Preclinical experiments show that isatuximab also eliminates CD38+ regulatory T cells, and thereby restores T-cell– and NK-cell–mediated antitumor immune responses.27 It is currently unknown whether MOR202 has similar immunomodulatory activities.
CD38 antibody monotherapy in MM
Single-agent activity in MM patients
MM patients with disease refractory to immunomodulatory drugs (IMiDs) and proteasome inhibitors have a very poor outcome.28 Based on the results from the GEN50129 and Sirius30 studies, daratumumab was approved as single agent in 2015 by the US Food and Drug Administration (FDA) and in 2016 by the European Medicines Agency (EMA) for relapsed/refractory MM. A pooled analysis of both monotherapy studies showed an overall response rate (ORR) of 31.1% (complete response [CR] in 4.7%), median duration of response of 7.6 months, median progression-free survival (PFS) of 4.0 months, and median overall survival (OS) of 20.1 months in patients treated with 16 mg/kg daratumumab (median 5 prior lines of therapy).31 Responses were observed across all subgroups including patients with renal impairment, extramedullary plasmacytoma, and those with triple- or quadruple-refractory disease, advanced age, or high-risk cytogenetics.30,-32 Pharmacokinetic studies show that the current 16 mg/kg daratumumab administration schedule leads to rapid and sustained target saturation in the majority of patients.33,34 Daratumumab has a manageable toxicity profile with 48% of patients experiencing infusion-related reactions (IRRs).31 With the aim of shortening infusion time, a phase 1 study is currently investigating the subcutaneous delivery of daratumumab in combination with recombinant human hyaluronidase.35 Preliminary data suggest that subcutaneous delivery is associated with a lower rate of IRRs as compared with IV daratumumab, with a comparable response rate and similar serum through concentrations in relapsed/refractory MM.35
These studies demonstrated the potent effects of targeting CD38 in MM, and supported the clinical development of additional agents in this class. MOR202 (4-16 mg/kg) combined with low-dose dexamethasone achieved partial response (PR) or better in 29% of patients (median 4 prior lines of therapy).36 Also, isatuximab at a dose of ≥10 mg/kg has single-agent activity with an ORR of 24.3% (median PFS, 3.7 months; median OS, 18.6 months) in relapsed/refractory MM patients with a median of 5 prior therapies.37,38
Determinants of response
There is a marked heterogeneity in the response of patients to CD38 antibodies as single agent. Mechanisms underlying the differential therapeutic efficacy probably include both host- and tumor-related factors.
Tumor-related factors
Cell surface expression of CD38 on MM cells was associated with extent of daratumumab-mediated ADCC and CDC in MM cell lines and primary samples.39 Similarly, daratumumab-treated patients who achieved at least PR, had higher baseline CD38 expression on their tumor cells, compared with patients with less than PR.40 However, given the overlap in CD38 expression between responding and nonresponding patients, CD38 levels alone do not explain the whole variability in response.40 Baseline expression levels of complement inhibitors on MM cells or soluble CD38 molecules did not affect the activity of daratumumab.40 Subgroup analysis suggests that CD38 antibodies are also active in patients with high-risk cytogenetics.30,38 However, these subgroups involve relatively small numbers of patients, and further analysis is required to assess the impact of high-risk cytogenetics on outcome. The contribution of specific mutations and activation status of signaling pathways to the variability in response to CD38 antibodies is currently unknown.
Bone marrow microenvironment
The bone marrow (BM) microenvironment confers protection against several anti-MM agents and also renders MM cells resistant to T cells.41 We showed that BM stromal cells also protect MM cells against daratumumab-mediated ADCC.42 This microenvironment-induced apoptosis resistance is possibly related to increased expression of antiapoptotic proteins such as survivin.42
Differences in the frequency of NK cells or monocytes in the BM microenvironment also explained part of the variability in daratumumab-mediated killing of primary MM cells.39 Activity of these cells may be affected by previous or concomitant therapies. Furthermore, inhibitory signals transmitted to effector cells by MM cells may also result in effector cell dysfunction. The impact of genetic variation of activating and inhibitory receptors on response requires further investigation.43
Acquired resistance
The majority of patients who initially respond to monotherapy eventually progress. Development of resistance toward daratumumab was associated with increased expression of the complement inhibitors CD55 and CD59 on MM cells, or the outgrowth of subpopulations with high expression of complement inhibitors.40 Conversely, reduction of CD55 and CD59 sensitized MM cells to daratumumab-mediated CDC.40
CD38 reduction on nondepleted tumor cells may also confer protection against daratumumab.40 However, significant CD38 reduction was already observed after the first daratumumab infusion, and in both responding and nonresponding patients including those with sustained clinical response,40 which suggests that the continuous pressure to maintain MM cells in a CD38−/low state seems to offer a clinical benefit by altering MM cell adhesion and reducing immunosuppressive adenosine levels in the BM microenvironment.44,45 Importantly, CD38 expression analysis was done by flow cytometry with a monoclonal antibody that binds to a different epitope than daratumumab, which excludes the possibility that binding of daratumumab masked the detection of CD38. The rapid daratumumab-mediated reduction of CD38 expression on surviving MM cells is probably the result of the selection of cells with low CD38 expression, release of tumor-derived CD38-carrying microvesicles,44,46 and trogocytosis.47 Interestingly, CD38 expression was preserved during treatment with MOR202.36 No clinical data are yet available for isatuximab.
Reduced biological activity mediated by anti-drug antibodies has not been observed with daratumumab or isatuximab up until now,29,30,48,49 whereas it is a rare event with MOR202.50
Other factors that may contribute to development of resistance are changes in frequency and activity of effector cells. In this respect, NK cells have high CD38 expression and are rapidly reduced after infusion of daratumumab,51,52 which may impair MM cell killing. However, NK-cell reduction was similar in responding and nonresponding patients.52
Retreatment
Preliminary evidence from a study with 2 patients suggests that retreatment of patients with daratumumab is feasible and effective.53 However, it is currently unknown whether a treatment-free interval is required in order to allow NK-cell recovery and CD38 expression levels to return to baseline on remaining MM cells.40,52 Another strategy is to maintain daratumumab, and add other therapeutic agents to potentiate any anti-MM effects of daratumumab.
Combination therapy with CD38 antibodies
MM is a very heterogeneous disease with multiple subclones with different susceptibility to specific agents. Therefore, combining multiple agents with overlapping toxicities and synergistic mechanisms of action is a successful strategy in improving clinical outcome. Based on the efficacy of CD38 antibodies, these agents are very attractive as a component of combination regimens.
IMiDs
The combination of a CD38 antibody with an IMiD such as lenalidomide and pomalidomide markedly enhanced NK-cell mediated ADCC.25,32,54,,-57 This potentiation is mainly related to the ability of IMiDs to increase NK-cell numbers and NK-cell activity.32,55 In addition, IMiDs also promote tumoricidal activity of macrophages and enhance ADCP.58 Furthermore, IMiDs increase the direct toxic effects of isatuximab25 and increase CD38 expression on regulatory T cells, resulting in enhanced isatuximab-mediated elimination of these cells.27 Interestingly, preclinical data show that IMiDs also synergize with daratumumab in IMiD-resistant tumors, indicating that the immune system of these patients can still respond to the immunomodulatory effects of these agents.32 No improvement in CDC has been observed with IMiDs.55
Altogether, this formed the preclinical rationale for the randomized phase 3 Pollux study, in which 569 patients with at least 1 prior therapy were randomized to lenalidomide-dexamethasone (Rd) with or without daratumumab (Table 1). Patients treated with daratumumab plus Rd (DRd) had higher rates of deeper responses, which translated into markedly better PFS (hazard ratio [HR], 0.37; PFS at 12 months, 83.2% vs 60.1%).59 The PFS advantage was observed across all subgroups including patients ≥65 years and those with previous lenalidomide exposure.59,60 Also, patients with high-risk cytogenetics [del(17p); t(4;14); t(14;16)] had better PFS with DRd compared with Rd (median PFS, 22.6 vs 10.2 months), but poor risk conferred by these cytogenetic abnormalities was not completely abrogated by adding daratumumab. Longer follow-up is needed to show potential OS benefit. Daratumumab also significantly increased minimal residual disease (MRD)-negativity rate (DRd vs Rd, 24.8% and 5.7%; threshold 10−5), which translated into significantly improved PFS.61,62 In patients with high-risk cytogenetics, MRD− status was only achieved in those treated with daratumumab.62 Other than IRRs, there was an increased incidence of grade 3/4 neutropenia, diarrhea, fatigue, and dyspnea when daratumumab was added to Rd, probably as a result of longer treatment exposure.59 Similar to prior findings with daratumumab monotherapy, addition of daratumumab to Rd induced clonal expansion of T cells.63 Furthermore, baseline T-cell richness was associated with improved PFS in the DRd arm.63 Based on these data, the FDA (2016) and EMA (2017) approved this triplet in MM patients with ≥1 prior therapy.
CD38 antibodies plus lenalidomide-dexamethasone in relapsed/refractory MM
| . | NCT02076009 (POLLUX)59,62 . | NCT0174996964 . | NCT0142118636,65 . | |
|---|---|---|---|---|
| Phase | Phase 3 | Phase 1b | Phase 1/2a | |
| Regimen | Rd | DRd | Isatuximab-Rd | MOR202-Rd |
| Treatments | Lenalidomide: 25 mg on days 1-21 per 28-d cycle (10 mg if CrCl 30-60 mL/min) | Daratumumab (16 mg/kg): weekly for 8 wk, then Q2W for 16 wk, thereafter Q4W | Isatuximab: 3, 5, or 10 mg/kg Q2W or 10, 20 mg/kg QW for 4 wk, and then Q2W | MOR202 8-16 mg/kg, weekly |
| Dexamethasone: 40 mg weekly (20 mg if age >75 y) | Lenalidomide: 25 mg on days 1-21 per 28-d cycle (10 mg if CrCl 30-60 mL/min) | Lenalidomide: 25 mg on days 1-21 per 28-d cycle (10 mg if CrCl 30-60 mL/min) | Lenalidomide: 25 mg on days 1-21 per 28-d cycle | |
| Treatment until progression | Dexamethasone: 40 mg weekly (20 mg if age >75 y) | Dexamethasone: 40 mg weekly | Dexamethasone: 40 mg weekly (20 mg if age >75 y) | |
| Treatment until progression | Treatment until progression | Treatment until progression or a maximum of 2 y | ||
| No. of patients | 283 | 286 | 57 (52 evaluable for response) | 15 (13 evaluable for response) |
| Median age, y | 65 | 65 | 61 | 64 |
| Median no. of prior therapies | 1 | 1 | 5 | 2 |
| Len-refractory, % | Len-refractory patients were excluded | Len-refractory patients were excluded | 82 | 13 |
| Bort-refractory, % | 16.3 | 19.9 | 65 | 27 |
| IRR, % | Not applicable | 47.7 | 56 | 6.7 |
| ≥PR, % | 76.4 | 92.9 | 55.7 (Len-refractory: 52.4%) | 84.6 |
| ≥VGPR, % | 44.2 | 75.8 | 36.5 (Len-refractory: 35.7%) | 23.1 |
| ≥CR, % | 19.2 | 43.1 | 3.8 (Len-refractory: 0.0%) | 0.0 |
| MRD− disease (10−5), % | 5.7 | 24.8 | NA | NA |
| PFS | 12-mo: 60.1% | 12 mo: 83.2% | Median: 8.5 mo 12 mo: 38.9% | Median: not reached |
| OS | 12 mo: 86.8% | 12 mo: 91.2% | Not reported | Median: not reached |
| . | NCT02076009 (POLLUX)59,62 . | NCT0174996964 . | NCT0142118636,65 . | |
|---|---|---|---|---|
| Phase | Phase 3 | Phase 1b | Phase 1/2a | |
| Regimen | Rd | DRd | Isatuximab-Rd | MOR202-Rd |
| Treatments | Lenalidomide: 25 mg on days 1-21 per 28-d cycle (10 mg if CrCl 30-60 mL/min) | Daratumumab (16 mg/kg): weekly for 8 wk, then Q2W for 16 wk, thereafter Q4W | Isatuximab: 3, 5, or 10 mg/kg Q2W or 10, 20 mg/kg QW for 4 wk, and then Q2W | MOR202 8-16 mg/kg, weekly |
| Dexamethasone: 40 mg weekly (20 mg if age >75 y) | Lenalidomide: 25 mg on days 1-21 per 28-d cycle (10 mg if CrCl 30-60 mL/min) | Lenalidomide: 25 mg on days 1-21 per 28-d cycle (10 mg if CrCl 30-60 mL/min) | Lenalidomide: 25 mg on days 1-21 per 28-d cycle | |
| Treatment until progression | Dexamethasone: 40 mg weekly (20 mg if age >75 y) | Dexamethasone: 40 mg weekly | Dexamethasone: 40 mg weekly (20 mg if age >75 y) | |
| Treatment until progression | Treatment until progression | Treatment until progression or a maximum of 2 y | ||
| No. of patients | 283 | 286 | 57 (52 evaluable for response) | 15 (13 evaluable for response) |
| Median age, y | 65 | 65 | 61 | 64 |
| Median no. of prior therapies | 1 | 1 | 5 | 2 |
| Len-refractory, % | Len-refractory patients were excluded | Len-refractory patients were excluded | 82 | 13 |
| Bort-refractory, % | 16.3 | 19.9 | 65 | 27 |
| IRR, % | Not applicable | 47.7 | 56 | 6.7 |
| ≥PR, % | 76.4 | 92.9 | 55.7 (Len-refractory: 52.4%) | 84.6 |
| ≥VGPR, % | 44.2 | 75.8 | 36.5 (Len-refractory: 35.7%) | 23.1 |
| ≥CR, % | 19.2 | 43.1 | 3.8 (Len-refractory: 0.0%) | 0.0 |
| MRD− disease (10−5), % | 5.7 | 24.8 | NA | NA |
| PFS | 12-mo: 60.1% | 12 mo: 83.2% | Median: 8.5 mo 12 mo: 38.9% | Median: not reached |
| OS | 12 mo: 86.8% | 12 mo: 91.2% | Not reported | Median: not reached |
Bort, bortezomib; CR, complete response; CrCl, creatinine clearance; DRd, daratumumab plus lenalidomide-dexamethasone; IRR, infusion-related reaction; Len, lenalidomide; MRD, minimal residual disease; NA, not assessed; OS, overall survival; PFS, progression-free survival; PR, partial response; Q2W, once every 2 weeks; Q4W, once every 4 weeks; QW, once weekly; Rd, lenalidomide-dexamethasone; VGPR, very-good partial response.
Isatuximab combined with Rd was evaluated in patients with more advanced MM (median of 5 prior lines of therapy; 82% lenalidomide-refractory). At least PR was achieved in 56% of the patients and median PFS was 8.5 months.64 Fifty-two percent of lenalidomide-refractory patients obtained PR or better, indicating clinical synergy between these drugs.64 Preliminary results of MOR202 combined with Rd also showed a good tolerability profile and evidence for long-lasting disease control.36,65
A phase 2 study showed that addition of daratumumab to pomalidomide-dexamethasone (Pd; DPd) is also effective with approximately a doubling of the response rate as compared with Pd alone (Table 2).66 Based on these data, DPd was recently approved (June 2017) by the FDA for MM patients with ≥2 prior therapies including lenalidomide and a proteasome inhibitor (PI). Similar efficacy was observed when isatuximab or MOR202 was combined with Pd.36,67 Phase 3 trials will further evaluate the value of adding daratumumab or isatuximab to Pd.
CD38 antibodies plus pomalidomide-dexamethasone in relapsed/refractory MM
| . | NCT0199897166 . | NCT0228377567,106 . | NCT0142118636,65 . |
|---|---|---|---|
| Phase | 1b | 1b | 1/2a |
| Regimen | Daratumumab-pom/dex | Isatuximab-pom/dex | MOR202-pom/dex |
| Treatments | Daratumumab (16 mg/kg): weekly for 8 wk, then Q2W for 16 wk, thereafter Q4W | Isatuximab 5, 10, or 20 mg/kg, weekly during first cycle, then every 2 wk | MOR202 8-16 mg/kg, weekly |
| Pomalidomide: 4 mg on days 1-21 per 28-d cycle | Pomalidomide: 4 mg on days 1-21/28 d cycle | Pomalidomide: 4 mg on days 1-21 per 28-d cycle | |
| Dexamethasone: 40 mg weekly (20 mg if age >75 y) | Dexamethasone: 40 mg weekly (20 mg if age ≥75 y) | Dexamethasone: 40 mg weekly (20 mg if age >75 y) | |
| Treatment until progression | Treatment until progression | Treatment until progression or a maximum of 2 y | |
| No. of patients | 103 | 20 (14 evaluable patients) | 11 (9 evaluable for response) |
| Median age, y | 64 | 65.5 | 66 |
| Median no. of prior therapies | 4 | 5.0 | 3 |
| Len-refractory, % | 89 | 75 | 100 |
| Bort-refractory, % | 71 | 45 | 36 |
| IRR, % | 50 (mostly grade ≤ 2) | 45 (all grade 1 or 2) | 0 |
| ≥PR | 60.2 | 64.3 | 55.6 |
| ≥VGPR | 41.7 | 35.7 | 22.2 |
| ≥CR | 16.5 | 7.1 | 22.2 |
| MRD− disease (10−5) among patients with CR or better, % | 29.4 | NA | NA |
| Median PFS, mo | 8.8 | Not reported | Not reached |
| Median OS, mo | 17.5 | Not reported | Not reached |
| . | NCT0199897166 . | NCT0228377567,106 . | NCT0142118636,65 . |
|---|---|---|---|
| Phase | 1b | 1b | 1/2a |
| Regimen | Daratumumab-pom/dex | Isatuximab-pom/dex | MOR202-pom/dex |
| Treatments | Daratumumab (16 mg/kg): weekly for 8 wk, then Q2W for 16 wk, thereafter Q4W | Isatuximab 5, 10, or 20 mg/kg, weekly during first cycle, then every 2 wk | MOR202 8-16 mg/kg, weekly |
| Pomalidomide: 4 mg on days 1-21 per 28-d cycle | Pomalidomide: 4 mg on days 1-21/28 d cycle | Pomalidomide: 4 mg on days 1-21 per 28-d cycle | |
| Dexamethasone: 40 mg weekly (20 mg if age >75 y) | Dexamethasone: 40 mg weekly (20 mg if age ≥75 y) | Dexamethasone: 40 mg weekly (20 mg if age >75 y) | |
| Treatment until progression | Treatment until progression | Treatment until progression or a maximum of 2 y | |
| No. of patients | 103 | 20 (14 evaluable patients) | 11 (9 evaluable for response) |
| Median age, y | 64 | 65.5 | 66 |
| Median no. of prior therapies | 4 | 5.0 | 3 |
| Len-refractory, % | 89 | 75 | 100 |
| Bort-refractory, % | 71 | 45 | 36 |
| IRR, % | 50 (mostly grade ≤ 2) | 45 (all grade 1 or 2) | 0 |
| ≥PR | 60.2 | 64.3 | 55.6 |
| ≥VGPR | 41.7 | 35.7 | 22.2 |
| ≥CR | 16.5 | 7.1 | 22.2 |
| MRD− disease (10−5) among patients with CR or better, % | 29.4 | NA | NA |
| Median PFS, mo | 8.8 | Not reported | Not reached |
| Median OS, mo | 17.5 | Not reported | Not reached |
dex, dexamethasone; pom, pomalidomide. Other abbreviations are explained in Table 1.
Proteasome inhibitors
Preclinical studies also showed synergy when CD38-targeting agents were combined with PIs.54,68,69 Similarly, in the Castor study, patients (≥1 prior line of therapy) treated with bortezomib-dexamethasone (Vd) with daratumumab (DVd) had a better response and PFS (HR, 0.39; 12-month PFS, 60.7% vs 26.9%), compared with Vd-treated patients (Table 3).48 Subgroup analysis showed that adding daratumumab was beneficial in all subgroups including patients ≥65 years and those with prior bortezomib exposure.48,70 Also, patients with high-risk cytogenetics achieved benefit from adding daratumumab to Vd (median PFS, 11.2 vs 7.2 months).71 Achievement of MRD negativity resulted in excellent PFS in both treatment arms, but the MRD-negativity rate was significantly higher in the daratumumab group (DVd vs Vd, 10.4% and 2.4%; threshold 10−5).61 MRD-negativity rate and PFS were superior with DRd compared with DVd.48,59 Apart from type of standard of care to which daratumumab was added, differences in terms of study design (continuous treatment with Rd vs fixed duration treatment with Vd) and patient characteristics (more prior lines of treatment in Castor; greater proportion of patients with standard-risk cytogenetics in Pollux) may also explain differences in depth and durability of response between both studies (Tables 1 and 3). Added toxicity of daratumumab consisted of IRRs.48 Furthermore, the incidence of grade 3/4 thrombocytopenia, neutropenia, lymphopenia, diarrhea, dyspnea, and hypertension was higher in the daratumumab group, which may be partly explained by a larger proportion of patients in the DVd group receiving the maximum of 8 cycles of bortezomib treatment when compared with the control group.48 The FDA (2016) and EMA (2017) approved DVd in patients with ≥1 prior treatment.
Bortezomib-dexamethasone with or without daratumumab in MM patients with at least 1 prior line of therapy (Castor study)
| . | NCT02136134 (CASTOR)48,61 . | |
|---|---|---|
| Phase | Phase 3 | |
| Regimen | Vd | DVd |
| Treatments | Bortezomib 1.3 mg/m2: days 1, 4, 8, 11 of 21-d cycle; 8 cycles | Daratumumab (16 mg/kg): weekly ×10, Q3W until end of Vd, then Q4W until progression |
| Dexamethasone 20 mg: days 1, 2, 4, 5, 8, 9, 11,12; 8 cycles (20 mg once weekly if age >75 y) | Bortezomib 1.3 mg/m2: days 1, 4, 8, 11 of 21-d cycle; 8 cycles | |
| Dexamethasone 20 mg: days 1, 2, 4, 5, 8, 9, 11,12; 8 cycles (20 mg once weekly if age >75 y) | ||
| No. of patients | 247 | 251 |
| Median age, y | 64 | 64 |
| Median no. of prior therapies | 2 | 2 |
| Previous IMiD therapy, % | 80.2 | 71.3 |
| Previous PI therapy, % | 69.6 | 67.3 |
| IRR, % | Not applicable | 45.3 |
| ≥PR, % | 63.2 | 82.9 |
| ≥VGPR, % | 29.1 | 59.2 |
| ≥CR, % | 9.0 | 19.2 |
| MRD− disease (10−5), % | 2.4 | 10.4 |
| 12-mo PFS, % | 26.9 | 60.7 |
| OS | Median OS, not evaluable; HR, 077 | Median OS, not evaluable; HR, 0.77 |
| . | NCT02136134 (CASTOR)48,61 . | |
|---|---|---|
| Phase | Phase 3 | |
| Regimen | Vd | DVd |
| Treatments | Bortezomib 1.3 mg/m2: days 1, 4, 8, 11 of 21-d cycle; 8 cycles | Daratumumab (16 mg/kg): weekly ×10, Q3W until end of Vd, then Q4W until progression |
| Dexamethasone 20 mg: days 1, 2, 4, 5, 8, 9, 11,12; 8 cycles (20 mg once weekly if age >75 y) | Bortezomib 1.3 mg/m2: days 1, 4, 8, 11 of 21-d cycle; 8 cycles | |
| Dexamethasone 20 mg: days 1, 2, 4, 5, 8, 9, 11,12; 8 cycles (20 mg once weekly if age >75 y) | ||
| No. of patients | 247 | 251 |
| Median age, y | 64 | 64 |
| Median no. of prior therapies | 2 | 2 |
| Previous IMiD therapy, % | 80.2 | 71.3 |
| Previous PI therapy, % | 69.6 | 67.3 |
| IRR, % | Not applicable | 45.3 |
| ≥PR, % | 63.2 | 82.9 |
| ≥VGPR, % | 29.1 | 59.2 |
| ≥CR, % | 9.0 | 19.2 |
| MRD− disease (10−5), % | 2.4 | 10.4 |
| 12-mo PFS, % | 26.9 | 60.7 |
| OS | Median OS, not evaluable; HR, 077 | Median OS, not evaluable; HR, 0.77 |
DVd, daratumumab-bortezomib-dexamethasone; HR, hazard ratio; IMiD, immunomodulatory drug; PI, proteasome inhibitor; Q3W, once every 3 weeks; Vd, bortezomib-dexamethasone. Other abbreviations are explained in Table 1.
Other combinations
The better understanding of host- and tumor-related factors that predict response has also resulted in the rational design of new daratumumab-based combinations. We showed that all-trans retinoic acid (ATRA) upregulates CD38 expression and reduces expression of complement inhibitors CD55 and CD59 on MM cells, leading to markedly improved daratumumab-mediated ADCC and CDC.39 Interestingly, ATRA also increases CD38 and reduces CD55 and CD59 levels on MM cells with acquired daratumumab resistance.40 A phase 1/2 study is currently enrolling patients to evaluate the value of ATRA in patients with daratumumab-refractory disease. Similarly, the pan–histone deacetylase inhibitor panobinostat also induces upregulation of CD38 on MM cells, leading to increased ADCC with daratumumab.74 CDC was not improved as a result of concomitant upregulation of CD55 and CD59.74
Various strategies to improve effector cell function, and thereby enhance ADCC/ADCP, are also evaluated in preclinical models. For example, NK-cell activity can be improved by blocking inhibitory killer immunoglobulin-like receptors (KIRs) with the anti-KIR antibody IPH2102 (lirilumab), which resulted in enhanced daratumumab-mediated lysis of MM cells.75 Also, inhibition of the CD47–signal-regulatory protein α (SIRPα) antiphagocytic axis76 or the programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) pathway, which delivers inhibitory signals to T cells, may enhance the activity of CD38 antibodies.77
Management aspects of CD38 antibodies
In the following section, we describe some practical management aspects that are characteristic for CD38 antibodies (Figure 3).78
Practical aspects of management of CD38 antibody therapy. The main adverse event associated with CD38 antibodies is the development of IRRs, which may prolong the duration of the CD38 antibody infusion. To prevent development of IRRs, it is important to administer appropriate pre- and postinfusion medication. We typically administer 1 to 3 hours prior to the daratumumab infusion preinfusion prophylaxis consisting of antihistamines (H1 receptor antagonist: eg, 2 mg of clemastine or 25-50 mg of diphenhydramine), acetaminophen (650-1000 mg), and corticosteroids (100 mg of methylprednisolone or equivalent for daratumumab monotherapy [following the second infusion, this can be lowered to 60 mg] and 20 mg of dexamethasone for combination therapy). Furthermore, we give all patients 10 mg of montelukast prior to the first daratumumab infusion. In case the patient experiences an IRR, we also consider montelukast in subsequent infusions. For patients on daratumumab monotherapy, we give as postinfusion medication corticosteroids (20 mg methylprednisolone or equivalent on each of the 2 days following the daratumumab infusion). For patients on daratumumab combination therapy, no additional methylprednisolone as postinfusion medication is needed if dexamethasone is administered the day after the daratumumab infusion as part of the background regimen (otherwise administration of 20 mg of methylprednisolone or equivalent can be considered on the day after the infusion). For patients with a history of chronic obstructive pulmonary disease, consider prescribing short- and long-acting bronchodilators and inhaled corticosteroids. For isatuximab and MOR202, which are currently not approved, we follow the study protocol for pre-and postinfusion prophylaxis. Herpes zoster prophylaxis should be considered in patients treated with CD38-targeted therapy. CD38 antibodies also interfere with laboratory tests such as serum protein electrophoresis (SPEP) and immunofixation electrophoresis (IFE), which may confound evaluation of response. CD38 antibodies also interfere with routine methods for compatibility testing for blood transfusion. Important management aspects of some other antibody classes are also shown.
Practical aspects of management of CD38 antibody therapy. The main adverse event associated with CD38 antibodies is the development of IRRs, which may prolong the duration of the CD38 antibody infusion. To prevent development of IRRs, it is important to administer appropriate pre- and postinfusion medication. We typically administer 1 to 3 hours prior to the daratumumab infusion preinfusion prophylaxis consisting of antihistamines (H1 receptor antagonist: eg, 2 mg of clemastine or 25-50 mg of diphenhydramine), acetaminophen (650-1000 mg), and corticosteroids (100 mg of methylprednisolone or equivalent for daratumumab monotherapy [following the second infusion, this can be lowered to 60 mg] and 20 mg of dexamethasone for combination therapy). Furthermore, we give all patients 10 mg of montelukast prior to the first daratumumab infusion. In case the patient experiences an IRR, we also consider montelukast in subsequent infusions. For patients on daratumumab monotherapy, we give as postinfusion medication corticosteroids (20 mg methylprednisolone or equivalent on each of the 2 days following the daratumumab infusion). For patients on daratumumab combination therapy, no additional methylprednisolone as postinfusion medication is needed if dexamethasone is administered the day after the daratumumab infusion as part of the background regimen (otherwise administration of 20 mg of methylprednisolone or equivalent can be considered on the day after the infusion). For patients with a history of chronic obstructive pulmonary disease, consider prescribing short- and long-acting bronchodilators and inhaled corticosteroids. For isatuximab and MOR202, which are currently not approved, we follow the study protocol for pre-and postinfusion prophylaxis. Herpes zoster prophylaxis should be considered in patients treated with CD38-targeted therapy. CD38 antibodies also interfere with laboratory tests such as serum protein electrophoresis (SPEP) and immunofixation electrophoresis (IFE), which may confound evaluation of response. CD38 antibodies also interfere with routine methods for compatibility testing for blood transfusion. Important management aspects of some other antibody classes are also shown.
Infusion-related reactions
CD38 antibodies have a favorable toxicity profile with IRRs as the main side effect. Adequate and timely management of IRRs (including pre- and postinfusion prophylaxis) is important to prevent discomfort to patients, more serious toxicity, prolonged infusion time, and treatment discontinuation (Figure 3). Daratumumab-associated IRRs occur in ∼50% of patients, are mostly grade 1/2, and consist mainly of respiratory conditions such as nasal congestion, cough, allergic rhinitis, throat irritation, and dyspnea.31,48,59,79 Nonrespiratory IRRs include chills and nausea.31 Most IRRs (95.8%) occur during the first infusion, and the incidence of IRRs decreases during second (7%) and subsequent infusions (7%).31 There is no relationship between maximum daratumumab serum levels and development of IRRs.34
Isatuximab-induced IRRs have similar characteristics as those mediated by daratumumab, and occur in 55% to 56% of patients.38,64 MOR202 treatment is associated with a lower incidence of IRRs (∼10%), which may be explained by low CDC activity.36 Therefore, MOR202 can be infused faster, compared with daratumumab or isatuximab.
IRRs are managed with temporary interruptions of the infusion, or extra antihistamines or corticosteroids.31 Importantly, the leukotriene receptor antagonist, montelukast, probably mitigates CD38 antibody-associated IRRs.80 Furthermore, inhalable corticosteroids and bronchodilators are recommended for patients with obstructive lung disorders.
Response evaluation
Various therapeutic antibodies, including CD38-targeting antibodies, can be detected as a monoclonal band in serum protein electrophoresis (SPEP) and serum immunofixation electrophoresis (IFE),81,82 which may confound interpretation of these assays and thus evaluation of response (Figure 4). As expected, CD38 antibodies as intact immunoglobulins do not interfere with the serum free light chain (sFLC) assay.83 Importantly, a recent update of the International Myeloma Working Group (IMWG) uniform response criteria clarified that CR requires disappearance of the original M protein associated with MM on IFE, and therefore CR is not affected by unrelated M proteins that are secondary to therapeutically administered monoclonal antibodies.84
Daratumumab interferes with the evaluation of response. (A) The mean trough and peak serum daratumumab concentrations at the end of weekly 16 mg/kg dosing are 573 μg/mL and 915 μg/mL, respectively.33,34 These concentrations decrease slightly when patients enter the less-intense dose periods.33,34 These concentrations are greater than the sensitivity for most SPEP and serum IFE assays, which explains that daratumumab and also other therapeutic antibodies can be detected as a monoclonal band in SPEP and IFE when patients are assessed for response. Comigration of daratumumab (IgG-κ) with an IgG-κ M-protein during SPEP and IFE can mask clearance of a patient’s endogenous M protein in response to treatment. The daratumumab-specific IFE reflex assay (DIRA) uses a highly specific mouse anti-daratumumab antibody that binds daratumumab and shifts its migration away from endogenous M protein on IFE gels. Of note, because the anti-daratumumab antibody is of mouse origin, it is not precipitated with antibodies directed at human immunoglobulins.82 The mouse anti-daratumumab antibody is therefore not detected as an additional band in the DIRA. (B) If an IgG-κ band remains that is not shifted completely by the DIRA, the patient is considered to have residual M protein, and disease monitoring should continue. Patients with a single IgG-κ band that is shifted completely by the DIRA are considered to have no remaining serum M protein and may have reached CR/sCR, and therefore are candidates for additional IMWG-required confirmatory testing (BM evaluation, free light-chain assay). Dara, daratumumab; G, IgG antisera; IHC, immunohistochemistry; κ, κ antisera; sCR, stringent CR; SP, total serum protein fix; VGPR, very-good partial response.
Daratumumab interferes with the evaluation of response. (A) The mean trough and peak serum daratumumab concentrations at the end of weekly 16 mg/kg dosing are 573 μg/mL and 915 μg/mL, respectively.33,34 These concentrations decrease slightly when patients enter the less-intense dose periods.33,34 These concentrations are greater than the sensitivity for most SPEP and serum IFE assays, which explains that daratumumab and also other therapeutic antibodies can be detected as a monoclonal band in SPEP and IFE when patients are assessed for response. Comigration of daratumumab (IgG-κ) with an IgG-κ M-protein during SPEP and IFE can mask clearance of a patient’s endogenous M protein in response to treatment. The daratumumab-specific IFE reflex assay (DIRA) uses a highly specific mouse anti-daratumumab antibody that binds daratumumab and shifts its migration away from endogenous M protein on IFE gels. Of note, because the anti-daratumumab antibody is of mouse origin, it is not precipitated with antibodies directed at human immunoglobulins.82 The mouse anti-daratumumab antibody is therefore not detected as an additional band in the DIRA. (B) If an IgG-κ band remains that is not shifted completely by the DIRA, the patient is considered to have residual M protein, and disease monitoring should continue. Patients with a single IgG-κ band that is shifted completely by the DIRA are considered to have no remaining serum M protein and may have reached CR/sCR, and therefore are candidates for additional IMWG-required confirmatory testing (BM evaluation, free light-chain assay). Dara, daratumumab; G, IgG antisera; IHC, immunohistochemistry; κ, κ antisera; sCR, stringent CR; SP, total serum protein fix; VGPR, very-good partial response.
However, M-protein monitoring is more complicated when the patient’s M protein and therapeutic antibody have the same heavy- and light-chain isotype, and also co-migrate into the same region. In this situation, the 2 bands cannot be differentiated from each other, without using additional tests such as the daratumumab-specific IFE reflex assay (DIRA) to distinguish the therapeutic from the patient’s M protein. This assay uses an anti-idiotype antibody that binds to daratumumab and alters its migration pattern to distinguish between endogenous M protein and therapeutic antibody.81,82 The DIRA assay can be used to determine whether additional testing, including determination of the sFLC ratio and BM evaluation, is warranted in patients with IgG-κ band and low measurable M protein (≤2 g/L) to assess the presence of (stringent)CR. A commercially available automated and standardized DIRA test is now available.85 Similar assays are under development for isatuximab and MOR202.86
Blood transfusion
Daratumumab binds to CD38 on test red blood cells, explaining why daratumumab-treated patients have pan-reactive indirect antiglobulin tests (IATs) observed on antibody screens (Figure 5).87,,-90 Positive IATs may persist for up to 6 months after cessation of daratumumab therapy.89,91 Remarkably, daratumumab-treated patients generally have negative direct antiglobulin tests, which is explained by rapid removal of CD38 from the red blood cell surface without altering levels of other proteins such as Kell or Duffy.89,90 Furthermore, daratumumab does not affect ABO/Rh typing of patient red blood cells.88,92
CD38 antibodies interfere with blood group compatibility testing. (A) CD38 antibodies bind to CD38 molecules present on reagent or donor red blood cells (RBCs), and thereby interfere with blood bank compatibility tests, such as antibody screening and crossmatching (both IATs). (B) Several strategies can be used to negate daratumumab interference with blood group compatibility testing to ensure that patients receive a timely transfusion. This includes a chemical method (dithiothreitol [DTT]) to denature CD38 so that daratumumab binding is reversed. Because the Kell blood group system is also sensitive to DTT treatment, K− units should be supplied after ruling out or identifying alloantibodies using DTT-treated RBCs. Approximately 9% of the population is reactive to the Kell blood group system, therefore, >90% of blood units will be Kell− and suitable for transfusion. Alternatively, binding of CD38 antibodies to RBCs can be prevented by using neutralizing agents such as anti-idiotype antibody or recombinant soluble CD38. Furthermore, extensive human erythrocyte antigen phenotyping or genotyping can be performed prior to starting CD38 antibody therapy. Genotyping is not impacted by CD38 antibodies and may therefore be performed at any time, also during CD38 antibody therapy. When patients require blood transfusions, blood products can be identified based on the results of phenotyping and genotyping. Importantly, once treatment with a CD38 antibody is discontinued, pan-agglutination may persist. The duration of this effect varies from patient to patient, but for daratumumab this effect may persist for up to 6 months. Mitigation methods should be used until pan-agglutination is no longer observed.
CD38 antibodies interfere with blood group compatibility testing. (A) CD38 antibodies bind to CD38 molecules present on reagent or donor red blood cells (RBCs), and thereby interfere with blood bank compatibility tests, such as antibody screening and crossmatching (both IATs). (B) Several strategies can be used to negate daratumumab interference with blood group compatibility testing to ensure that patients receive a timely transfusion. This includes a chemical method (dithiothreitol [DTT]) to denature CD38 so that daratumumab binding is reversed. Because the Kell blood group system is also sensitive to DTT treatment, K− units should be supplied after ruling out or identifying alloantibodies using DTT-treated RBCs. Approximately 9% of the population is reactive to the Kell blood group system, therefore, >90% of blood units will be Kell− and suitable for transfusion. Alternatively, binding of CD38 antibodies to RBCs can be prevented by using neutralizing agents such as anti-idiotype antibody or recombinant soluble CD38. Furthermore, extensive human erythrocyte antigen phenotyping or genotyping can be performed prior to starting CD38 antibody therapy. Genotyping is not impacted by CD38 antibodies and may therefore be performed at any time, also during CD38 antibody therapy. When patients require blood transfusions, blood products can be identified based on the results of phenotyping and genotyping. Importantly, once treatment with a CD38 antibody is discontinued, pan-agglutination may persist. The duration of this effect varies from patient to patient, but for daratumumab this effect may persist for up to 6 months. Mitigation methods should be used until pan-agglutination is no longer observed.
The interference with routine methods for compatibility testing for blood transfusion puts patients who require transfusion at risk for delays in receiving compatible blood, as underlying alloantibodies cannot be detected.93 Standard serologic methods to eliminate pan-reactive antibodies fail to resolve the interference,93 but blood banks can use a variety of mitigation methods to safely and timely provide blood products for patients treated with daratumumab.93 First, denaturation of CD38 by dithiothreitol (DTT) negates daratumumab interference and allows identification of underlying clinically significant alloantibodies.88 A disadvantage of the DTT method is the disruption of a limited number of other blood group antigens with the Kell antigen as the most relevant DTT-sensitive red blood cell antigen in routine clinical practice. Therefore, Kell− units should be provided when using the DTT method, unless the patient is known to be Kell+.87,88 In a multicenter international study, this approach was shown to be robust and reproducible.87 A neutralization method with the use of a daratumumab idiotype antibody or recombinant soluble CD38 is also effective, but currently not widely available.88,89 A third approach is red blood cell phenotyping or genotyping in patients who will be treated with a CD38 antibody.92 Phenotyping should be done before CD38 therapy is started, whereas genotyping can be performed after CD38 treatment has started. In case of transfusion, phenotypically or genotypically matched red blood cell units should be given. We also provide our patients with blood transfusion cards indicating that CD38 antibody therapy is being received. Although daratumumab interferes with blood typing and cross-matching, no adverse events associated with blood transfusions have been described.30,31,48,59,94,95
The other CD38 antibodies also interfere in the IAT,89 and similar mitigation strategies can be used as clinically indicated.
New applications for CD38 therapy
Other plasma cell dyscrasias
CD38 antibodies are currently also being evaluated in smoldering MM and certain monoclonal gammopathies with renal significance (Table 4). Furthermore, preliminary results show that daratumumab is effective and safe in patients with advanced immunoglobulin light-chain amyloidosis.96,97
Selected ongoing or planned studies with CD38-targeting antibody-containing regimens in other conditions
| Study . | Phase . | Patients . | Treatment . |
|---|---|---|---|
| NCT02841033 | 1/2 | Relapsed or refractory AL amyloidosis | Daratumumab as single agent |
| NCT02816476 (AMYDARA) | 2 | Patients with AL amyloidosis not in VGPR or better after previous treatment | Daratumumab as single agent |
| NCT03067571 | 2 | AML or high-risk myelodysplastic syndrome (relapsed or refractory) | Daratumumab as single agent |
| NCT03011034 | 2 | Transfusion-dependent patients with low or intermediate-1 risk myelodysplastic syndrome who are relapsed or refractory to erythropoiesis-stimulating agents | Daratumumab as single agent |
| NCT03095118 | 2 | PGNMID and C3GN | Daratumumab as single agent |
| NCT03098550 | 1/2 | Advanced or metastatic solid tumors | Daratumumab plus nivolumab |
| NCT02488759 (CheckMate358) | 1/2 | Virus-associated tumors such as squamous cell carcinoma of the head and neck, cervix, and anal canal | Nivolumab or nivolumab combinations, including nivolumab plus daratumumab |
| NCT02060188 (CheckMate142) | 2 | Recurrent and metastatic colon cancer | Nivolumab or nivolumab combinations, including nivolumab plus daratumumab |
| NCT03023423 | 1/2 | Previously treated advanced or metastatic non–small cell lung cancer | Daratumumab plus atezolizumab vs atezolizumab |
| NCT02413489 (Carina) | 2 | Relapsed/refractory CD38+ mantle cell lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma | Daratumumab as single agent |
| NCT02927925 | 2 | Relapsed/refractory NKTCL, nasal type | Daratumumab as single agent |
| NCT01084252 | 1/2 | Relapsed/refractory CD38+ hematological malignancies such as B-cell non-Hodgkin lymphoma, MM, AML, B-ALL, and CLL | Isatuximab as single agent |
| NCT02999633 | 2 | Relapsed or refractory T-ALL and T-LBL | Isatuximab as single agent |
| Study . | Phase . | Patients . | Treatment . |
|---|---|---|---|
| NCT02841033 | 1/2 | Relapsed or refractory AL amyloidosis | Daratumumab as single agent |
| NCT02816476 (AMYDARA) | 2 | Patients with AL amyloidosis not in VGPR or better after previous treatment | Daratumumab as single agent |
| NCT03067571 | 2 | AML or high-risk myelodysplastic syndrome (relapsed or refractory) | Daratumumab as single agent |
| NCT03011034 | 2 | Transfusion-dependent patients with low or intermediate-1 risk myelodysplastic syndrome who are relapsed or refractory to erythropoiesis-stimulating agents | Daratumumab as single agent |
| NCT03095118 | 2 | PGNMID and C3GN | Daratumumab as single agent |
| NCT03098550 | 1/2 | Advanced or metastatic solid tumors | Daratumumab plus nivolumab |
| NCT02488759 (CheckMate358) | 1/2 | Virus-associated tumors such as squamous cell carcinoma of the head and neck, cervix, and anal canal | Nivolumab or nivolumab combinations, including nivolumab plus daratumumab |
| NCT02060188 (CheckMate142) | 2 | Recurrent and metastatic colon cancer | Nivolumab or nivolumab combinations, including nivolumab plus daratumumab |
| NCT03023423 | 1/2 | Previously treated advanced or metastatic non–small cell lung cancer | Daratumumab plus atezolizumab vs atezolizumab |
| NCT02413489 (Carina) | 2 | Relapsed/refractory CD38+ mantle cell lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma | Daratumumab as single agent |
| NCT02927925 | 2 | Relapsed/refractory NKTCL, nasal type | Daratumumab as single agent |
| NCT01084252 | 1/2 | Relapsed/refractory CD38+ hematological malignancies such as B-cell non-Hodgkin lymphoma, MM, AML, B-ALL, and CLL | Isatuximab as single agent |
| NCT02999633 | 2 | Relapsed or refractory T-ALL and T-LBL | Isatuximab as single agent |
AL, amyloid light chain; AML, acute myeloid leukemia; B-ALL, B-cell acute lymphoblastic leukemia; C3GN, C3 glomerulopathy associated with monoclonal gammopathy; CLL, chronic lymphocytic leukemia; NKTCL, NK-/T-cell lymphoma; PGNMID, proliferative glomerulonephritis with monoclonal immune deposit; T-ALL, T-cell acute lymphoblastic leukemia; T-LBL, T-lymphoblastic lymphoma. Other abbreviations are explained in Table 1.
Other hematological malignancies
CD38 antibodies also have activity against lymphoma cell lines and in a lymphoma mouse model.20,21,24,69 Furthermore, daratumumab exerts significant cytotoxicity against chronic lymphocytic leukemia cells via ADCC and ADCP, but without significant CDC, probably as a result of high complement inhibitor expression.98 In addition, daratumumab interferes with CD38 signaling and reduces CLL adhesion, migration, and homing.98
CD38 is also strongly expressed on NK cells, which explains the sustained response with daratumumab in a patient with relapsed/refractory nasal-type extranodal NK-cell–T-cell lymphoma.99 Preclinical studies have also shown activity of CD38 antibodies against T-cell acute lymphoblastic leukemia (ALL) and B-cell ALL,24 Waldenström macroglobulinemia,100 and acute myeloid leukemia (AML).101 However, the BM microenvironment impairs the anti-AML activity of daratumumab.101 Several studies with CD38 antibodies in other CD38+ hematologic malignancies are currently ongoing (Table 4).
Solid tumors
CD38 antibodies may also have therapeutic potential beyond the treatment of hematologic malignancies (Table 4). Because daratumumab has immunomodulatory effects via elimination of CD38+ immune-suppressor cells,5 CD38 antibodies may even be effective in CD38− tumors. Interestingly, preclinical lung cancer studies suggest that CD38 confers resistance to PD-L1 blockade by inhibiting CD8+ T-cell function, which explains the marked efficacy of combination therapy of PD-L1 blocker and CD38 antibody.77 This forms the preclinical rationale of various ongoing studies with daratumumab plus PD-1/PD-L1–neutralizing antibodies.77
Autoimmune and allergic diseases
CD38 antibodies may also be active in autoantibody-mediated diseases by eradicating autoantibody-producing plasma cells. Indeed, daratumumab was effective in the treatment of refractory autoimmune hemolytic anemia.102 Similarly, daratumumab reduces total and allergen-specific IgE levels by depleting IgE-producing plasma cells, and this suggests potential value of CD38 antibodies in the management of severe allergy.103
Conclusions and future prospects
Although CD38 is widely expressed, CD38-targeting antibodies have manageable toxicity. Furthermore, by virtue of distinct mechanisms of action, CD38 antibodies have impressive single-agent activity in heavily pretreated MM patients, including those refractory to newer agents such as pomalidomide and carfilzomib. CD38 antibodies can also safely be added to backbone regimens, markedly increasing their efficacy. There is currently a lack of direct comparisons among different treatment regimens for early relapsed MM. However, the PFS advantage observed with daratumumab added to lenalidomide and bortezomib is unprecedented. Furthermore, a meta-analysis has identified DRd as the most effective treatment option in relapsed/refractory MM, followed by other triplets including DVd and Rd plus elotuzumab, ixazomib, or carfilzomib.104 Biomarkers need to be identified that predict response to these different combinations to further improve and personalize treatment. Choice of treatment of patients who become refractory to CD38 antibody-based therapy should be made on an individual basis taking into account tumor and patient characteristics, as well as treatment history. Because CD38 antibodies reduce NK-cell numbers,52 it is currently unknown whether antibodies that depend on NK-cell–mediated ADCC, such as elotuzumab, are equally effective before and after CD38 antibody therapy. Furthermore, the concept of adding another backbone regimen to the CD38-targeting antibody should also be further explored.
We also expect that CD38 antibodies will increasingly be incorporated into first-line regimens over the next few years given their efficacy and manageable toxicity profile. Preliminary results demonstrate that incorporation of daratumumab in standard regimens, such as Vd, bortezomib-thalidomide-dexamethasone, bortezomib-melphalan-prednisone, or carfilzomib-lenalidomide-dexamethasone, did not result in significant additional toxicity, other than IRRs.105 Various studies are currently evaluating the role of CD38 antibodies in induction, consolidation, and maintenance in both transplant-eligible and elderly patients with newly diagnosed MM (Table 5). Furthermore, CD38 antibodies are also being evaluated in smoldering MM to prevent progression to active MM (Table 5). Also, the impact of combining CD38 antibodies with other immunotherapeutic antibodies is of particular interest going forward as the immuno-oncology field in MM expands. Finally, treatment of patients with CD38 antibodies also requires bidirectional communication between the clinician and laboratory/blood bank when assessing therapeutic responses to minimize errors or delays in providing compatible red blood cell units.
Selected ongoing and planned studies with CD38-targeting antibody-containing regimens in newly diagnosed MM or smoldering MM
| Study . | Phase . | Patients . | Treatment . |
|---|---|---|---|
| NCT02316106 (Centaurus) | 2 | Intermediate or high-risk smoldering MM | 3 dose schedules of daratumumab as single agent (short, intermediate, and long treatment duration) |
| NCT02960555 | 2 | High-risk smoldering MM | Isatuximab as single agent |
| Cassiopeia (IFM 2015-01; HOVON 131; NCT02541383) | 3 | NDMM, transplant eligible | Randomization 1: VTD induction therapy, followed by high-dose melphalan plus autologous stem cell rescue, followed by VTD consolidation vs VTD with daratumumab induction therapy, followed by high-dose melphalan plus autologous stem cell rescue, followed by VTD with daratumumab consolidation |
| Randomization 2: daratumumab as single agent in maintenance vs observation only | |||
| NCT02874742 | 2 | NDMM, transplant eligible | RVD + daratumumab induction–high-dose melphalan plus autologous stem cell rescue, followed by RVD plus daratumumab consolidation, followed by daratumumab plus lenalidomide maintenance |
| vs | |||
| RVD induction–high-dose melphalan plus autologous stem cell rescue, followed by RVD consolidation, followed by lenalidomide maintenance | |||
| EMN study | 3 | NDMM, transplant eligible | Randomization 1: KRD induction therapy, followed by high-dose melphalan plus autologous stem cell rescue, followed by KRD consolidation vs KRD with daratumumab induction therapy, followed by high-dose melphalan plus autologous stem cell rescue, followed by KRD with daratumumab consolidation |
| Randomization 2: daratumumab or daratumumab plus lenalidomide maintenance | |||
| NCT02955810 | 1 | NDMM, transplant eligible | CyBorD plus daratumumab induction–high-dose melphalan plus autologous stem cell rescue, followed by CyBorD plus daratumumab consolidation, followed by maintenance with daratumumab (low-risk) or daratumumab plus bortezomib (high-risk) |
| NCT02951819 | 2 | Untreated NDMM and relapsed MM | CyBorD plus daratumumab induction, followed by daratumumab plus dexamethasone maintenance |
| NCT02252172 (Maia) | 3 | NDMM, transplant ineligible | Rd vs DRd |
| NCT02195479 (Alcyone) | 3 | NDMM, transplant ineligible | VMP vs VMP plus daratumumab |
| Hovon143 | 2 | NDMM, transplant ineligible and frail or unfit | Ixazomib-dexamethasone plus daratumumab induction, followed by ixazomib plus daratumumab maintenance |
| NCT03012880 | 2 | NDMM, 18 y and older | Ixazomib-Rd plus daratumumab |
| NCT01998971 | 1b | NDMM, irrespective of transplant eligibility for Vd, VTD, and KRD, and transplant ineligible for VMP | Daratumumab combined with Vd, VTD, KRD, or VMP |
| NCT02513186 | 1 | NDMM, transplant ineligible | Isatuximab plus CyBorD or VRD |
| NCT03104842 | 2 | NDMM, transplant eligible and high-risk cytogenetic abnormalities | Isatuximab plus KRD induction, followed by high-dose melphalan plus autologous stem cell rescue, followed by isatuximab plus KRD consolidation, followed by isatuximab plus KR maintenance |
| NDMM, transplant ineligible and high-risk cytogenetic abnormalities | Isatuximab plus KRD induction followed by isatuximab plus KR maintenance |
| Study . | Phase . | Patients . | Treatment . |
|---|---|---|---|
| NCT02316106 (Centaurus) | 2 | Intermediate or high-risk smoldering MM | 3 dose schedules of daratumumab as single agent (short, intermediate, and long treatment duration) |
| NCT02960555 | 2 | High-risk smoldering MM | Isatuximab as single agent |
| Cassiopeia (IFM 2015-01; HOVON 131; NCT02541383) | 3 | NDMM, transplant eligible | Randomization 1: VTD induction therapy, followed by high-dose melphalan plus autologous stem cell rescue, followed by VTD consolidation vs VTD with daratumumab induction therapy, followed by high-dose melphalan plus autologous stem cell rescue, followed by VTD with daratumumab consolidation |
| Randomization 2: daratumumab as single agent in maintenance vs observation only | |||
| NCT02874742 | 2 | NDMM, transplant eligible | RVD + daratumumab induction–high-dose melphalan plus autologous stem cell rescue, followed by RVD plus daratumumab consolidation, followed by daratumumab plus lenalidomide maintenance |
| vs | |||
| RVD induction–high-dose melphalan plus autologous stem cell rescue, followed by RVD consolidation, followed by lenalidomide maintenance | |||
| EMN study | 3 | NDMM, transplant eligible | Randomization 1: KRD induction therapy, followed by high-dose melphalan plus autologous stem cell rescue, followed by KRD consolidation vs KRD with daratumumab induction therapy, followed by high-dose melphalan plus autologous stem cell rescue, followed by KRD with daratumumab consolidation |
| Randomization 2: daratumumab or daratumumab plus lenalidomide maintenance | |||
| NCT02955810 | 1 | NDMM, transplant eligible | CyBorD plus daratumumab induction–high-dose melphalan plus autologous stem cell rescue, followed by CyBorD plus daratumumab consolidation, followed by maintenance with daratumumab (low-risk) or daratumumab plus bortezomib (high-risk) |
| NCT02951819 | 2 | Untreated NDMM and relapsed MM | CyBorD plus daratumumab induction, followed by daratumumab plus dexamethasone maintenance |
| NCT02252172 (Maia) | 3 | NDMM, transplant ineligible | Rd vs DRd |
| NCT02195479 (Alcyone) | 3 | NDMM, transplant ineligible | VMP vs VMP plus daratumumab |
| Hovon143 | 2 | NDMM, transplant ineligible and frail or unfit | Ixazomib-dexamethasone plus daratumumab induction, followed by ixazomib plus daratumumab maintenance |
| NCT03012880 | 2 | NDMM, 18 y and older | Ixazomib-Rd plus daratumumab |
| NCT01998971 | 1b | NDMM, irrespective of transplant eligibility for Vd, VTD, and KRD, and transplant ineligible for VMP | Daratumumab combined with Vd, VTD, KRD, or VMP |
| NCT02513186 | 1 | NDMM, transplant ineligible | Isatuximab plus CyBorD or VRD |
| NCT03104842 | 2 | NDMM, transplant eligible and high-risk cytogenetic abnormalities | Isatuximab plus KRD induction, followed by high-dose melphalan plus autologous stem cell rescue, followed by isatuximab plus KRD consolidation, followed by isatuximab plus KR maintenance |
| NDMM, transplant ineligible and high-risk cytogenetic abnormalities | Isatuximab plus KRD induction followed by isatuximab plus KR maintenance |
CyBorD, cyclophosphamide-bortezomib-dexamethasone; DRd, daratumumab plus lenalidomide-dexamethasone; KR, carfilzomib and lenalidomide; KRD, carfilzomib-lenalidomide-dexamethasone; NDMM, newly diagnosed MM; Rd, lenalidomide-dexamethasone; Vd, bortezomib-dexamethasone; VMP, bortezomib-melphalan-prednisone; VRD or RVD, bortezomib-lenalidomide-dexamethasone; VTD, bortezomib-thalidomide-dexamethasone.
Acknowledgments
The authors thank Victor Muñoz Sanz (Sanz Serif Research + Design Agency) for creating Figures 1, 2, and 3. The authors also thank Caline Sakabedoyan for help with the DIRA image and blood transfusion interference image.
Authorship
Contribution: All authors performed literature searches and prepared the manuscript.
Conflict-of-interest disclosure: N.W.C.J.v.d.D. has received research support from Janssen Pharmaceuticals, Amgen, Celgene, and Bristol-Myers Squibb (BMS), and serves on advisory boards for Janssen Pharmaceuticals, Amgen, Celgene, BMS, Novartis, and Servier. P.G.R. has served on advisory boards for Millennium Pharmaceuticals, Celgene, Novartis, Janssen Pharmaceuticals, and BMS. F.M. has received research support from Janssen Pharmaceuticals, Celgene, Tusk Therapeutics, and Centrose, and serves on advisory boards for Centrose and Tusk Therapeutics.
Correspondence: Niels W. C. J. van de Donk, Department of Hematology, VU University Medical Center, De Boelelaan 1117, 1081HV Amsterdam, The Netherlands; e-mail: n.vandedonk@vumc.nl.


![Figure 3. Practical aspects of management of CD38 antibody therapy. The main adverse event associated with CD38 antibodies is the development of IRRs, which may prolong the duration of the CD38 antibody infusion. To prevent development of IRRs, it is important to administer appropriate pre- and postinfusion medication. We typically administer 1 to 3 hours prior to the daratumumab infusion preinfusion prophylaxis consisting of antihistamines (H1 receptor antagonist: eg, 2 mg of clemastine or 25-50 mg of diphenhydramine), acetaminophen (650-1000 mg), and corticosteroids (100 mg of methylprednisolone or equivalent for daratumumab monotherapy [following the second infusion, this can be lowered to 60 mg] and 20 mg of dexamethasone for combination therapy). Furthermore, we give all patients 10 mg of montelukast prior to the first daratumumab infusion. In case the patient experiences an IRR, we also consider montelukast in subsequent infusions. For patients on daratumumab monotherapy, we give as postinfusion medication corticosteroids (20 mg methylprednisolone or equivalent on each of the 2 days following the daratumumab infusion). For patients on daratumumab combination therapy, no additional methylprednisolone as postinfusion medication is needed if dexamethasone is administered the day after the daratumumab infusion as part of the background regimen (otherwise administration of 20 mg of methylprednisolone or equivalent can be considered on the day after the infusion). For patients with a history of chronic obstructive pulmonary disease, consider prescribing short- and long-acting bronchodilators and inhaled corticosteroids. For isatuximab and MOR202, which are currently not approved, we follow the study protocol for pre-and postinfusion prophylaxis. Herpes zoster prophylaxis should be considered in patients treated with CD38-targeted therapy. CD38 antibodies also interfere with laboratory tests such as serum protein electrophoresis (SPEP) and immunofixation electrophoresis (IFE), which may confound evaluation of response. CD38 antibodies also interfere with routine methods for compatibility testing for blood transfusion. Important management aspects of some other antibody classes are also shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/1/10.1182_blood-2017-06-740944/4/m_blood740944f3.jpeg?Expires=1769674719&Signature=geQv6vHJfXBLvsNGfrL-8ycZma47gBFlPy3dp0E4ql16Y5mOFQMK1onXliQjFjVxPHwRmnWyZiAL51DmGZ1WQLLdmTWhQdrr0obn75VDZkj5laRTyfAD~iqaCs8gWe75tSyiVU2ho8A659RYuNuDSa-BouXWTo5vJ3Yy~NwQuWWZsx7zWz5XUuFfm~WoYISO3zZertOHpYfoaf6jW2JYQmN2ZaKLKjxz0N3oab1d~Aqrjz-wUlPSoYQG-NsRGUyvt9aq77nlK~cAI8-9CJydBZcAzBXY9Exj8BslQDMvQpcNFZbySPITPJSDCgZET2HynyEt6b5g2ztrDdD6qfyfLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
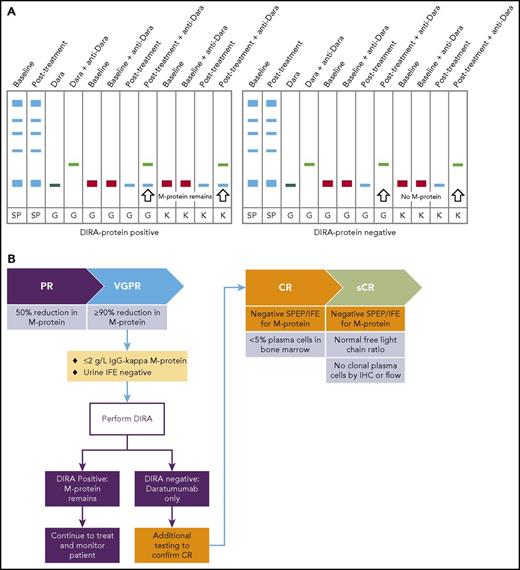
![Figure 5. CD38 antibodies interfere with blood group compatibility testing. (A) CD38 antibodies bind to CD38 molecules present on reagent or donor red blood cells (RBCs), and thereby interfere with blood bank compatibility tests, such as antibody screening and crossmatching (both IATs). (B) Several strategies can be used to negate daratumumab interference with blood group compatibility testing to ensure that patients receive a timely transfusion. This includes a chemical method (dithiothreitol [DTT]) to denature CD38 so that daratumumab binding is reversed. Because the Kell blood group system is also sensitive to DTT treatment, K− units should be supplied after ruling out or identifying alloantibodies using DTT-treated RBCs. Approximately 9% of the population is reactive to the Kell blood group system, therefore, >90% of blood units will be Kell− and suitable for transfusion. Alternatively, binding of CD38 antibodies to RBCs can be prevented by using neutralizing agents such as anti-idiotype antibody or recombinant soluble CD38. Furthermore, extensive human erythrocyte antigen phenotyping or genotyping can be performed prior to starting CD38 antibody therapy. Genotyping is not impacted by CD38 antibodies and may therefore be performed at any time, also during CD38 antibody therapy. When patients require blood transfusions, blood products can be identified based on the results of phenotyping and genotyping. Importantly, once treatment with a CD38 antibody is discontinued, pan-agglutination may persist. The duration of this effect varies from patient to patient, but for daratumumab this effect may persist for up to 6 months. Mitigation methods should be used until pan-agglutination is no longer observed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/1/10.1182_blood-2017-06-740944/4/m_blood740944f5.jpeg?Expires=1769674719&Signature=kkQK77pdT3d6OX6GFRL7hYc6rLYiIXR~cqPOh9W1a2NZO5OLw1TRXgJjG9YkWWgPfh4RyPc32ukMseRGKLdu~RnVcyhbku7IHE9ftBAERswiUMBg3hBASuSDAndMrO93a7TV25Uvu5NMcTbvWVJ9rI8vSgAm4baahr-8GyjJvc2KnhXrrdbWTRg2RPKFMFDRjB0u4Xt9OnTnybNwxL5~fJfEwBCk~OXlN8JvdY-2PlN-9wJsvjzXBy3etcNl1hoeEvObNrFEG-e0~cHFZbDpNCS9mMJcJQXyVmvEzIthNq8IfCcxEmVvttxZNQZnQQroLikgZR7gXwF0OlO1lAm7Pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal