Key Points
A new T-cell immunotherapy has been developed to prevent or manage relapse of leukemia following HCT.
Memory CD8+ and CD4+ T cells are engineered with a minor histocompatibility antigen–targeting TCR, CD8 coreceptor, and safety switch.
Leukemia relapse remains the major cause of allogeneic hematopoietic stem cell transplantation (HCT) failure, and the prognosis for patients with post-HCT relapse is poor. There is compelling evidence that potent selective antileukemic effects can be delivered by donor T cells specific for particular minor histocompatibility (H) antigens. Thus, T-cell receptors (TCRs) isolated from minor H antigen–specific T cells represent an untapped resource for developing targeted T-cell immunotherapy to manage post-HCT leukemic relapse. Recognizing that several elements may be crucial to the efficacy and safety of engineered T-cell immunotherapy, we developed a therapeutic transgene with 4 components: (1) a TCR specific for the hematopoietic-restricted, leukemia-associated minor H antigen, HA-1; (2) a CD8 coreceptor to promote function of the class I–restricted TCR in CD4+ T cells; (3) an inducible caspase 9 safety switch to enable elimination of the HA-1 TCR T cells in case of toxicity; and (4) a CD34-CD20 epitope to facilitate selection of the engineered cell product and tracking of transferred HA-1 TCR T cells. The T-cell product includes HA-1 TCR CD4+ T cells to augment the persistence and function of the HA-1 TCR CD8+ T cells and includes only memory T cells; naive T cells are excluded to limit the potential for alloreactivity mediated by native TCR coexpressed by HA-1 TCR T cells. We describe the development of this unique immunotherapy and demonstrate functional responses to primary leukemia by CD4+ and CD8+ T cells transduced with a lentiviral vector incorporating the HA-1 TCR transgene construct.
Introduction
Relapse occurs following allogeneic hematopoietic cell transplantation (HCT) in approximately one-third of patients with acute leukemia who undergo the procedure, and most patients subsequently die of their disease.1,2 T-cell immunotherapy using chimeric antigen receptors (CARs) is highly effective for treating CD19+ B-lineage acute lymphoblastic leukemia (B-ALL) even in the post-HCT setting, but novel T-cell immunotherapies are required for patients with other leukemia types.3,,-6
Genes encoding T-cell receptor (TCR) α and β chains, previously isolated from high-avidity antigen-specific T-cell clones, can provide an “off-the-shelf” reagent to produce antigen-specific immunotherapy by TCR transfer.7,,,,,-13 In contrast to CARs, which can only recognize cell surface molecules, natural TCRs recognize peptides derived from intracellular or surface proteins. Minor histocompatibility (H) antigens are peptides derived from normal polymorphic self-proteins that differ in amino acid sequence between HCT recipients and donors.14,15 Alloreactive donor T cells that recognize minor H antigens on recipient epithelial cells cause graft-versus-host-disease (GVHD) after HLA-matched HCT. However, some minor H antigens are expressed predominantly or exclusively on hematopoietic cells, and donor T cells specific for hematopoietic-restricted minor H antigens can provide a potent and selective antileukemic effect.14,15 TCRs derived from hematopoietic-restricted minor H antigen–specific T cells represent an untapped resource for the development of gene-modified T-cell immunotherapy to manage leukemia relapse post-HCT.7,9,16
The minor H antigen, HA-1H, is a compelling target for immunotherapy post-HCT.15,17,,,,,-23 HA-1H is a peptide (VLHDDLLEA; henceforth called HA-1) presented by a common HLA allele (HLA-A*0201) and encoded by a DNA sequence spanning a single nucleotide polymorphism (RS_1801284) with a balanced phenotypic distribution within the HMHA1 gene.17 HMHA1-encoded protein is highly expressed in leukemia and normal hematopoietic cells, but not normal nonhematopoietic cells.24,-26 The immunogenicity of HA-1 (VLHDDLLEA, genotype RS_1801284 A/G or A/A) relates predominantly to the lower affinity and unstable binding of the corresponding nonimmunogenic sequence (VLRDDLLEA, genotype RS_1801284 G/G) to HLA-A*0201.27,28 In HA-1–mismatched HCT, the G/G (HA-1–) donor immune system is not tolerant to HA-1 as it is to self-antigens. Thus, high-avidity T cells from HA-1– donors can be primed against HA-1 and mediate a graft-versus-leukemia effect. HA-1+ recipients with an HA-1– donor have lower rates of relapse than HA-1–compatible pairs, and HA-1–specific T-cell clonal expansions associate with remissions in HA-1+ leukemia patients who receive donor lymphocyte infusions (DLIs) from HA-1– donors to treat post-HCT relapse.20,-22,29 HA-1–specific T cells do not damage the normal HA-1– donor–derived hematopoietic system in HA-1–mismatched HCT and do not cause toxicity in nonhematopoietic recipient tissues because they have negligible HMHA1 expression.24,-26,30
In this article, we describe the development and optimization of a novel T-cell therapy. We cloned high-affinity HA-1–specific TCRs into a lentiviral vector (LV) and showed that HA-1 TCR–transduced T cells produced HA–specific killing of primary leukemia. To facilitate efficacy and minimize toxicity, we included a CD8 coreceptor to promote TCR function in CD4+ T cells, a safety switch to permit eradication of HA-1 TCR T cells in case of toxicity, and a selection/tracking marker in the transgene. We strategically included CD4+ T cells, expressing the class I–restricted TCR and a CD8 coreceptor, because CD4+ T helper cells can augment antitumor cytotoxic T lymphocyte (CTL) responses by facilitating CD8+ T-cell trafficking to the site of the antigen, enhancing clonal expansion at the tumor site and preventing activation-induced cell death.31,,,,,,,-39
Methods
Generation of HA-1–specific T-cell clones
Using a CD8+ T-cell isolation kit and anti-CD45RO immunomagnetic beads (Miltenyi Biotec), CD8+ naive T cells (TN) were isolated from HLA A*0201+, HA-1– (RS_1801284, G/G) normal donor peripheral blood mononuclear cells (PBMCs). Autologous dendritic cells (DCs) were pulsed with 1 μg/mL HA-1 peptide (VLHDDLLEA) for 3 to 6 hours at 37°C. Purified CD8+ TN were combined in complete cytotoxic T lymphocyte (CTL) medium with peptide-pulsed DCs at a TN to DC ratio of 30:1 and cocultured in 96-well plates at 6 × 104 T cells per well, supplemented with 10 ng/mL interleukin-12 (IL-12) from initiation and 10 ng/mL IL-15 from day 7. On day 11 through 13, cells were evaluated for HA-1–specific cytotoxicity in split-well micro-chromium release assays (CRAs; μCRAs). T-cell lines that lysed T2 cells pulsed with 1 μg/mL HA-1 peptide (>20% lysis and more than fivefold more lysis of peptide-pulsed vs -unpulsed targets) were subsequently cloned by limiting dilution using anti-CD3 monoclonal antibody (mAb), IL-2, and feeder cells. Clones were screened by μCRAs on day 11 through 13. T-cell clones from wells showing specific cytotoxicity, using the above criteria, were expanded using anti-CD3 mAb, IL-2, and feeder cells by the rapid expansion protocol.40 The specificity of expanded clones was evaluated by CRAs, HA-1/HLA-A2 multimer staining, and intracellular cytokine staining (ICC) (supplemental Methods, available on the Blood Web site).
Cloning of the TCR into an LV
RNA was extracted from each HA-1–specific T-cell clone. To identify full TCR regions, 5′-first-strand complementary DNA (cDNA) amplification and rapid amplification of cDNA ends (RACE) polymerase chain reaction (PCR) were performed using a SMARTer RACE cDNA Amplification Kit (Clontech Laboratories). Briefly, cDNA was synthesized from RNA using 5′ CDS Primer A, SMARTer IIA oligo, and SMARTScribe Reverse Transcriptase. Subsequently, the cDNA was used to perform a RACE PCR reaction using Phusion High-Fidelity DNA Polymerase, and gene-specific primers for the TCR α (α) (5′-GGTGAATAGGCAGACAGACTT−3′) or TCR β (β) chain (5′-GTGGCCAGGCACACCAGTGT-3′)n. The RACE PCR product was purified and sequenced to identify the TCR α and β chains. IMGT/V-QUEST software was used to define the TCR variable, diversity, and joining regions.
Complementary cysteine residues at positions 48 (Thr to Cys) and 57 (Ser to Cys) were incorporated into the constant domains of the TCR α and β genes to increase exogenous TCR pairing and decrease mispairing with the endogenous TCR.41 To ensure coordinated gene expression, the TCR chains were separated by codon diversified 2A elements from the porcine teschovirus (P2A). The transgenes were codon-optimized to enhance expression,42 synthesized by GeneArt (Life Technologies), and cloned into the pRRLSIN.cPPT.MSCV.WPRE LV by restriction digestion and ligation.
Subsequently, genes for the inducible caspase 9 (iCasp9) safety switch,43,,,,-48 truncated EGFR,49 RQR8,50 myc-tag,51 and the CD8 coreceptor (NP_004922.1; βM1 and α chain separated by a codon-diversified P2A) were codon-optimized and synthesized by GeneArt. The myc-tag and CD34 epitope sequences were inserted immediately 3′ of the leader peptide from the TCR α or β chains, or the CD8 α chain or β chain. The safety switch and/or CD8 coreceptor were inserted by restriction digestion into the LV, separated by codon diversified P2A elements, before or after the TCR, respectively.
Transduction, purification, and expansion of T cells
CD8+ and CD4+ T cells were purified from normal donor PBMCs using T-cell immunomagnetic isolation kits (Miltenyi Biotec) and activated with Dynabeads Human T-Activator CD3/CD28 (ThermoFisher Scientific) in 50 IU/mL IL-2 for 24 hours. T cells were then transduced with LV supernatant in 1 µg/mL hexadimethrine bromide (Polybrene), centrifuged at 800 g for 90 minutes, and returned to 37°C, 5% carbon dioxide. Four days after transduction, the T cells were stained with HA-1 multimer (Immudex) and anti-CD8 or anti-CD4 fluorescent mAb. HA1+CD8+ and/or HA1+CD4+ T cells were sorted to >95% purity using a FACSAria II Cell Sorter (BD Pharmingen) and expanded using the rapid expansion protocol. After 10 days, T cells were evaluated by flow cytometry and functional assays.
CD45RA– depletion and large-scale cell processing
Normal donor PBMCs (3-10 × 109 ) were exposed to anti-CD45RA and anti-CD14 immunomagnetic microbeads and an AutoMACs Pro or CliniMACs Separator (Miltenyi Biotec) to produce a CD45RA– CD14– fraction; 108 cells were set aside as the CD4+-enriched fraction, and remaining CD45RA– CD14– cells were CD8+-enriched by CD4+ cell depletion with anti-CD4 immunomagnetic beads. CD3/CD28 Dynabeads were used to activate ∼108 CD45RA–CD14–CD8+ and 108 CD45RA–CD14–CD4+ cells at T cell-to-bead ratios of 1:3 in CTL medium with 50 U/mL IL-2. The cells were incubated at 2 × 106 cells/mL in 12-well plates for 24 hours at 37°C, 5% carbon dioxide and then transduced with the LV transgene construct (iCasp9-HA1TCR2-RQR-CD8). After 4 days, HA-1+CD34+CD4+ and HA-1+CD34+CD8+ cells were sorted by fluorescence-activated cell sorting and 1 to 3 × 106 sorted CD4+ and CD8+ T cells were separately expanded in CTL medium with anti-CD3 mAb, IL-2, and feeder cells, in gas-permeable rapid expansion device (G-REX100M) flasks (Wilson Wolf). On day 10 through 14 of expansion, CD4+ and CD8+ T cells were independently selected using anti-CD34 immunomagnetic microbeads and a MACs Separator to ensure the removal of any untransduced T cells and then evaluated by flow cytometry and functional assays.
See supplemental Methods for additional information.
Results
Isolation of HA-1–specific T-cell clones and TCRs
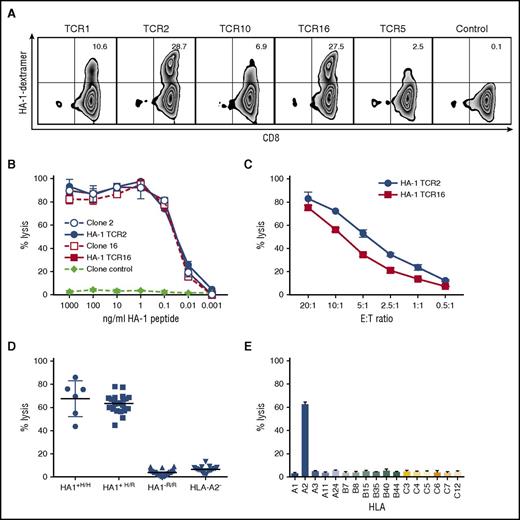
Normal donors with an HA-1– genotype (RS_1801284 G/G) are not tolerant to HA-1 and are likely to have high-avidity HA-1–specific T cells in their TN repertoire. To isolate HA-1–specific T cells and TCRs, we first performed HLA and HA-1 genotyping on normal donor PBMCs. We identified 2 HLA-A*0201(A2)+HA-1– donors and used a method we previously developed to prime rare TN against minor H antigens in vitro and derive T-cell clones from which antigen-specific TCRs could be isolated.52 Eight HA-1–specific CD8+ T-cell clones were obtained from 2 donors. The T cells specifically bound HA-1/HLA-A2 multimers (supplemental Figure 1A) and lysed HA-1 peptide-pulsed T2 cells at low peptide concentrations, implying high functional avidity (supplemental Figure 1B). The T cells killed HA-1+ (RS_1801284 A/A, A/G) A2+ lymphoblastoid cell lines (LCLs), but not HA-1– or A2– cells, demonstrating specific recognition of physiological quantities of HA-1 antigen naturally processed and presented on the cell surface with the HLA-restricting allele (data not shown). They also specifically killed HA-1+A2+ primary acute myeloid leukemia (AML) and AML cell lines (supplemental Figure 1C). We sequenced the TCR Vβ and Vα genes from each clone (supplemental Table 1). Although there was heterogeneity, 7 of the 8 T-cell clones used the same Vβ (TRBV 7-9), consistent with previous reports of preferential use of this Vβ by HA-1–specific T cells.53,54
HA-1 TCR–transduced T cells efficiently and specifically kill HA1+ target cells
Six unique HA-1 TCR sequences were first cloned into the retroviral vector MP71. Five transduced TCRs functioned in the Jurkat cell line and were subsequently codon-optimized to maximize expression.42 Complementary cysteine residues were introduced at positions 48 (Thr to Cys) and 57 (Ser to Cys) to maximize α− and β-chain pairing and minimize the potential for mispairing with the endogenous TCR.41 The optimized TCR sequences were then cloned into a multicistronic LV. All 5 TCR LV constructs transduced primary CD8+ T cells; however, TCR2 and TCR16 LV produced higher TCR expression than the others and were further evaluated (Figure 1A). HA-1 TCR2– and TCR16–transduced T cells efficiently lysed T2 cells pulsed with low concentrations of HA-1 peptide, comparable with lysis by the T-cell clones from which they were derived (half maximum lysis at 10-50 pm) (Figure 1B). HA-1 TCR–transduced CD8+ T cells also killed HA-1+ LCLs at low effector-to-target (E:T) ratios (Figure 1C) and consistently specifically killed all HA-1+ homozygous and heterozygous, HLA-A2+ LCLs, but never killed HA-1– or A2– targets (Figure 1D-E). Because HA-1 TCR16–transduced T cells consistently demonstrated slightly lower level of killing of HA1+ targets (Figure 1C and data not shown) than HA-1 TCR2 T cells, the HA1 TCR2 (hereafter referred to as HA-1 TCR) was chosen for further evaluation.
HA-1 TCR–transduced CD8+T cells kill HA1+target cells. (A) Flow cytometry showing HLA-A2/HA-1 multimer staining of CD8+ T cells transduced with HA-1 TCR LV (TCR 1, 2, 10, 16, 5) and CD8+ T cells transduced with TCRs specific for a different minor H antigen (control). (B-E) CRAs to evaluate specific lytic activity. (B) Lysis of T2 target cells pulsed with HA-1 peptide at various concentrations by TCR-transduced CD8+ T cells (solid lines and symbols; TCR2, blue circles; TCR16, red squares), HA-1–specific T-cell clones (dashed lines, open symbols: clone 2, blue circles; clone 16, red squares), or T-cell clone control (diamonds). (C) Lysis of HLA-A2+HA-1+ LCL by CD8+ T cells transduced with HA-1 TCR2 (circles) or HA-1 TCR16 (squares) at various E:T ratios. (D) Lysis of HLA-A2+/HA-1+ homozygous (H/H) (circles, n = 7), HLA-A2+/HA1+ heterozygous (H/R) (squares, n = 22), HLA-A2+/HA-1– (R/R) (triangles, n = 17), or HLA-A2–negative (inverted triangles, n = 41) hematopoietic cell (LCL) targets by HA-1 TCR2–transduced CD8+ T cells. (E) Lysis of LCL with common HLA alleles by HA-1 TCR2–transduced CD8+ T cells. *An E:T ratio of 20:1 was used unless otherwise specified. Data comparable with that shown in panels D and E were also obtained with HA-1 TCR16 (data not shown).
HA-1 TCR–transduced CD8+T cells kill HA1+target cells. (A) Flow cytometry showing HLA-A2/HA-1 multimer staining of CD8+ T cells transduced with HA-1 TCR LV (TCR 1, 2, 10, 16, 5) and CD8+ T cells transduced with TCRs specific for a different minor H antigen (control). (B-E) CRAs to evaluate specific lytic activity. (B) Lysis of T2 target cells pulsed with HA-1 peptide at various concentrations by TCR-transduced CD8+ T cells (solid lines and symbols; TCR2, blue circles; TCR16, red squares), HA-1–specific T-cell clones (dashed lines, open symbols: clone 2, blue circles; clone 16, red squares), or T-cell clone control (diamonds). (C) Lysis of HLA-A2+HA-1+ LCL by CD8+ T cells transduced with HA-1 TCR2 (circles) or HA-1 TCR16 (squares) at various E:T ratios. (D) Lysis of HLA-A2+/HA-1+ homozygous (H/H) (circles, n = 7), HLA-A2+/HA1+ heterozygous (H/R) (squares, n = 22), HLA-A2+/HA-1– (R/R) (triangles, n = 17), or HLA-A2–negative (inverted triangles, n = 41) hematopoietic cell (LCL) targets by HA-1 TCR2–transduced CD8+ T cells. (E) Lysis of LCL with common HLA alleles by HA-1 TCR2–transduced CD8+ T cells. *An E:T ratio of 20:1 was used unless otherwise specified. Data comparable with that shown in panels D and E were also obtained with HA-1 TCR16 (data not shown).
HA-1 TCR–transduced T cells specifically kill leukemic cells
Recognition and killing of primary leukemia cells and cell lines by HA-1 TCR–transduced cells were assessed. HA-1 TCR–transduced CD8+ T cells expressed CD107a after stimulation with HA-1+A2+ primary AML of various molecular and morphological subtypes and B-ALL, but not HA-1– AML or B-ALL, reflecting specific recognition of HA-1+ leukemia and T-cell degranulation (Figure 2A-B). Moreover, HA-1 TCR CD8+ T cells specifically killed HA-1+A2+ AML at low E:T ratios, and the activity of TCR-transduced cells was similar to that of the T-cell clone from which TCR2 was derived (Figure 2C-D). HA-1 TCR CD8+ T cells also killed B-ALL and T-lineage acute lymphoblastic leukemia, AML, and T-cell lymphoma cell lines that naturally express or had been transduced to express HLA A*0201 (supplemental Figure 2) and HA-1 (Figure 2E-F), confirming the specificity of the killing and that HA-1 TCR CD8+ T cells can effectively target lymphoid as well as myeloid malignancies.
HA-1 TCR–transduced CD8+T cells kill leukemic cells. (A-B) HA-1–specific expression of CD107a on HA-1 TCR2–transduced CD8+ T cells showing degranulation after 5 hours of coculture (1:1) with a panel of (A) primary AML samples and (B) primary B-ALL samples. (C-F) CRAs showing lysis of leukemia and lymphoma targets by HA-1 TCR–transduced CD8+ T cells; lysis of (C) primary HA-1+ AML or HA-1– AML by HA-1 TCR–transduced CD8+ T cells (blue bars) and HA-1–specific T-cell clone 2 (gray bars); (D) HLA-A2+/HA-1+ primary AML (AML1) at various E:T ratios; (E) B-ALL lines (1) BALL-1 and (2) RS4;11, T-ALL lines (1) MOLT4, (2) CEM, (3) RPMI-8402, and (4) HSB-2, and AML line NB-4; and (F) T-cell lymphoma (SUP-M2 HLA-A2+, HA-1+; SU-DHL-1 HLA-A2+ HA-1–) cell lines. In panel E, HLA-A2– and/or HA-1– (wild-type [WT]) cell lines were transduced (TD) with LV encoding *HLA-A2 or **HLA-A2 and HA-1 minigene or &HA-1 minigene if the WT was HLA-A2– or had a HA-1– genotoype. An E:T ratio of 20:1 was used unless otherwise specified.
HA-1 TCR–transduced CD8+T cells kill leukemic cells. (A-B) HA-1–specific expression of CD107a on HA-1 TCR2–transduced CD8+ T cells showing degranulation after 5 hours of coculture (1:1) with a panel of (A) primary AML samples and (B) primary B-ALL samples. (C-F) CRAs showing lysis of leukemia and lymphoma targets by HA-1 TCR–transduced CD8+ T cells; lysis of (C) primary HA-1+ AML or HA-1– AML by HA-1 TCR–transduced CD8+ T cells (blue bars) and HA-1–specific T-cell clone 2 (gray bars); (D) HLA-A2+/HA-1+ primary AML (AML1) at various E:T ratios; (E) B-ALL lines (1) BALL-1 and (2) RS4;11, T-ALL lines (1) MOLT4, (2) CEM, (3) RPMI-8402, and (4) HSB-2, and AML line NB-4; and (F) T-cell lymphoma (SUP-M2 HLA-A2+, HA-1+; SU-DHL-1 HLA-A2+ HA-1–) cell lines. In panel E, HLA-A2– and/or HA-1– (wild-type [WT]) cell lines were transduced (TD) with LV encoding *HLA-A2 or **HLA-A2 and HA-1 minigene or &HA-1 minigene if the WT was HLA-A2– or had a HA-1– genotoype. An E:T ratio of 20:1 was used unless otherwise specified.
CD8 coreceptor function in CD4+ HA-1 TCR cells
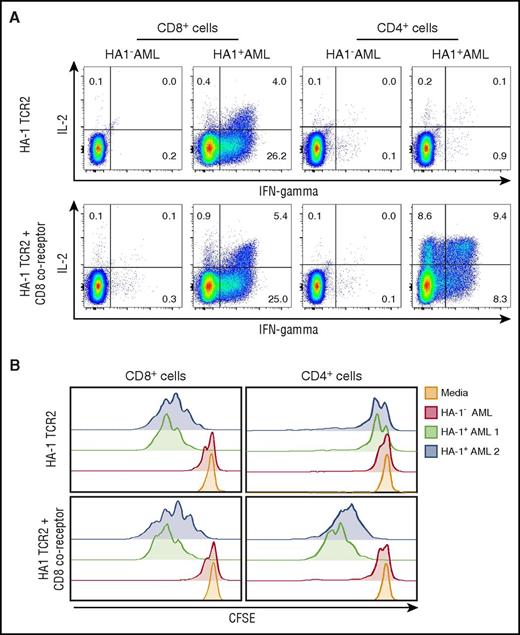
The inclusion of CD4+ T cells in an immunotherapy cell product can provide antigen-induced IL-2 secretion and augment persistence and function of transferred cytotoxic CD8+ T cells.31,,,,,,,-39 However, the optimal function of many class I–restricted TCRs in CD4+ T cells requires the transfer of a CD8 coreceptor to enhance sensitivity of the TCR to class I HLA peptide complexes. CD4 coreceptors differ in structure to CD8 and cannot effectively substitute for CD8 coreceptors.55,56 Accordingly, relatively high HA-1 peptide concentrations were required to induce cytolytic activity in CD4+ T cells transduced with an HA-1 TCR alone, and HA-1 TCR CD4+ T cells did not recognize cell lines or leukemia, implying CD8 coreceptor dependency of the TCR (data not shown). Therefore, we investigated options for including a CD8 coreceptor in the transgene construct. CD8 coreceptors exist on the surface of human conventional αβ TCR T cells, typically as dimers of CD8α and β chains, and there are 5 β chain variants with different intracytoplasmic tail sequences (βM1-5).57,58 We created constructs of HA-1 TCR2 with CD8α and/or each of the β chains as full-length or truncated variants. When used to transduce primary CD4+ T cells, the α and β chains were expressed on the cell surface; αβ dimers and α monomers increased HA-1/HLA-A2 multimer binding by TCR-transduced T cells to a greater extent than did β monomers (supplemental Figure 3A). CD8 α and β chains with truncations of the intracellular chain components did not increase multimer binding above the TCR alone in contrast to findings by other groups (supplemental Figure 3A).59 Transduction with the CD8 α and βM1 or βM4 variants improved HA-1 TCR function in CD4+ T cells more than did the CD8 α and βM2 or βM5 chains (supplemental Figure 3B). In some functional assays, incorporation of the βM1 or βM4 chain improved the CD4+ T-cell function to a greater extent than CD8 α monomers (supplemental Figure 3B-C). βM1 provided greater specificity of response than did βM4. We therefore selected the βM1 variant and confirmed that CD4+ T cells transduced with a multicistronic LV that included CD8 α and βM1 sequences as well as the HA-1 TCR secreted both IL-2 and interferon-γ (IFN-γ) and proliferated when cocultured with HA-1+ AML cells (Figure 3A-B). Moreover, in vitro, HA-1 TCR CD8+ T cells appear to function somewhat better when cocultured with HA-1 TCR CD4+ T cells (supplemental Figure 4).
CD8 coreceptor enhances the function of class I–restricted, HA-1 TCR–transduced CD4+T cells. (A) Intracellular cytokine assay showing IL-2 and IFN-γ production by CD8+ T cells (left) and CD4+ T cells (right) transduced with HA-1 TCR2 LV (upper panels) or HA-1 TCR2-CD8 coreceptor LV (lower panels) in response to HLA-A2+/HA-1+ AML or HLA-A2+/HA-1– AML; (B) carboxyfluorescein diacetate succinimidyl ester (CFSE) assay showing proliferation of CD8+ T cells (left) and CD4+ T cells (right) transduced with HA-1–specific TCR2 LV (upper panels) or HA-1 TCR2-CD8 coreceptor LV (lower panels) in response to HLA-A2+/HA-1+ primary AML (green and blue), HLA-A2+/HA-1– AML (red), or media control (orange).
CD8 coreceptor enhances the function of class I–restricted, HA-1 TCR–transduced CD4+T cells. (A) Intracellular cytokine assay showing IL-2 and IFN-γ production by CD8+ T cells (left) and CD4+ T cells (right) transduced with HA-1 TCR2 LV (upper panels) or HA-1 TCR2-CD8 coreceptor LV (lower panels) in response to HLA-A2+/HA-1+ AML or HLA-A2+/HA-1– AML; (B) carboxyfluorescein diacetate succinimidyl ester (CFSE) assay showing proliferation of CD8+ T cells (left) and CD4+ T cells (right) transduced with HA-1–specific TCR2 LV (upper panels) or HA-1 TCR2-CD8 coreceptor LV (lower panels) in response to HLA-A2+/HA-1+ primary AML (green and blue), HLA-A2+/HA-1– AML (red), or media control (orange).
Introduction of a safety switch into the HA-1 TCR transgene construct
To ensure that HA-1 TCR–transduced T cells can be rapidly depleted in case of any unexpected toxicity, we tested 4 safety switch constructs previously described by other groups as follows: (1) the iCasp9 is based on the fusion of human caspase 9 to a modified human FK-binding protein, allowing conditional dimerization; when exposed to a synthetic dimerizing drug, iCasp9 becomes activated and initiates rapid death of cells expressing this construct43,,,,-48 ; (2) the truncated human EGFR (tEGFR) is a polypeptide devoid of extracellular N-terminal ligand-binding domains and intracellular receptor tyrosine kinase activity, but retains type I transmembrane cell surface localization and a binding epitope for the pharmaceutical-grade anti-EGFR mAb, cetuximab49 ; (3) RQR8 is a compact marker and safety switch for T cells, combining target epitopes from both CD34 and CD20 antigens presented on a truncated CD8 coreceptor stalk; RQR8 is bound by the pharmaceutical-grade anti-CD20 mAb, rituximab50 ; and (4) Myc-tagged TCRs incorporate a 10-amino acid tag of the human c-Myc protein that is bound by a tag-specific mAb.51 Binding of the respective mAbs to tEGFR, RQR8, or myc-tag provides a target for complement-dependent or antibody-dependent cellular cytotoxicity and the elimination of transduced cells. Safety-switch molecules were cloned into our TCR2 LV, and T cells transduced with iCasp9- HA-1 TCR, tEGFR-HA1 TCR, RQR8-HA-1 TCR, or Myc-tagged HA1-TCR all demonstrated HA-1 TCR expression and HA1+ target cell recognition similar to recognition by T cells transduced with the HA-1 TCR alone (data not shown).
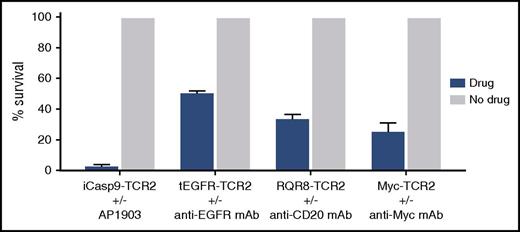
To test the ability of the safety switches to eliminate T cells, we incubated transduced T cells for 24 hours with the optimal concentration (supplemental Figure 5) of the respective drug, specifically the dimerizer, AP1903, for iCasp9/HA-1 TCR and complement plus appropriate mAb (anti-EGFR mAb for tEGFR-HA1 TCR, anti-CD20 mAb for RQR8-HA-1 TCR, and anti-Myc tag mAb for Myc-tagged HA1-TCR in all other constructs). All safety-switch–transduced T cells were susceptible to their respective trigger (Figure 4). However, iCasp9 with AP1903 consistently provided the most rapid and complete elimination of transduced T cells and was selected for further evaluation.
Evaluation of safety switches in HA-1 TCR2–transduced T cells. Comparison of survival of CD8+ T cells transduced with LV constructs, including HA-1 TCR2, and various safety switches after exposure to a safety-switch–activating drug (blue bars) or no drug (gray bar). Survival percentage of iCasp9-TCR2–, tEGFR-TCR2–, RQR8-TCR2–, and Myc-TCR2–transduced CD8+ T cells was measured after 24 hours of incubation with the optimal concentration of the respective drug: chemical inducer of dimerization (AP1903), anti-EGFR mAb (cetuximab) + complement, anti-CD20 (rituximab) + complement, anti-myc mAb + complement, or media control only. Residual HA-1 TCR2–transduced T cells were quantified by flow cytometry.
Evaluation of safety switches in HA-1 TCR2–transduced T cells. Comparison of survival of CD8+ T cells transduced with LV constructs, including HA-1 TCR2, and various safety switches after exposure to a safety-switch–activating drug (blue bars) or no drug (gray bar). Survival percentage of iCasp9-TCR2–, tEGFR-TCR2–, RQR8-TCR2–, and Myc-TCR2–transduced CD8+ T cells was measured after 24 hours of incubation with the optimal concentration of the respective drug: chemical inducer of dimerization (AP1903), anti-EGFR mAb (cetuximab) + complement, anti-CD20 (rituximab) + complement, anti-myc mAb + complement, or media control only. Residual HA-1 TCR2–transduced T cells were quantified by flow cytometry.
Design and selection of the final HA-1 TCR transgene construct
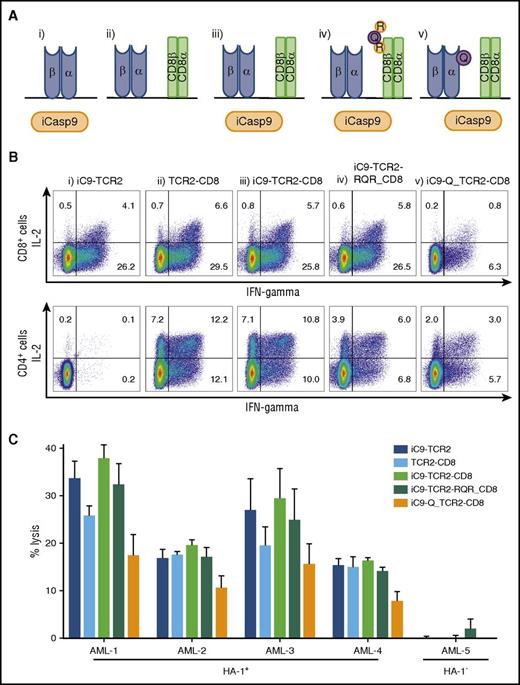
The final stage in the construction of our HA-1 TCR transgene involved the incorporation and evaluation of both the iCasp9 and the CD8αβM1 coreceptor with the HA-1 TCR2. To assist in the selection and tracking of transduced T cells, we explored options for the inclusion of a surface CD34 tag that is recognized by clinically compliant, CD34-binding immunomagnetic beads. Specifically, we modified the RQR8 sequence50 and nested either the minimal CD34 epitope (Q) into the α chain of the HA-1 TCR or incorporated the CD34 and 2 CD20 epitopes (RQR) into the β chain of our full-length functional CD8 αβM1 coreceptor. We then created and compared 5 LV transgene constructs: (1) iCasp9-HA1 TCR2; (2) HA1-TCR2-CD8 coreceptor; (3) iCasp9-HA1 TCR2-CD8; (4) iCasp9-HA1 TCR2-RQR-CD8; and (5) iCasp9-CD34-HA1 TCR2-CD8 (Figure 5A). All constructs produced T cells that specifically secreted cytokine and killed HA-1+ but not HA-1– AML cells and had similar function. The exception was the iCasp9-CD34-HA1 TCR2-CD8 construct, in which the CD34 epitope was embedded in the TCR α chain, which performed poorly (Figure 5B-C). We selected the iCasp9-HA1 TCR2-RQR-CD8 transgene, which contains all desired elements and functioned as well as the less complex constructs.
Evaluation of CD8+T cells transduced with constructs, including HA-1 TCR2, and safety switch and/or CD8 coreceptor and/or a selection marker. (A) Drawing illustrating the composition of the 5 different LV transgene constructs evaluated: (i) iCasp9(iC9)-HA1 TCR2; (ii) HA1 TCR2-CD8 coreceptor (CD8); (iii) iC9-HA1 TCR2-CD8; (iv) iC9-HA1 TCR2-RQR-CD8; and (v) iC9-CD34-HA1 TCR2-CD8. (B) Intracellular cytokine staining showing production of IL-2 and IFN-γ by CD8+ and CD4+ T cells transduced with the LV transgene constructs in response to HLA-A2/HA-1+ primary AML. (C) CRAs showing specific lysis of HLA-A2+/HA-1+ AML (AML 1-4) or HLA-A2+/HA-1– AML (AML 5) by CD8+ T cells transduced with the LV transgene constructs.
Evaluation of CD8+T cells transduced with constructs, including HA-1 TCR2, and safety switch and/or CD8 coreceptor and/or a selection marker. (A) Drawing illustrating the composition of the 5 different LV transgene constructs evaluated: (i) iCasp9(iC9)-HA1 TCR2; (ii) HA1 TCR2-CD8 coreceptor (CD8); (iii) iC9-HA1 TCR2-CD8; (iv) iC9-HA1 TCR2-RQR-CD8; and (v) iC9-CD34-HA1 TCR2-CD8. (B) Intracellular cytokine staining showing production of IL-2 and IFN-γ by CD8+ and CD4+ T cells transduced with the LV transgene constructs in response to HLA-A2/HA-1+ primary AML. (C) CRAs showing specific lysis of HLA-A2+/HA-1+ AML (AML 1-4) or HLA-A2+/HA-1– AML (AML 5) by CD8+ T cells transduced with the LV transgene constructs.
Clinical-scale production and evaluation of HA-1 TCR T cells
The cellular composition of T-cell immunotherapy products can have important downstream effects on the persistence and function of antigen-specific T cells after adoptive T-cell transfer.39,60,,,-64 In general, the infusion of antigen-specific T cells derived from younger T-cell subsets, including TN, T memory stem cells, and central memory T cells appears advantageous. In the context of post-HCT T-cell immunotherapy, it is also important to consider the potential for GVHD mediated by the native TCR of donor T cells. TN cause severe GVHD in murine models, and depletion of CD45RA+ TN from PBSC grafts reduces the risk of severe and/or chronic GVHD in humans.65,,,,,,,-73 It is also desirable to include both CD4+ and CD8+ cells specific for the same antigen because CD4+ T helper cells can enhance anti-tumor CTL responses by enhancing clonal expansion at the tumor site and preventing activation-induced cell death.31,,,,,,,-39 We therefore designed our donor T-cell product to first be depleted of CD45RA+ TN to minimize the risk of serious GVHD, and depleted of CD14+ monocytes to optimize LV transduction efficiency, before separating CD8+- and CD4+-enriched fractions to ensure a consistent CD4:CD8 composition and activating and transducing the T cells with the iCasp9-HA1 TCR2-RQR-CD8 LV.
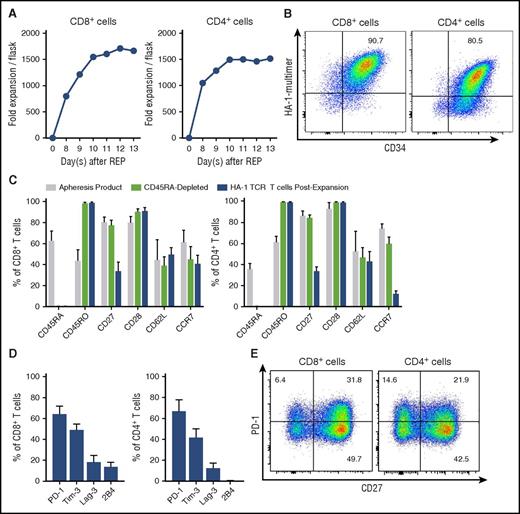
In a series of clinical-scale experiments, we depleted CD45RA+ TN and CD14+ cells from the donor apheresis product, activated and transduced with the iCasp9-HA1 TCR2-RQR-CD8 LV, flow sorted using HA-1/HLA-A2 multimers and CD34 mAb 4 to 5 days later, and cultured in G-Rex flasks. The CD4+ and CD8+ HA-1 TCR memory T cells expanded efficiently, with an average 2000-fold expansion (Figure 6A), retained expression of the HA-1 TCR (Figure 6B; supplemental Figure 6), had a predominantly CD45RO+CD28+ phenotype with variable expression of CD62L, CCR7, and CD27 (Figure 6C), and included cells that did not express exhaustion markers, such as PD-1 (Figure 6D-E). The expanded CD8+ and CD4+ HA-1 TCR T cells retained their ability to specifically kill and secrete cytokines in response to HA-1 stimulation, including by HA-1+ leukemia cell lines, and many HA-1 TCR CD8+ and CD4+ cells secreted multiple cytokines (Figure 7). The expanded CD8+ and CD4+ HA-1 TCR T cells were also enriched using anti-CD34 immunomagnetic beads to remove any untransduced T cells (supplemental Figure 7A) and were efficiently eliminated by exposure to the AP1903 dimerizer drug (supplemental Figure 7B). Finally, we evaluated the native TCRs in HA-1 TCR CD4+ and CD8+ T cells in the cell product using TCR immunosequencing (Adaptive Biotechnologies) and observed a diverse polyclonal population (supplemental Figure 8). Moreover, the product contained numerous very low–frequency TCRs, a finding that has recently been associated with the potential for persistence and expansion after adoptive T-cell transfer.74
Evaluation of clinical-scale iC9-HA-1TCR2-RQR-CD8–transduced T cells. Evaluation of iC9-HA-1TCR2-RQR-CD8–transduced (transduced) CD8+ (left) and CD4+ (right) T cells. (A) Growth of transduced T cells. (B) HA-1 TCR multimer binding and CD34 expression on transduced T cells by flow cytometry with HA-1/HLA-A2 multimer staining. (C) Expression of costimulatory and homing molecules on T cells at the time of the apheresis (gray), after CD45RA depletion (light green), and after transduction and expansion (blue) (n = 5). (D-E) Expression of “exhaustion” markers on HA-1 TCR CD8+ and CD4+ in the final cell product (n = 3) (D) and a representative example (E).
Evaluation of clinical-scale iC9-HA-1TCR2-RQR-CD8–transduced T cells. Evaluation of iC9-HA-1TCR2-RQR-CD8–transduced (transduced) CD8+ (left) and CD4+ (right) T cells. (A) Growth of transduced T cells. (B) HA-1 TCR multimer binding and CD34 expression on transduced T cells by flow cytometry with HA-1/HLA-A2 multimer staining. (C) Expression of costimulatory and homing molecules on T cells at the time of the apheresis (gray), after CD45RA depletion (light green), and after transduction and expansion (blue) (n = 5). (D-E) Expression of “exhaustion” markers on HA-1 TCR CD8+ and CD4+ in the final cell product (n = 3) (D) and a representative example (E).
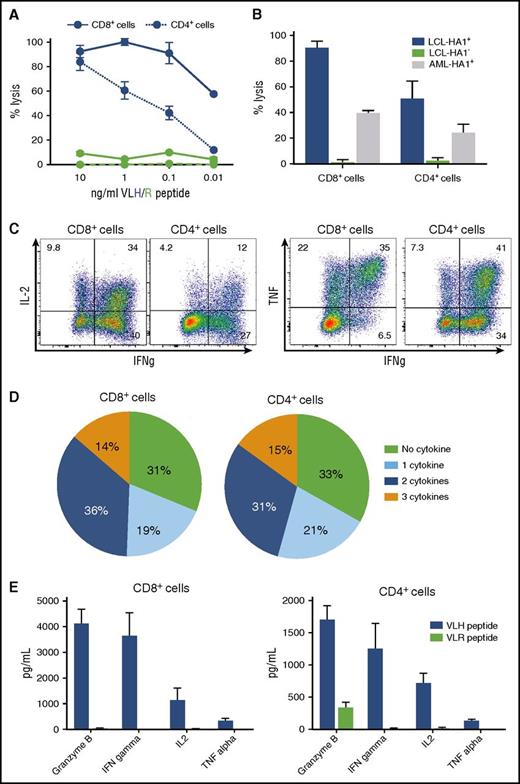
Clinical scale HA-1 TCR2-RQR-CD8–transduced T cells retain functional recognition of HA1+target cells. Functional evaluation of HA-1 TCR2-RQR-CD8–transduced CD8+ and CD4+ T cells in the final T-cell product. (A) Lysis of target T2 cells pulsed with a range of VLH (blue) and VLR (green) peptide concentrations by CD8+ (solid lines) and CD4+ T cells (dashed lines) in CRAs at an E:T ratio 20:1. (B) Lysis of HA-1+ A2+ LCL (blue), HA-1– A2+ LCL (green), and AML HA1+ A2+ cell lines (THP-1; gray) by CD8+ and CD4+ T cells in CRAs. (C) IL-2, IFN-γ, and tumor necrosis factor–α production by T cells in response to stimulation by T2 cells pulsed with 10 ng/mL of HA-1 peptide. (D) Pie charts displaying the number of cytokines secreted by T cells. (E) Concentration of cytokines and granzyme B in media 24 hours after stimulation of T cells by T2 cells pulsed with VLH (blue) and VLR (green) peptides measured by multiplex immunoassay.
Clinical scale HA-1 TCR2-RQR-CD8–transduced T cells retain functional recognition of HA1+target cells. Functional evaluation of HA-1 TCR2-RQR-CD8–transduced CD8+ and CD4+ T cells in the final T-cell product. (A) Lysis of target T2 cells pulsed with a range of VLH (blue) and VLR (green) peptide concentrations by CD8+ (solid lines) and CD4+ T cells (dashed lines) in CRAs at an E:T ratio 20:1. (B) Lysis of HA-1+ A2+ LCL (blue), HA-1– A2+ LCL (green), and AML HA1+ A2+ cell lines (THP-1; gray) by CD8+ and CD4+ T cells in CRAs. (C) IL-2, IFN-γ, and tumor necrosis factor–α production by T cells in response to stimulation by T2 cells pulsed with 10 ng/mL of HA-1 peptide. (D) Pie charts displaying the number of cytokines secreted by T cells. (E) Concentration of cytokines and granzyme B in media 24 hours after stimulation of T cells by T2 cells pulsed with VLH (blue) and VLR (green) peptides measured by multiplex immunoassay.
Discussion
We report the development of a new T-cell immunotherapy for managing post-HCT leukemia relapse. The significance of the work is threefold. First, we used T cells genetically modified to express a TCR specific for a promising new target antigen. Second, we created a therapeutic transgene that includes a CD8+ coreceptor, allowing a class I–restricted TCR to function in transduced CD4+ as well as CD8+ T cells. Despite the complexity of the transgene that incorporates the TCR, CD8 coreceptor, a safety switch, and a tracking marker, we demonstrate the successful clinical-scale transduction and expansion of CD4+ and CD8+ HA-1 TCR T cells and confirm the function of all transgene elements in the final cell product. Third, our T-cell product is designed specifically for HCT recipients, excluding TN from the product to mitigate the risk of inducing GVHD.
HA-1 is expressed on AML and ALL cells at sufficient levels to induce reliable recognition, killing, cytokine elaboration, and proliferation by HA-1 TCR–transduced T cells. Approximately 25% of individuals present both HA-1 and the HLA-A*0201–restricting allele. Allowing for either an HLA-matched or haploidentical donor with a suitable HA-1 or HLA-A2 mismatch, ∼15% to 20% of patients with post-HCT leukemic relapse would be suitable for HA-1 TCR T-cell immunotherapy. Moreover, HA-1 TCR immunotherapy could provide proof-of-principle for T-cell therapies targeting other hematopoietic-restricted minor H antigens in the future. In contrast with somatic mutations, minor H antigens like HA-1 arise from germ line polymorphisms, will be expressed on all leukemic clones in an individual, and are relatively unlikely to escape T-cell surveillance due to clonal evolution. HA-1–specific T cells can deliver a potent antileukemic effect following HCT or DLI, but adequate reactions do not reliably occur in all HA-1+ HCT recipients, and other T cells in the HCT graft or DLI can cause GVHD. Adoptive T-cell immunotherapy targeting HA-1 has the potential to amplify the antileukemic effect and separate the therapeutic effect from GVHD. Because HA-1 is not expressed on HA-1– donor hematopoietic cells and is minimally expressed on recipient nonhematopoietic tissues, the risk of graft failure, or GVHD and other toxicities, is minimized. As we have demonstrated, gene-modified TCR T-cell immunotherapy provides a feasible strategy for delivering an antigen-specific T-cell product in 2 to 3 weeks and allows control of the cell dose and composition to maximize efficacy and safety.
A key feature of our TCR T-cell product is the inclusion of a CD8 coreceptor in the transgene to enable function of the class I–restricted TCR in CD4+ T cells. We are unaware of publications of clinical trials of any TCR T-cell immunotherapy that incorporates a CD8 coreceptor. The inclusion of functional HA-1–specific CD4+ T cells is key because CD4+ T cells can be directly cytotoxic and can also enhance antitumor CD8+ CTL responses.31,,,,,,,-39 However, most class I–restricted TCRs of moderate to moderately high affinity require coexpression of CD8 for full functional activity. The CD8 coreceptor assists in stabilizing HLA/peptide/TCR binding and brings the intracellular kinase, Lck, into contact with the TCR/CD3 complex.55,56 Although affinity maturation could be used to obtain a very high–affinity HA-1–specific, CD8-independent TCR, very high–affinity TCRs carry the risk of cross-reactivity, especially in CD8+ T cells, and could therefore reduce the ability of TCRs specific for HA-1 (VLHDDLLEA) expressed in the recipient to distinguish from the allelic variant (VLRDDLLEA) in donor hematopoietic cells.56,75 To maximize TCR function in CD4+ T cells, but avoid cross-reactivity, we chose to introduce both a TCR and CD8 coreceptor. We have demonstrated that transduction with both elements allows CD4+ T cells to specifically respond to HA-1+ leukemia, but not HA-1– cells, and preserves specific HA-1 antigen recognition by CD8+ T cells. If CD4+ T cells transduced with a class I–restricted HA-1 TCR persist and function after infusion into patients and contribute to the efficacy of immunotherapy, our approach will serve as a model for the inclusion of CD8 coreceptors in therapeutic TCR transgenes in future T-cell immunotherapies targeting a variety of hematological and nonhematological malignancies. Of note, the inclusion of the CD8 coreceptor, in addition to the RQR, iCasp9, and HA-1 TCR results in a complex construct. Although all individual elements are of human origin, the complexity, and specifically the junction sequences, add some risk of immunogenicity. Because our T-cell immunotherapy product is intended for use in the post-HCT setting, the risk of immune-mediated rejection of the cells is relatively low and can be further mitigated by the administration of fludarabine before the T-cell infusions.6
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Riddell, J. Radich, and R. Walter for providing cell lines, N. Culores and the staff of the Fred Hutchinson Cancer Research Center process development and cell production facilities for their technical assistance, A. Chapuis and F. Wegener for sharing their experience with T cell “exhaustion marker” flow cytometry analysis, and D. Banker for assisting with manuscript preparation.
The work was supported in part by the Damon Runyon Cancer Research Foundation and the Richard Lumsden Foundation, Alex’s Lemonade Stand Foundation and Cure4Cam Childhood Cancer Organization, the Leukemia and Lymphoma Society, Unravel Pediatric Cancer, the Bezos family, and the National Cancer Institute, National Institutes of Health (grant K23 CA154532).
Authorship
Contribution: R.G.D. and T.C. designed and performed the research, analyzed data, and contributed to writing the manuscript; D.S. and I.M.-R. designed and performed the research and analyzed the data; M.A.B. performed the research and analyzed the data; K.F. performed the research; and M.B. designed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie Bleakley, Clinical Research Division, Mailstop D3-100, Fred Hutchinson Cancer Research Center, Seattle, WA 98109; e-mail: mbleakle@fredhutch.org.

![Figure 2. HA-1 TCR–transduced CD8+ T cells kill leukemic cells. (A-B) HA-1–specific expression of CD107a on HA-1 TCR2–transduced CD8+ T cells showing degranulation after 5 hours of coculture (1:1) with a panel of (A) primary AML samples and (B) primary B-ALL samples. (C-F) CRAs showing lysis of leukemia and lymphoma targets by HA-1 TCR–transduced CD8+ T cells; lysis of (C) primary HA-1+ AML or HA-1– AML by HA-1 TCR–transduced CD8+ T cells (blue bars) and HA-1–specific T-cell clone 2 (gray bars); (D) HLA-A2+/HA-1+ primary AML (AML1) at various E:T ratios; (E) B-ALL lines (1) BALL-1 and (2) RS4;11, T-ALL lines (1) MOLT4, (2) CEM, (3) RPMI-8402, and (4) HSB-2, and AML line NB-4; and (F) T-cell lymphoma (SUP-M2 HLA-A2+, HA-1+; SU-DHL-1 HLA-A2+ HA-1–) cell lines. In panel E, HLA-A2– and/or HA-1– (wild-type [WT]) cell lines were transduced (TD) with LV encoding *HLA-A2 or **HLA-A2 and HA-1 minigene or &HA-1 minigene if the WT was HLA-A2– or had a HA-1– genotoype. An E:T ratio of 20:1 was used unless otherwise specified.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/1/10.1182_blood-2017-07-791608/4/m_blood791608f2.jpeg?Expires=1769911316&Signature=oqbauDDvEaVX0X5yy3byc0QvwE1rtT0jT-eUWjRup79eeeIC5Yd1WM7NhGBYpOKbDNXnZ0BwMd1OWwg3ad4sgrNu-VBrfFDdZmWa8jWZhPr5fzGtgxbpHU~0q-X6mE12ZauiS8~~UzronfVeogSohQ~bVLDV5yZurlpVJFNcshRRcoLrzC5eeERFPmAjIBNWG3zfVWHpbvWdRDKQ~c9Zp70j1YxcCNoI1ELyhfsI1I26r4l~ofiRy1M7uqxUH71nqkTeyShHgvpYcoVK1aVq904c~6prdDB8eVw4o9EHdpdWKqOKLAVxC7ijepMqXVz4HoB0fQ883NvI8QsiGbejAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal