Key Points
Daratumumab is highly active in AL amyloidosis.
Abstract
The majority of patients with immunoglobulin light chain amyloidosis (AL) fail to achieve a complete response (CR) to standard light chain suppressive chemotherapy, and almost all patients eventually experience hematologic relapse and progression of organ involvement. Additional well-tolerated treatment options are needed. We present our retrospective experience of 25 consecutive previously treated AL patients who received daratumumab, a CD38-directed monoclonal antibody approved for the treatment of multiple myeloma. Daratumumab was administered at 16 mg/kg weekly for 8 weeks, then every 2 weeks for 8 doses, and then every 4 weeks. Patients had received a median of 3 prior lines of therapy, with a previous hematologic CR in only 5 patients. The overall hematologic response rate to daratumumab was 76%, including CR in 36% and very good partial response in 24%. Median time to response was 1 month. Therapy was well tolerated, even among the 72% of patients with cardiac AL involvement. Grade 1-2 infusion reactions occurred in 15 patients, but no grade 3 or 4 reactions were observed. Daratumumab is a highly effective agent that produced rapid and deep hematologic responses without unexpected toxicity in our cohort of heavily pretreated AL patients.
Introduction
Immunoglobulin light chain (LC) amyloidosis (AL) is a protein deposition disease characterized by varying degrees of organ dysfunction, morbidity, and early death from toxicity of misfolded LCs, usually produced by a clonal plasma cell population.1 Cardiac involvement is common, and patients with severe cardiac dysfunction have a poor prognosis, with a median survival of as little as 6 months.2 Successful LC suppressive therapy, measured by consensus hematologic response criteria, is associated with improved rates of organ response and survival.3,4 Although upfront LC suppressive therapy yields high hematologic overall response rates, the majority of patients do not achieve a complete hematologic response to therapy, allowing ongoing toxic amyloid deposition.5 Improvement in involved organ function is even less common. Furthermore, the underlying plasma cell dyscrasia is not curable, and almost all patients eventually relapse, requiring additional LC suppressive therapy.
Daratumumab is a human immunoglobulin G1κ (IgG1κ) monoclonal antibody targeting the CD38 surface antigen on plasma cells that has proven efficacy in multiple myeloma.6 Although the biology of the clonal plasma cell in AL is distinct from that of multiple myeloma, with a lower proliferation index,7 clonal plasma cells in AL do express surface CD38, providing a rationale for the use of daratumumab in AL amyloidosis.8
Study design
Patients
This is a retrospective analysis of consecutive patients followed at Stanford University for biopsy-proven AL confirmed by immunohistochemistry or mass spectroscopy; patients were treated with daratumumab and dexamethasone from January 2016 to December 2016. The study was approved by the Stanford University Institutional Review Board. Patients with previously treated AL were eligible for analysis. Hematologic response was defined per consensus guidelines.4 Of note, daratumumab is detectable as an IgGκ band on serum immunofixation, confounding response assessment. To maintain purity of complete response (CR) designation, patients with a detectable IgGκ band possibly consistent with daratumumab posttreatment were not considered to have obtained CR unless they had confirmed history of involved λ-free LC disease.
Treatment
Daratumumab was administered intravenously at 16 mg/kg weekly for 8 weeks, followed by every other week for 8 doses, and then every 4 weeks. Doses were administered over 8 hours with 500 mL intravenous fluids, except for the first dose, which was split in half and given on 2 consecutive days. If well tolerated, doses could be administered over a minimum of 4 hours. All patients received premedication with acetaminophen (650 mg), diphenhydramine (50 mg), and dexamethasone (20 mg). After the initial infusion, dexamethasone was tapered per physician discretion.
Results and discussion
Twenty-five patients received a median of 12 (3-35) infusions of daratumumab. All patients were evaluable for toxicity and 24 were evaluable for hematologic response. Clinical characteristics are shown in Table 1.9,10 The median age was 66 years, and 72% of patients had cardiac involvement, including 1 patient with NT-proBNP >8500 pg/mL. Median number of prior treatments was 3; all patients had previously received bortezomib or carfilzomib. Eighteen (72%) patients had been treated with both proteasome inhibitors and immunomodulatory agents, and 4 (16%) had undergone prior autologous stem cell transplant with melphalan-based conditioning.
Baseline characteristics of n = 25 patients
| Variable . | Percentage, % . | Median (range) . |
|---|---|---|
| Age, y | — | 66 (50-76) |
| Male | 68 | — |
| Time from diagnosis, mo | — | 30 (6-103) |
| No. prior lines of treatment | — | 3 (1-5) |
| Prior bortezomib or carfilzomib | 100 | |
| Prior IMiD and PI | 72 | — |
| Prior high-dose therapy + autologous HCT | 16 | |
| Best prior hematologic response = CR | 20 | — |
| Best prior hematologic response <VGPR | 44 | — |
| Time from last therapy, mo | — | 1.0 (0.25-58) |
| Refractory to prior line of therapy | 60 | — |
| Relapsed from prior line of therapy | 12 | — |
| Rising free light chain level from prior line of therapy (not meeting relapsed criteria) | 20 | — |
| Response plateau at PR to prior therapy | 8 | — |
| Serum albumin, g/dL | — | 3.0 (1.7-3.9) |
| dFLC at start of therapy, mg/dL | — | 7.4 (0.4-28) |
| M-protein, g/dL | — | Negative (0-0.6) |
| Cardiac involvement (18 patients) | 72 | — |
| NT-proBNP before therapy (cardiac patients), pg/L | — | 2058 (263-12 205) |
| Troponin I, ng/mL | — | 0.1 (<0.1-0.2) |
| Mayo 2004 cardiac stage I | 17 | — |
| Mayo 2004 cardiac stage II | 44 | — |
| Mayo 2004 cardiac stage III | 39 | — |
| Renal involvement (17 patients) | 68 | — |
| Estimated GFR <50 | 81 | 47 (10-90) |
| Proteinuria >5 g/24 h | 29 | 3432 (91-15 125) |
| ≥3 organs involved | 24 | — |
| Variable . | Percentage, % . | Median (range) . |
|---|---|---|
| Age, y | — | 66 (50-76) |
| Male | 68 | — |
| Time from diagnosis, mo | — | 30 (6-103) |
| No. prior lines of treatment | — | 3 (1-5) |
| Prior bortezomib or carfilzomib | 100 | |
| Prior IMiD and PI | 72 | — |
| Prior high-dose therapy + autologous HCT | 16 | |
| Best prior hematologic response = CR | 20 | — |
| Best prior hematologic response <VGPR | 44 | — |
| Time from last therapy, mo | — | 1.0 (0.25-58) |
| Refractory to prior line of therapy | 60 | — |
| Relapsed from prior line of therapy | 12 | — |
| Rising free light chain level from prior line of therapy (not meeting relapsed criteria) | 20 | — |
| Response plateau at PR to prior therapy | 8 | — |
| Serum albumin, g/dL | — | 3.0 (1.7-3.9) |
| dFLC at start of therapy, mg/dL | — | 7.4 (0.4-28) |
| M-protein, g/dL | — | Negative (0-0.6) |
| Cardiac involvement (18 patients) | 72 | — |
| NT-proBNP before therapy (cardiac patients), pg/L | — | 2058 (263-12 205) |
| Troponin I, ng/mL | — | 0.1 (<0.1-0.2) |
| Mayo 2004 cardiac stage I | 17 | — |
| Mayo 2004 cardiac stage II | 44 | — |
| Mayo 2004 cardiac stage III | 39 | — |
| Renal involvement (17 patients) | 68 | — |
| Estimated GFR <50 | 81 | 47 (10-90) |
| Proteinuria >5 g/24 h | 29 | 3432 (91-15 125) |
| ≥3 organs involved | 24 | — |
GFR, glomerular filtration rate; HCT, hematocrit; IMid, immunomodulatory agent; NT-proBNP, N-terminal pro b-type natriuretic peptide; PI, proteasome inhibitor; PR, partial response; VGPR, very good partial response.
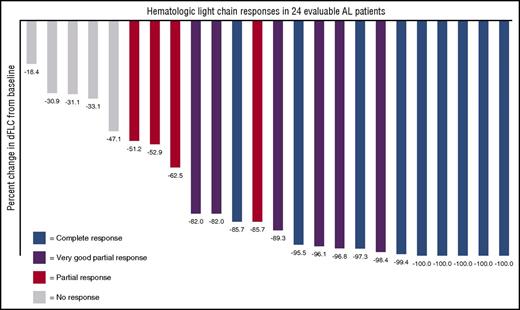
The 24 evaluable patients had a reduction in their difference between involved and uninvolved free light chains (dFLCs) as displayed in Figure 1. Nineteen of all 25 patients (76%) achieved a hematologic response to daratumumab, 9 of which (36%) achieved a CR, 6 (24%) achieved a VGPR, and 4 (16%) achieved a partial response. Of note, even among 11 patients with <VGPR to any prior therapy, daratumumab resulted in 3 CRs and 5 VGPRs. Responses were rapid, with a median time to deepest hematologic response of 1 month (7-188 days).
Waterfall plot demonstrating percent reduction of the dFLC in response to daratumumab. Best hematologic response is color coded; 100% of patients had a decrease in the dFLC.
Waterfall plot demonstrating percent reduction of the dFLC in response to daratumumab. Best hematologic response is color coded; 100% of patients had a decrease in the dFLC.
The tolerability profile of daratumumab was similar to that in myeloma studies, with 15 patients (60%) experiencing grade 1-2 infusion reactions. There were no ≥grade 3 infusion reactions. Two patients were hospitalized for the first dose solely to facilitate administration of the 2-day infusion. Among the 18 patients with cardiac AL involvement at the time of starting daratumumab, therapy was similarly well tolerated, without need for adjustment in diuretic dosing or decompensated heart failure related to drug infusion. Hematologic toxicity was minimal: only 1 patient with baseline anemia and chronic renal insufficiency required red cell transfusion. Four patients were hospitalized for management of grade 3-4 clinical events: 1 after a spontaneous pneumothorax, 1 had decompensated heart failure, and 2 were hospitalized for infections.
At the time of this report, 14 of 25 patients (56%) continue therapy with daratumumab. Eleven patients (44%) discontinued therapy: 7 (28%) from a lack of response or a plateau of response at PR, progression after PR, or personal preference. Four other patients (16%) discontinued daratumumab after grade 3-4 clinical events as described previously, all after 8 to 17 infusions achieving VGPR or CR.
Overall, 2 patients had hematologic progression during the study period. One patient achieved a CR but discontinued therapy because of infection, and experienced disease progression 7 months later. A second patient achieved a partial response after 4 doses, but discontinued therapy 3 months later because of hematologic progression. Two (8%) patients included in this report died from complications of AL amyloidosis, both after discontinuing daratumumab. One patient discontinued daratumumab for progressive disease and 1 for personal preference after 6 infusions and having achieved VGPR. At a median follow-up of 7 months from the start of daratumumab, both median duration of response and median progression-free survival have not yet been reached (supplemental Tables 1 and 2; supplemental Figures 1 and 2).
Historical response rates for patients with pretreated AL are typically derived from small series. The combination of bortezomib and dexamethasone produced hematologic responses in 6 pretreated patients (33% CR), with a median of 1 prior line of therapy,11 whereas cyclophosphamide plus dexamethasone resulted in response rates up to 73.9% in relapsed patients (21.7% CR) and up to 94% in a predominantly treatment-naïve population.12,13 Newer agents are showing efficacy in the pretreated setting, with carfilzomib producing a 78% overall hematologic response rate, although no CRs were seen.14 Pomalidomide in combination with dexamethasone in previously treated AL had a near 50% overall hematologic response rate, with only 1 (3%) CR observed in a population in which the majority of patients had not previously received both a proteasome inhibitor and immunomodulatory agent.15
Frequently, these responses are of limited duration (14.1-month median progression-free survival in the pomalidomide study and not yet reported in those receiving carfilzomib) and therapeutic options for relapsed patients remain limited.
We here report that daratumumab produced rapid and deep hematologic responses in a cohort of AL patients who had received multiple prior therapies, which is a select group of patients given their disease duration and survival. We demonstrate superior depth of response seen in a more heavily pretreated patient cohort than other reported regimens. Daratumumab was well tolerated, even among patients with advanced cardiac AL involvement. Further, neuropathy, commonly observed with other regimens, was not a concern. Although this is a small cohort, the study provides evidence for daratumumab’s activity in AL amyloidosis and compares favorably with other reported options in the setting of pretreated AL amyloidosis. In our patient cohort, daratumumab therapy was continued until disease progression; however, the need for continuous therapy adds additional cost and the benefit is unexplored. Prospective studies of daratumumab alone or in combination with chemotherapy in patients with AL amyloidosis are warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Patricia Ulloa and Marie Lugtu for their contributions in caring for the patients and abstracting data.
Authorship
Contribution: G.P.K., S.L.S., and M.L. designed the study; and G.P.K., S.L.S., R.A.L., S.A., R.M.W., and M.L. wrote the manuscript.
Conflict-of-interest disclosure: M.L. has received research funding from Gilead, Novartis, and Celgene, and has engaged in consultancy and received research funding from Prothena, Amgen, Takeda, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Michaela Liedtke, Department of Medicine, Stanford University School of Medicine, 300 Pasteur Dr, Lane 210, Stanford, CA 94305; e-mail: mliedtke@stanford.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal