Key Points
rhTPO is a potentially effective and safe treatment option for ITP during pregnancy.
Abstract
The aim of this study was to determine the safety and efficacy of recombinant human thrombopoietin (rhTPO) for the management of immune thrombocytopenia (ITP) during pregnancy. Pregnant patients with ITP were enrolled in the study if they had a platelet count less than 30 × 109/L, were experiencing bleeding manifestations, had failed to respond to corticosteroids and/or intravenous immunoglobulin (IVIG), and had developed refractoriness to platelet transfusion. Thirty-one patients received rhTPO at an initial dose of 300 U/kg once daily for 14 days. Twenty-three patients responded (74.2%), including 10 complete responders (>100 × 109/L) and 13 responders (30-100 × 109/L). It appears that rhTPO ameliorated the bleeding symptoms remarkably, even in the nonresponders. rhTPO was well tolerated. Dizziness, fatigue, and pain at an injection site were reported in 1 patient each. No congenital disease or developmental delays were observed in the infants in a median follow-up of 53 (range, 39-68) weeks. In conclusion, rhTPO is a potentially safe and effective treatment choice for patients with ITP during pregnancy. Our work has paved the way for further study on the clinical application of rhTPO and other thrombopoietic agents for the management of ITP during pregnancy. This study is registered at www.clinicaltrials.gov as NCT02391272.
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by a transient or persistent decrease in platelet count.1 Both increased platelet destruction caused by autoantibodies to platelet membrane glycoproteins and insufficient platelet production are involved in ITP.2-6 ITP is the most common cause of thrombocytopenia in early pregnancy,7 associated with 5% of all thrombocytopenia cases in pregnant women.8-10 Patients with severe thrombocytopenia (platelet count <20 × 109/L) are at risk for spontaneous bleeding, postpartum hemorrhage, and placental abruption.8
Platelet IgG type autoantibody can pass through the placental barrier and result in fetal or transient neonatal thrombocytopenia. From 8.9% to 14.7% newborns of pregnant women with ITP have severe thrombocytopenia, and the incidence of intracranial hemorrhage (ICH) is approximately 1.5%.11
Similar to nonpregnant adult patients with ITP, first-line treatments include corticosteroids and intravenous immunoglobulin (IVIG). In a recent retrospective study, Sun et al reported that the response rates of pregnant patients with ITP to either IVIG or corticosteroids were less than 40% and might be lower than that of the nonpregnant patients.12 The secondary options are limited if the patients fail to respond to the primary treatments.
Recombinant human thrombopoietin (rhTPO) is a full-length glycosylated TPO with a molecular weight of 90 000 Daltons, expressed in Chinese hamster ovary cells and purified using bioengineering techniques. rhTPO is a recombinant form of the c-Mpl ligand. It maintains an amino acid sequence identical to the endogenous TPO, but further glycosylated.13 It has been proven to be active in both human and animal models.14,15 rhTPO was approved by the China State Food and Drug Administration for the treatment of chronic ITP refractory to first-line therapy. A multicenter randomized trial revealed that rhTPO rapidly increased platelet counts with a total response rate of 60.3% in patients with steroid-resistant ITP.14 However, rhTPO has not been tested for the management of patients with ITP in pregnancy. Here, we performed a prospective multicenter open-labeled study to investigate whether rhTPO is clinically applicable for ITP in pregnancy.
Methods
Study design
This multicenter, open-labeled, single-arm study aimed to determine the safety and efficacy of rhTPO in patients with corticosteroid/IVIG-resistant ITP in pregnancy. Eight hospitals in China participated in this study. The protocol was approved by the ethics committees of all participating hospitals. Informed consent was obtained from each patient in accordance with the Declaration of Helsinki. All authors have full access to primary clinical trial data.
Patients
Patients from 8 centers in China were enrolled. The confirmation and reconfirmation of the diagnosis of ITP were based on the international consensus report on the investigation and management of primary immune thrombocytopenia11 and consensus of Chinese experts on diagnosis and treatment of adult primary immune thrombocytopenia (version 2016).16 Enrollment criteria included pregnant women between 18 and 50 years of age with bleeding manifestations and failure to respond to corticosteroids and/or IVIG (can be with stable dose of corticosteroids on enrollment) who were refractory to platelet transfusion. Patients who had 2 serial 1-hour posttransfusion corrected count increments of less than 10 × 109/L were considered as platelet refractoriness. Patients’ platelet counts were below 30 × 109/L. Gestational age was more than 12 weeks.
Patients with the following conditions or diagnoses were excluded: virus-induced thrombocytopenia; heart, kidney, liver, or lung dysfunction; severe immunodeficiency; other autoimmune diseases, positive antinuclear antibodies, anti-cardiolipin antibodies, lupus anticoagulant, or direct Coombs test. Patients were also excluded if they had received chemotherapy or anticoagulants within 3 months before screening or other second-line ITP-specific treatments within 3 months before screening.
Procedures
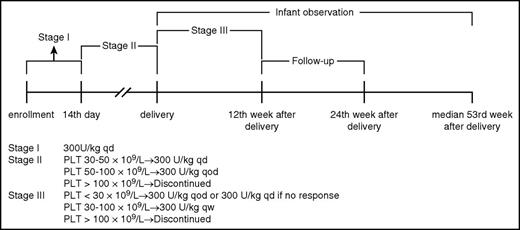
All eligible participants received rhTPO at an initial dose of 300 U/kg once daily subcutaneously for 14 days and then received sequential maintenance therapy. To reduce the risk for thrombocytosis during maintenance,17 dose was tapered to 300 U/kg every other day when platelet counts exceeded 50 × 109/L, and treatment discontinued when platelet counts rose above 100 × 109/L. After delivery, the dose was further tapered to 300 U/kg every week, and adjusted if the platelet count could not maintain above 30 × 109/L. If the platelet count did not reach 30 × 109/L or above within 2 weeks or fell below 30 × 109/L and did not achieve 30 × 109/L within 2 weeks after dose adjustment, then treatment was discontinued (Figure 1). If patients experienced severe bleeding symptoms, platelet transfusion was permitted. Any requirements for additional ITP-specific intervention were considered treatment failure.
Dose scheme of rhTPO. PLT, platelet; qd, daily; qod, every other day; qw, every week.
Dose scheme of rhTPO. PLT, platelet; qd, daily; qod, every other day; qw, every week.
Outcomes
The primary end point was a platelet count greater than 30 × 109/L on the 14th day of the study. The platelet counts were determined at each collaborative center. Complete response was defined as a platelet count of at least 100 × 109/L and the absence of bleeding. Response was defined as a platelet count more than 30 × 109/L and increase of at least twice the baseline. Nonresponse was defined as a platelet count below 30 × 109/L. We considered the following secondary endpoints: adverse events in mothers and neonates; neonatal platelet counts at birth, day 3 and day 7 if necessary (thrombocytopenic neonates), and day 42; and stillbirth, premature birth (before 37 weeks of gestation), and birth weight <2.5 kg. Adverse events were recorded and graded according to the Common Terminology Criteria for Adverse Events, Version 4.0. All patients were assessed weekly for the efficacy and safety of the treatment in pregnancy and at 4-week intervals for 24 weeks after delivery. After birth, infants’ physical measures (eg, length, weight, head circumferences, chest circumference, abdominal circumference) and development assessment (eg, gross motor skills, fine motor skills, adaptive capacity, language, and social behavior) were performed monthly by pediatricians according to China Child Growth Standards and Gesell Developmental Scale.18
Assessment of bleeding was performed on days 1 and 14, according to the reported scoring system by the Gruppo Italiano Malattie Ematologiche dell’Adulto ITP Working Party.19 Bleeding severity was graded as follows: grade 0, absence of bleeding; grade 1, petechiae; grade 2, ecchymoses and/or dripping with moderate loss of blood; grade 3, major mucous hemorrhage with copious loss of blood without sequelae; and grade 4, major mucous and/or parenchymal hemorrhage with copious loss of blood with sequelae and/or life-threatening or deadly.
In previous studies, neutralizing antibodies targeting endogenous TPO were found in patients who received pegylated human recombinant megakaryocytic growth and development factor.13,20,21 Therefore, serum anti-TPO antibodies were measured by enzyme-linked immunosorbent assay 4 weeks after the first dose, at the end of rhTPO treatment, and 6 months after delivery.22
Given concerns about whether rhTPO could pass through the placenta, cord blood TPO levels were measured in neonates of healthy women and of patients with ITP treated with rhTPO, using enzyme-linked immunosorbent assay kits (R&D Systems) according to the manufacturer's instructions. Serum TPO levels in ITP mothers before and after rhTPO treatment were also measured to prove the measurability of exogenous TPO.
Statistical analysis
All participants who received at least 1 dose of rhTPO were included in primary and safety analyses. Baseline characteristics of the study population were summarized using descriptive statistical methods. Correlations were analyzed using Spearman’s correlation. Differences between groups were analyzed using Fisher’s exact test or t test. A P value less than .05 was considered statistically significant. Data management and statistical analyses were performed using Statistical Package for the Social Sciences, version 22.0 (SPSS, Chicago, IL). This study is registered at www.clinicaltrials.gov as NCT02391272.
Results
Patients
Between March 20, 2015, and November 17, 2015, 32 pregnant patients with ITP were enrolled in the study. In 1 patient, the diagnosis changed from ITP to aplastic anemia before treatment initiated. All the mothers with ITP were followed up to the end of the 24th week after delivery. The median follow-up time for infants was 53 (range, 39-68) weeks.
The median age of the pregnant ITP patients was 26 years (interquartile range [IQR], 24-33 years), and 93.5% (29/31) were primigravidae (Table 1). The median gestational age at the time of enrollment was 24 weeks (IQR, 16-27 weeks) (Table 1). The median baseline platelet count was 10 × 109/L (IQR, 6-12 × 109/L) (Table 1). Three patients had underlying pregnancy-induced hypertension, and 4 had pregnancy-related diabetes (Table 1). Seventy-four percent (23/31) of these patients were diagnosed with ITP before pregnancy, and 25.8% (8/31) were diagnosed during pregnancy (Table 1). None of the enrolled pregnant patients with ITP had ever responded to previous treatments. Although some patients did have temporary increase of platelet count, none of their peak platelet counts exceeded 30 × 109/L (Table 1). All patients were heavily transfused and became refractory to platelet transfusion before enrollment (Table 1). The median total amount of platelets transfused per patient was 10 units (IQR, 6-17 units; Table 1). Ten patients were receiving a stable dose (IQR, 15-30 mg/day) of prednisone on enrollment (Table 1). On the day of enrollment, most patients were graded with a bleeding score of 1 with petechiae or a score of 2 with hematuria (Table 2). Two patients were graded with a score of 3 with gingival and vagina bleeding, respectively (Table 2).
Baseline characteristics of patients enrolled in the study (n = 31)
| Characteristic . | All patients (n = 31) . |
|---|---|
| Age, median (IQR), y | 26 (24-33) |
| Age of gestation, median (IQR), wk | 24 (16-27) |
| 13-17 wk, n (%) | 11 (35.5) |
| 18-22 wk, n (%) | 5 (16.1) |
| 23-27 wk, n (%) | 8 (25.8) |
| 28-32 wk, n (%) | 4 (12.9) |
| ≥33 wk, n (%) | 3 (9.7) |
| Platelet count on enrollment, median (IQR), ×109/L | 10 (6-12) |
| Primigravida, n (%) | 29 (93.5) |
| Underlying disease, n (%) | |
| Hypertension | 3 (9.7) |
| Diabetes | 4 (12.9) |
| Diagnosis of ITP, n (%) | |
| Before pregnancy | 23 (74.2) |
| In pregnancy | 8 (25.8) |
| Prior therapies, n (%) | |
| Dex+PLT transfusion | 7 (22.6) |
| Pred+PLT transfusion | 8 (25.8) |
| IVIG+PLT transfusion | 9 (29.0) |
| Dex+IVIG+PLT transfusion | 2 (6.5) |
| Pred+IVIG+PLT transfusion | 4 (12.9) |
| Dex+Pred+IVIG+PLT transfusion | 1 (3.2) |
| Peak platelet count during previous treatments, n (%) | |
| <30 × 109/L | 31 (100) |
| ≥30 × 109/L | 0 (0) |
| Total amount of platelets transfused per patient (unit)*, median (IQR) | 10 (6-17) |
| Platelet transfusion refractoriness, n (%) | 31 (100) |
| Patients on stable dose (15-30 mg/d) of Pred upon enrollment, n (%) | 10 (32.3) |
| Characteristic . | All patients (n = 31) . |
|---|---|
| Age, median (IQR), y | 26 (24-33) |
| Age of gestation, median (IQR), wk | 24 (16-27) |
| 13-17 wk, n (%) | 11 (35.5) |
| 18-22 wk, n (%) | 5 (16.1) |
| 23-27 wk, n (%) | 8 (25.8) |
| 28-32 wk, n (%) | 4 (12.9) |
| ≥33 wk, n (%) | 3 (9.7) |
| Platelet count on enrollment, median (IQR), ×109/L | 10 (6-12) |
| Primigravida, n (%) | 29 (93.5) |
| Underlying disease, n (%) | |
| Hypertension | 3 (9.7) |
| Diabetes | 4 (12.9) |
| Diagnosis of ITP, n (%) | |
| Before pregnancy | 23 (74.2) |
| In pregnancy | 8 (25.8) |
| Prior therapies, n (%) | |
| Dex+PLT transfusion | 7 (22.6) |
| Pred+PLT transfusion | 8 (25.8) |
| IVIG+PLT transfusion | 9 (29.0) |
| Dex+IVIG+PLT transfusion | 2 (6.5) |
| Pred+IVIG+PLT transfusion | 4 (12.9) |
| Dex+Pred+IVIG+PLT transfusion | 1 (3.2) |
| Peak platelet count during previous treatments, n (%) | |
| <30 × 109/L | 31 (100) |
| ≥30 × 109/L | 0 (0) |
| Total amount of platelets transfused per patient (unit)*, median (IQR) | 10 (6-17) |
| Platelet transfusion refractoriness, n (%) | 31 (100) |
| Patients on stable dose (15-30 mg/d) of Pred upon enrollment, n (%) | 10 (32.3) |
Dex, dexamethasone; Pred, prednisone.
Apheresis platelet product: 2.5 ×1011/unit.
Bleeding score before and after treatment
| Bleeding score . | Enrollment, n (%) . | Day 14, n (%) . |
|---|---|---|
| 0 | 0 (0) | 25 (80.6) |
| 1 | 19 (61.3) | 4 (12.9) |
| 2 | 10 (32.3) | 2 (6.5) |
| 3 | 2 (6.4) | 0 (0) |
| 4 | 0 (0) | 0 (0) |
| Bleeding score . | Enrollment, n (%) . | Day 14, n (%) . |
|---|---|---|
| 0 | 0 (0) | 25 (80.6) |
| 1 | 19 (61.3) | 4 (12.9) |
| 2 | 10 (32.3) | 2 (6.5) |
| 3 | 2 (6.4) | 0 (0) |
| 4 | 0 (0) | 0 (0) |
Response
Of these patients, 74.2% (23/31) responded to the initial 14-day rhTPO therapy, including 10 with complete response and 13 with response. Eight patients were nonresponsive, although their platelet counts rose mildly (Figure 2). The median platelet count of responders was 100 × 109/L (IQR, 36-160 × 109/L) on day 14. On day 14, most participants were graded 0 (25/31, 80.6%; Table 2). It appears that the bleeding symptom of 26 patients was ameliorated. Among 23 responders, only 1 still had hemorrhage (petechiae). In 4 nonresponders, although their platelet counts did not meet the response level, the bleeding grade improved. The response rates of patients with and without stable dose of prednisone at the time of enrollment were 70.0% (7/10) and 76.2% (16/21), and the median platelet counts on day 14 were 84 × 109/L (IQR, 28-141 × 109/L) and 36 × 109/L (IQR, 25-127 × 109/L). There was no significant difference (P > .999; P = .264) between these 2 groups. In addition, peak platelet counts during previous treatments had no influence on whether or not the patients responded to rhTPO (P = .492).
One responder had a transient loss of response during maintenance after delivery. Although influenza was diagnosed at the same time, the association between influenza and the decrease of platelet count was undetermined. After dose adjustment of rhTPO from 300 U/kg every week to 300 U/kg every other day, the platelet count exceeded 50 × 109/L in the next visit.
Platelet counts gradually dropped after withdrawal of rhTPO. The relapse-free survival rates (platelet count at least 30 × 109/L) at weeks 4 and 12 after withdrawal of rhTPO were 69.6% (16/23) and 21.7% (5/23), respectively (Figure 3A).
Dynamic change of platelet counts. (A) Platelet counts of responders (n = 23). (B) Platelet counts of thrombocytopenic neonates (n = 9). Dots indicate the platelet count of patients. Dashed line indicates platelet count 30 × 109/L.
Dynamic change of platelet counts. (A) Platelet counts of responders (n = 23). (B) Platelet counts of thrombocytopenic neonates (n = 9). Dots indicate the platelet count of patients. Dashed line indicates platelet count 30 × 109/L.
Safety of mothers
Safety and adverse events were evaluated in all 31 participants. rhTPO was well tolerated. Only mild previously reported adverse events were observed, including 1 case of dizziness, 1 of fatigue, and 1 of pain at injection site (Table 3).14,23 There were no new adverse events reported during the observation period, and no adverse event-related study withdrawals. No obstetric conditions were reported. No anti-TPO antibody was detected among the participants.
Adverse events recorded
| Adverse events . | n (%) . |
|---|---|
| Patients (n = 31) | |
| Dizziness | 1 (3.2) |
| Fatigue | 1 (3.2) |
| Pain at injection site | 1 (3.2) |
| Newborns (n = 31) | |
| Thrombocytopenia | 9 (29.0) |
| Abdominal distension | 1 (3.2) |
| Adverse events . | n (%) . |
|---|---|
| Patients (n = 31) | |
| Dizziness | 1 (3.2) |
| Fatigue | 1 (3.2) |
| Pain at injection site | 1 (3.2) |
| Newborns (n = 31) | |
| Thrombocytopenia | 9 (29.0) |
| Abdominal distension | 1 (3.2) |
Adverse events recorded in all 31 patients who take at least 1 dose of rhTPO and in their infants.
Safety of infants
The safety of the 31 infants was also evaluated. Seven pregnancies (22.6%, including 4 responders and 3 nonresponders) were delivered by cesarean, and 24 by vaginal deliveries. In all 31 newborns, the median age of gestation was 39 weeks (IQR, 37-40 weeks; 3 cases were <37 weeks; Table 4). The median birth weight was 3.1 kg (IQR, 2.9-3.5 kg; 2 had birth weight <2.5 kg; Table 4). The median platelet count at birth was 132 × 109/L (IQR, 97-176 × 109/L). There was no correlation between maternal and neonatal platelet counts (P = .151). Platelet count less than 100 × 109/L was found in 9 neonates at birth (Table 4). Although some of them experienced a temporary decrease on day 3, none of them had bleeding nor had platelet count drop below 50 × 109/L. Thrombocytopenia in these neonates recovered within 42 days spontaneously (Figure 3B). Both of the multiparas were diagnosed as ITP before the first pregnancy. The neonatal platelet counts of 2 multiparas in the first and current pregnancy were 121 × 109/L and 146 × 109/L and 132 × 109/L and 107×109/L, respectively.
Perinatal outcome in pregnant women with ITP
| Perinatal outcome . | All newborns (n = 31) . |
|---|---|
| Live birth, n (%) | 31(100) |
| Stillbirth, n (%) | 0 (0) |
| Age of gestation, median (IQR), wk | 39 (37-40) |
| Preterm births (before 37th wk), n (%) | 3 (9.7) |
| Birth weight, median (IQR), kg | 3.1 (2.9-3.5) |
| Birth weight <2.5 kg, n (%) | 2 (6.5) |
| Birth length, median (IQR), cm | 50.2 (48.4-51.3) |
| Birth head circumference, median (IQR), cm | 34.3 (33.6-35.2) |
| Birth chest circumference, median (IQR), cm | 33.1 (31.9-34.5) |
| Birth abdominal circumference, median (IQR), cm | 33.0 (32.0-34.3) |
| Platelet count at birth, n (%) | |
| <50 × 109/L | 0 (0) |
| 50-100 × 109/L | 9 (29.0) |
| Hemorrhagic complications, n (%) | 0 (0) |
| Neonatal death, n (%) | 0 (0) |
| Perinatal outcome . | All newborns (n = 31) . |
|---|---|
| Live birth, n (%) | 31(100) |
| Stillbirth, n (%) | 0 (0) |
| Age of gestation, median (IQR), wk | 39 (37-40) |
| Preterm births (before 37th wk), n (%) | 3 (9.7) |
| Birth weight, median (IQR), kg | 3.1 (2.9-3.5) |
| Birth weight <2.5 kg, n (%) | 2 (6.5) |
| Birth length, median (IQR), cm | 50.2 (48.4-51.3) |
| Birth head circumference, median (IQR), cm | 34.3 (33.6-35.2) |
| Birth chest circumference, median (IQR), cm | 33.1 (31.9-34.5) |
| Birth abdominal circumference, median (IQR), cm | 33.0 (32.0-34.3) |
| Platelet count at birth, n (%) | |
| <50 × 109/L | 0 (0) |
| 50-100 × 109/L | 9 (29.0) |
| Hemorrhagic complications, n (%) | 0 (0) |
| Neonatal death, n (%) | 0 (0) |
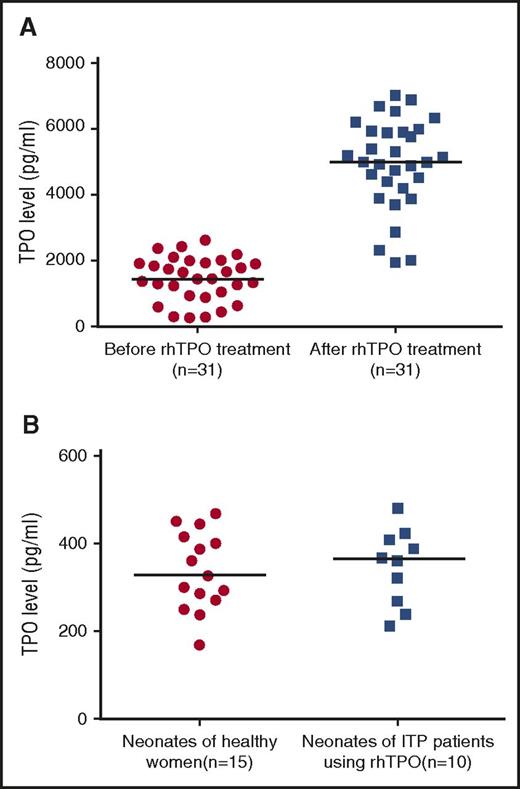
The median serum TPO levels in 31 mothers with ITP before and after rhTPO treatment were 1434.98 pg/mL (IQR, 932.06-1932.95 pg/mL) and 4987.23 pg/mL (IQR, 4198.54-5932.98 pg/mL), which showed a significant difference (P < .001) (Figure 4A). The median cord blood TPO levels in neonates of 15 healthy women and 10 pregnant patients with ITP treated with rhTPO were 327.30 pg/mL (IQR, 270.68-416.48 pg/mL) and 364.93 pg/mL (IQR, 261.37-412.35 pg/mL), respectively (Figure 4B). There was no significant difference between the 2 groups (P = .789).
TPO level in different groups. (A) Serum TPO level of pregnant patients with ITP before and after rhTPO treatment. (B) Cord blood TPO level in neonates of healthy women and of patients with ITP, using rhTPO. Horizontal line indicates median TPO level.
TPO level in different groups. (A) Serum TPO level of pregnant patients with ITP before and after rhTPO treatment. (B) Cord blood TPO level in neonates of healthy women and of patients with ITP, using rhTPO. Horizontal line indicates median TPO level.
No congenital disease or developmental delays of newborns were observed during a median follow-up of 53 (range, 39-68) weeks. One case of abdominal distension was reported, which was spontaneously resolved during follow-up (Table 3).
Discussion
Treatment options for ITP in pregnancy are limited if patients fail to respond to IVIG and corticosteroids. This is the first study to determine the efficiency and safety of rhTPO in the management of ITP in pregnancy. In our study, the total response rate to rhTPO in pregnant patients with ITP was at least the same as in nonpregnant patients with ITP, if not better.14 The stable dose of prednisone on enrollment had no influence on their response to rhTPO. rhTPO raised the platelet count in responders within 2 weeks, which significantly reduced the hemorrhage risk for patients with ITP in pregnancy. Furthermore, it seems that rhTPO ameliorates the bleeding symptoms remarkably, even in the nonresponders. Patients with ITP in pregnancy can be divided into 2 subgroups: patients with ITP diagnosed before pregnancy and those diagnosed during pregnancy. The former may experience exacerbation or relapse during their pregnancy.24,25 The managements of these 2 subgroups are identical. The outcomes are similar.26 Therefore, in our study, we do not stratify the enrolled patients.
rhTPO behaved as a linear pharmacokinetic. When it was subcutaneously administered at 150, 300, and 600 U/kg in healthy subjects, its absorption half-life (T1/2Ka) might increase with the dose (2.5-4.2 hours), and the time to peak concentration was 9.0-11.8 hours, which was similar among the 3 dose levels, indicating the dose level does not affect its absorption. In addition, the value of area under curve was approximately proportional to the dose administered, showing a roughly linear relationship. In vivo, rhTPO had a prolonged half-life and an extended elimination phase (38.7-46.3 hours), possibly because of the large molecular weight and great glycosylation of the agent.27 rhTPO was mainly excreted by kidney.28 In our study, 69.6% (16/23) and 21.7% (5/23) patients did not require treatment at weeks 4 and 12 after withdrawal of rhTPO.
In a recent retrospective study with a large sample size, Sun et al reported that the efficacy of corticosteroids and IVIG might be lower in pregnant than in nonpregnant patients with ITP.12 The management of pregnant patients with ITP remains a big challenge. Previous reports on secondary treatments are limited by either the retrospective nature or a small sample size. Splenectomy can be performed in the second trimester.11 Cyclosporin A has been used in other autoimmune disease and did not have significant toxicity to mother or fetus during pregnancy.29,30 It is reported that azathioprine has shown safety in pregnant patients with systemic lupus erythematosus and renal transplantation,31-33 but the time to response may be 3 to 6 months. These treatments all focused on reducing the destruction of platelets. Romiplostim, a thrombopoietin receptor agonist, has been used in a few cases of pregnant patients with ITP.34,35 However, a recently published case report indicated that romiplostim monotherapy was insufficient to maintain stable platelet counts in a pregnant patient with ITP. Labor was induced for thrombocytopenia episodes at 33 weeks and 6 days. The newborn had a grade III intraventricular hemorrhage that led to mild delay of motor skills and benign external hydrocephalus. The infant was also found to have adrenal insufficiency and phimosis. The relationship between these congenital diseases and TPO receptor agonists is still not clear.34
rhTPO was well tolerated by pregnant patients with ITP. In previous studies, the most frequently reported adverse events of rhTPO are fever, upper respiratory infection, pain at injection site, dizziness, and so on.14,23 The safety data in our study were consistent with previous reports. There was no new adverse event reported during the observation period and no adverse event–related study withdrawals.
Previous studies showed that patients using thrombopoietic agents, such as rhTPO, romiplostim, and eltrombopag, were at risk for thrombosis.36-38 However, the rate of thrombosis did not increase further with an extended period of treatment and does not appear to be related to platelet count.37 Whether these events were a result of the administration of thrombopoietic agents or a prompt increase of platelet count is unknown.39 And in some other studies, no higher incidence of thromboembolic events was reported.40,41 Most thromboembolic events have been observed in patients with at least 1 additional risk factor for thrombosis, such as comorbidities, splenectomy, hospitalization, long-term steroid therapy, or previous history of vascular disease.36,38,39,42 Pregnant women are subject to thrombosis because of their hypercoagulable state. Therefore, thrombosis is 1 of the major issues in the management of ITP in pregnancy with such agents. In our study, we adopted personalized dose adjustment; that is, we adjusted the dose of rhTPO according to each patient’s platelet count and maintained platelet count between 50 and 100 × 109/L to reduce the risk for thrombocytosis. Although no thromboembolic events occurred, it must be carefully monitored to avoid such thrombotic complication for the mothers during the use of rhTPO.
The anti-TPO antibodies were also taken into consideration in this study. There was another recombinant thrombopoietin molecule, pegylated human recombinant megakaryocytic growth and development factor, which was terminated in 1998 because of neutralizing autoantibodies against endogenous TPO in a few patients and healthy controls, resulting in persistent thrombocytopenia.13,20,21 Different from pegylated human recombinant megakaryocytic growth and development factor (a truncated and nonglycosylated TPO), rhTPO is a full-length and glycosylated TPO produced by Chinese hamster ovary cells that is almost identical to the endogenous TPO. Previous studies reported that 1% to 3% of patients with ITP were found to develop transient, low-titer, and nonneutralized anti-TPO antibodies after multidosing, subcutaneous injection.23,43 No anti-TPO antibodies were detected in our study, although the limited sample size precludes definitive conclusion.
In our study, the incidence of premature labor, birth weight, and platelet count of neonates were consistent with previous reports of pregnancy outcomes in patients with ITP.12,26,44
Pregnancy-associated autoimmune neonatal thrombocytopenia is a self-limited disease with a platelet nadir at postnatal days 4 and 5, after which most counts will rise to normal levels within 1 month.25 In our study, neonatal thrombocytopenia was noted in 9 neonates. Considering they were all asymptomatic with platelet count above 50 × 109/L, we did not give them any treatment. All of them spontaneously recovered on repeat measurement on the 42nd day of age. The neonatal platelet counts of 2 multiparas this time were comparable to those in first pregnancy.
ICH is a serious event for newborns. However, the methods for prevention and management of ICH remain controversial. The evidence of early detection of ICH in neonates by ultrasound is limited.45 Williams et al recommended that scanning and prophylaxis be considered after traumatic delivery.46 Also, it was recommended that ultrasound of fetal head should be performed when platelet count was less than 50 × 109/L.47 Fortunately, there was no such case in our study; therefore, no ultrasound was performed. Because all neonates in our study were asymptomatic with a nadir platelet count higher than 50 × 109/L, we inferred that none of the babies had ICH.
Another problem that hinders the use of novel thrombopoietin mimetics in the management of pregnant ITP is that they may cross the placenta and influence the fetus. rhTPO has a molecular weight of 90 000 Daltons and has a theoretical advantage over the currently marketed nonpeptide TPO mimetic, eltrombopag, which has a molecular weight of <1000 Daltons. Although there are multiple mechanisms that can influence placental passage, the threshold for placental passage is considered to be less than 5000 Daltons. The currently marketed peptide mimetic, romiplostim, with a molecular weight of 60 000 Daltons, is an exception. Romiplostim contains the CH2 and CH3 domains of the IgG-Fc, which enable it to bind Fc receptors.48 This structure not only contributes to the long circulating half-life of romiplostim49 but also promotes its transportation across the placenta.50-52 In rodents, 12 days after romiplostim administration, the drug was detected in both fetal serum and amniotic fluid.53 There is a paucity of data regarding whether eltrombopag could pass though the placenta, although some declared it could.36 For these reasons, the application of eltrombopag and romiplostim in pregnancy should be more cautious. rhTPO, with a molecular weight of 90 000 Daltons, could not pass through the placenta theoretically. Whether there is any active transport mechanism through c-Mpl remains unknown. In our study, the significant difference of serum TPO levels before and after rhTPO treatment showed the measurability of exogenous TPO. Cord blood TPO levels in neonates of patients with ITP treated with rhTPO showed no difference with those in neonates of healthy pregnant women, which provided certain evidence that rhTPO could not pass through the placenta.
This study demonstrates that rhTPO is a potentially safe, effective, and fast-acting treatment of pregnant patients with ITP who are refractory to fist-line therapy and platelet transfusion. Our work has paved the way for further study on the clinical application of rhTPO and other thrombopoietic agents in the management of ITP in pregnancy.
Presented orally in abstract form at the 58th annual meeting of the American Society of Hematology, Orlando, FL, 5 December 2016.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alexandra H. Marshall (Marshall Medical Communications) for editing the manuscript.
This work was supported by grants from National Natural Science Foundation of China (No. 81470284, No. 81500094), the Major Research plan of the National Natural Science Foundation of China (No. 91442204), State Key Clinical Specialty of China for Blood Disorders, the Key Clinical Research Project of Public Health Ministry of China 2010-2012, the Natural Science Foundation of Shandong Province (No. 2016ZRE27681), Taishan Scholar of Shandong Province, and Thrombocytopenia Funding from Yeehong Business School of Shenyang Pharmaceutical University (TCP funding).
Authorship
Contribution: Z.K. analyzed the data and wrote the first draft of the manuscript; P.Q. and S.X. coordinated the study, analyzed the data, and edited the manuscript; H.Z. collected the data and edited the manuscript; H.L., R.Y., X.L., J.L., Z.L., G.J., Z.C., Y.B., Y.W., and L.S. provided clinical input and collected the data; J.P., J.M., and M.H. designed the study protocol, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ming Hou, Department of Hematology, Qilu Hospital, Shandong University, Jinan 250012, China; e-mail: qlhouming@sina.com.cn; and Jun Ma, Harbin Institute of Hematology & Oncology, Harbin the First Hospital, Harbin, Heilongjiang 150010, China; e-mail: majun0322@126.com; and Jun Peng, Department of Hematology, Qilu Hospital, Shandong University, Jinan 250012, China; e-mail: junpeng88@sina.com.
References
Author notes
Z.K., P.Q., and S.X. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal