Key Points
Eculizumab, an anti–complement C5 mAb, blocked killing of meningococci by whole blood from healthy immunized adults.
Blocking the AP with ACH-4471, a small molecule in development for PNH, had much less of an effect on meningococcal killing.
Abstract
Eculizumab, a humanized anti–complement C5 monoclonal antibody (mAb) for treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome, blocks the terminal complement pathway required for serum bactericidal activity (SBA). Because treated patients are at >1000-fold increased risk of meningococcal disease, vaccination is recommended; whether vaccination can protect by opsonophagocytic activity in the absence of SBA is not known. Meningococci were added to anticoagulated blood from 12 healthy adults vaccinated with meningococcal serogroup B and serogroup A, C, W, Y vaccines. Bacterial survival was measured after 3-hour incubation in the presence of eculizumab or control complement factor D inhibitor ACH-4471, which blocks the complement alternative pathway (AP) and is in phase 2 development for treatment of PNH. In the absence of inhibitors, colony formation units (CFUs) per milliliter in blood from all 12 immunized subjects decreased from ∼4000 at time 0 to sterile cultures at 3 hours. In the presence of eculizumab, there was a >22-fold increase in geometric mean CFUs per milliliter (90 596 and 114 683 CFU/mL for serogroup B and C strains, respectively; P < .0001 compared with time 0). In the presence of ACH-4471, there was a >12-fold decrease (23 and 331 CFU/mL, respectively; P < .0001). The lack of meningococci killing by blood containing eculizumab resulted from inhibition of release of C5a, a C5 split product needed for upregulation of phagocytosis. The results provide an explanation for the large number of cases of meningococcal disease in immunized patients being treated with eculizumab and suggest that vaccination may provide better protection against meningococcal disease in patients treated with an AP-specific inhibitor.
Introduction
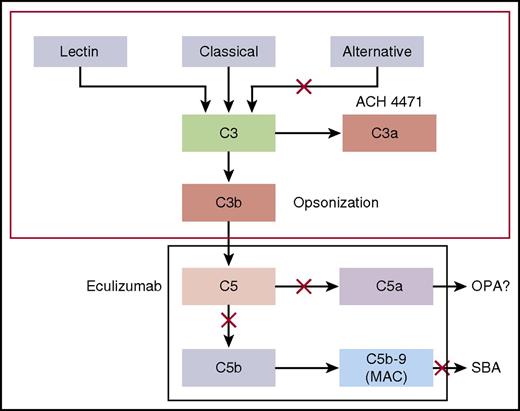
Dysregulated complement activation is the basis of a number of diseases, including paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). Eculizumab is a humanized monoclonal antibody (mAb) that received accelerated approval by the US Food and Drug Administration in 2007 for treatment of PNH.1-3 The mAb was subsequently shown to be effective and approved for treatment of aHUS.2,3 Eculizumab is a terminal complement pathway inhibitor that binds C5 and prevents formation of the membrane attack complement (MAC; Figure 14 ).2,3,5-9 Although eculizumab has dramatically improved quality of life, patients with terminal complement blockade are expected to be at a >1000-fold increased risk of invasive meningococcal disease than the general population because formation of a MAC is required for meningococcal serum bactericidal activity (SBA).10 This risk was underscored by 3 cases of meningococcal disease among 196 patients treated with eculizumab in clinical trials supporting drug licensure for treatment of PNH.1 The eculizumab package insert carries a “black-box” warning of the risk of potentially fatal meningococcal disease in treated patients.
Complement pathways. Model by which the CPs and complement APs mediate SBA or OPA killing of meningococci. Blocking cleavage of C5 or C7 in the terminal complement pathway prevents formation of MAC, which is required for complement-mediated SBA. In the absence of MAC, antimeningococcal antibodies may offer protection by eliciting antibody-Fc–mediated and/or C3b/iC3b complement receptor–mediated OPA. However, complement receptor–mediated phagocytic uptake as well as phagocytic cell chemotaxis is promoted by C5a, a split product of C5. Thus, blockage of release of C5a by eculizumab could abrogate this additional protective mechanism.4
Complement pathways. Model by which the CPs and complement APs mediate SBA or OPA killing of meningococci. Blocking cleavage of C5 or C7 in the terminal complement pathway prevents formation of MAC, which is required for complement-mediated SBA. In the absence of MAC, antimeningococcal antibodies may offer protection by eliciting antibody-Fc–mediated and/or C3b/iC3b complement receptor–mediated OPA. However, complement receptor–mediated phagocytic uptake as well as phagocytic cell chemotaxis is promoted by C5a, a split product of C5. Thus, blockage of release of C5a by eculizumab could abrogate this additional protective mechanism.4
To mitigate risk of meningococcal disease, the US Advisory Committee on Immunization Practices (ACIP) recommends that all patients treated with eculizumab be immunized with a quadrivalent meningococcal serogroup A, C, W, Y conjugate vaccine and a serogroup B vaccine, preferably beginning immunization at least 2 weeks before treatment.11,12 However, despite vaccination, cases of meningococcal disease have occurred.13-16 These cases raise concerns that meningococcal vaccination may be incompletely efficacious in persons treated with eculizumab because of impaired opsonophagocytic (OPA) killing as a consequence of inhibiting cleavage of C5 to release C5a (Figure 1).4 C5a is a potent proinflammatory peptide that upregulates phagocytic cell complement receptors and oxidative burst.4,17,18
In the present study, we investigated the effect of eculizumab on killing of meningococci by whole blood from healthy adults immunized with meningococcal serogroup A, C, W, Y, and serogroup B vaccines using a previously described ex vivo whole-blood model of meningococcal sepsis.4 The model is particularly useful because it incorporates important mechanisms by which serum antibodies confer protection against meningococcal disease (SBA and OPA). As a control complement inhibitor, we investigated in parallel ACH-4471, a small-molecule inhibitor of complement factor D, which selectively blocks the alternative pathway (AP)19 and currently is in phase 2 clinical investigation as an oral therapeutic agent.20
Methods
Subjects and blood samples
The study included 12 healthy microbiologists or health care workers (Table 1) who had been immunized with meningococcal vaccines as part of previous immunogenicity studies21,22 or at Employee Health, UCSF Benioff Children’s Hospital Oakland because of occupational exposure to meningococci. Five were men and 7 were women. The median age was 31.5 years (range, 23-65 years). All subjects had received at least 1 dose of a meningococcal A, C, W, Y polysaccharide or conjugate vaccine with the last dose given 8 to 132 months before blood sample collection. Subjects 001 to 006 also had been immunized with 3 doses of a serogroup B vaccine called MenB-FHbp (Trumenba; Pfizer) at 0, 2, and 6 months, with the last dose 6 to 12 months before blood sample collection. Subjects 007 to 012 had been immunized with 2 or 3 doses of a serogroup B vaccine called MenB-4C (Bexsero; GSK), with the last dose 2 to 9 months before blood sample collection. Blood samples were obtained with informed consent and the protocol and consent forms were approved by the institutional review board of UCSF Benioff Children’s Hospital Oakland (protocol 2016-068). The blood samples were treated with lepirudin, which is an anticoagulant that functions as a thrombin inhibitor and does not affect complement activity.4,18
Description of the immunized adults who provided blood samples
| Subject no. . | Age, y . | Sex . | MenB vaccine* . | Schedule . | Interval since last dose, mo . | MenA, C, Y, W vaccine† . | Interval since last dose, mo . |
|---|---|---|---|---|---|---|---|
| 001 | 50 | M | MenB-FHbp | 0, 1, 6 mo | 11-12 | MCV4-DT | 60 |
| 002 | 60 | M | MenB-FHbp | 0, 1, 6 mo | 11 | MCV4-DT (also MPSV4) | 120 |
| 003 | 28 | M | MenB-FHbp | 0, 1, 6 mo | 8 | MCV4-DT | 96 |
| 004 | 65 | F | MenB-FHbp | 0, 1, 6 mo | 9 | MCV4-DT (also MPSV4) | 24 |
| 005 | 33 | F | MenB-FHbp | 0, 1, 6 mo | 6 | MCV4-DT | 120 |
| 006 | 30 | F | MenB-FHbp | 0, 1, 6 mo | 11 | MCV4-DT | 132 |
| 007 | 23 | F | MenB-4C | 0, 1 mo, 1 y | 9 | MCV4-DT | 11 |
| 008 | 24 | F | MenB-4C | 0, 1 mo, 1 y | 6 | MCV4-DT | 11-12 |
| 009 | 35 | M | MenB-4C | 0, 1 mo | 6-8 | MCV4-DT | 8 |
| 010 | 25 | M | MenB-4C | 0, 1 mo, 1 y | 3-4 | MCV4-DT | 24 |
| 011 | 28 | F | MenB-4C | 0, 1 mo | 5-6 | MCV4-CRM | 12-13 |
| 012 | 41 | F | MenB-4C | 0, 1 mo | 2 | MCV4-DT | 60 |
| Subject no. . | Age, y . | Sex . | MenB vaccine* . | Schedule . | Interval since last dose, mo . | MenA, C, Y, W vaccine† . | Interval since last dose, mo . |
|---|---|---|---|---|---|---|---|
| 001 | 50 | M | MenB-FHbp | 0, 1, 6 mo | 11-12 | MCV4-DT | 60 |
| 002 | 60 | M | MenB-FHbp | 0, 1, 6 mo | 11 | MCV4-DT (also MPSV4) | 120 |
| 003 | 28 | M | MenB-FHbp | 0, 1, 6 mo | 8 | MCV4-DT | 96 |
| 004 | 65 | F | MenB-FHbp | 0, 1, 6 mo | 9 | MCV4-DT (also MPSV4) | 24 |
| 005 | 33 | F | MenB-FHbp | 0, 1, 6 mo | 6 | MCV4-DT | 120 |
| 006 | 30 | F | MenB-FHbp | 0, 1, 6 mo | 11 | MCV4-DT | 132 |
| 007 | 23 | F | MenB-4C | 0, 1 mo, 1 y | 9 | MCV4-DT | 11 |
| 008 | 24 | F | MenB-4C | 0, 1 mo, 1 y | 6 | MCV4-DT | 11-12 |
| 009 | 35 | M | MenB-4C | 0, 1 mo | 6-8 | MCV4-DT | 8 |
| 010 | 25 | M | MenB-4C | 0, 1 mo, 1 y | 3-4 | MCV4-DT | 24 |
| 011 | 28 | F | MenB-4C | 0, 1 mo | 5-6 | MCV4-CRM | 12-13 |
| 012 | 41 | F | MenB-4C | 0, 1 mo | 2 | MCV4-DT | 60 |
All subjects were microbiologists or health care workers who had been immunized as part of previous immunogenicity studies,21,22 or at Employee Health at UCSF Benioff Children’s Hospital Oakland because of occupational exposure to N meningitidis.
MenB-FHbp (Trumbemba; Pfizer Vaccines); MenB-4C (Bexsero; GSK).
MCV4-DT (Menactra; Sanofi); MPSV4 (Menomune; Sanofi); MCV4-CRM (Menveo; GSK).
Reagents
Eculizumab (anti-C5) was obtained for in vitro research purposes only from residual liquid (typically 2 mL) in vials after completing infusion of a patient. The small-molecule complement factor D inhibitor, ACH-4471 (supplemental Figure 1, available on the Blood Web site),19 was provided by Achillion Pharmaceuticals, Inc. The anti-C7 mAb and the C5a receptor (C5aR) antagonist were purchased, respectively, from Quidel and Tocris. Lepirudin (Refludan) was purchased from Hoechst. All reagents were divided into aliquots and stored at −70°C until needed unless otherwise specified.
Bacterial strains
The serogroup B strains were case isolates, designated H44/76 (clonal complex, sequence type, ST-32) from Norway during a period of hyperendemic disease23 ; CH855 (ST-269) from an outbreak at Ohio University24 (referred to as the Ohio University strain); and CH861 (ST-269) from Quebec, Canada, collected in 2013 during a period of hyperendemic disease25 (referred to as the Quebec strain). The serogroup C case isolate, 4243 (ST-11) was from Dallas, TX.26 All 4 strains express factor H–binding protein (FHbp) subfamily B proteins, which are included in recombinant forms in both of the licensed serogroup B vaccines. All 4 strains also express neisserial heparin-binding antigen, which is included in recombinant form in the MenB-4C vaccine. The Ohio University strain also expresses a NadA protein, which is included in recombinant form in MenB-4C.
Complement assays
The effect of inhibitors on the activity of the complement AP or the classic pathway (CP) was tested using commercially available Wieslab assays according to the manufacturer’s instructions. These assays measure the amount of MAC formation by enzyme-linked immunosorbent assay (ELISA) following activation of the specific pathway (Euro Diagnostica). The effect of inhibitors on CP-mediated hemolysis was assessed using a commercially available CP hemolytic assay (CP50; Complement Tech) according to the manufacturer’s instructions. Fifty-percent inhibition of activity (IC50) values were determined by variable-slope 4-parameter nonlinear regression analysis using GraphPad Prism 7.0.
Whole-blood OPA bactericidal assay
Eculizumab, ACH-4471, anti-C7 mAb, and C5aR antagonist were diluted in Dulbecco phosphate-buffered saline (DPBS) containing 1% bovine serum albumin (BSA) to achieve final concentrations in blood of 50 µg/mL (eculizumab), 4 µM (ACH-4471), 200 µg/mL (anti-C7 mAb), and 10 µM (C5aR antagonist), respectively. Sixty microliters of DPBS-BSA with or without an inhibitor were added individually or in combination (see “Results”) to tubes containing 260 µL of freshly obtained anticoagulated blood. Following incubation at room temperature for 20 minutes, ∼1000 to 1500 colony formation units (CFUs) of bacteria were added (∼3000 -4700 CFU/mL). The tubes were further incubated at 37°C in a CO2 incubator using a rotator (multitube tube with a fixed speed; Thermo Fisher) for 1 and/or 3 hours, at which time 50 µL, 10 µL, and 10 µL of a 1/10 dilution (equivalent to 1 µL) were removed and plated onto chocolate agar to quantify surviving CFUs per milliliter. To ascertain input CFUs per milliliter, aliquots were also removed at time 0 from control tubes containing buffer alone or the subjects’ plasma that had been heated to 56°C for 30 minutes to inactivate complement.
Statistical methods
Data are presented as mean or, where appropriate, geometric mean CFUs per milliliter, which was calculated from log10-transformed data using Prism version 7.00 (GraphPad). Test samples with sterile cultures were assigned a CFU per milliliter of 50 (half the lower limit of detection). Because of small sample sizes with little variation, all data were assumed to follow normal distribution because there were no obvious skewed distributions. Comparisons of geometric means of the respective CFUs per milliliter between different time points were performed using a paired Student t test on log-transformed data. Comparisons among inhibitor treatments were done by repeated measurement 1-way analysis of variance with Holm-Sidak multiple comparisons.
Results
Effect of complement inhibitors on CP and AP activity
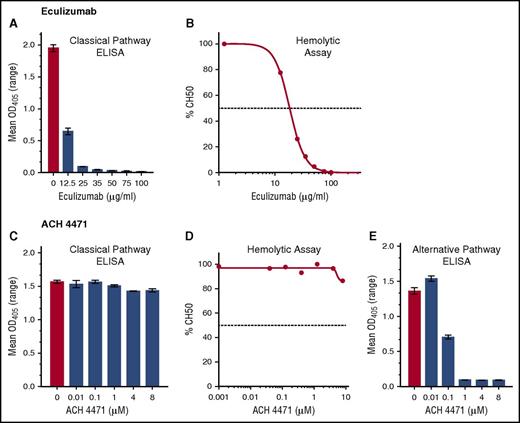
Eculizumab inhibited CP activity in a dose-dependent manner as measured by CP Wieslab (Figure 2A) and CP hemolysis assays (CH50) (Figure 2B). The control inhibitor ACH-4471, which is specific for factor D in the AP, completely blocked AP activity at concentrations as low as 1 µM (0.58 µg/mL) in the AP Wieslab assay (Figure 2E) but not in the counterpart CP Wieslab assay (Figure 2C) nor the CP hemolytic assay (Figure 2D) at the highest concentration tested (8 µM). For subsequent studies, we used eculizumab at a concentration of 50 µg/mL, which is in excess of the concentration required to completely block CP activity in vitro, but within the range in serum samples from patients treated with the drug5 ; we used ACH-4471 at 4 µM (2.3 µg/mL), which is fourfold higher than the concentration required to completely block AP activity in vitro and also in excess of concentrations projected for efficacy in patients.20
Effect of eculizumab (anti-C5) and ACH-4471 (a complement factor D inhibitor) on complement pathways. (A,C) CP activity measured by CP Wieslab. (B,D) CP activity measured by hemolytic assay. Horizontal dashed lines represent IC50, which was 18.3 µg/mL for eculizumab. (E) AP activity measured by AP Wieslab assay. At a concentration of 1 µM ACH-4471 (0.58 µg/mL), complete inhibition was achieved.
Effect of eculizumab (anti-C5) and ACH-4471 (a complement factor D inhibitor) on complement pathways. (A,C) CP activity measured by CP Wieslab. (B,D) CP activity measured by hemolytic assay. Horizontal dashed lines represent IC50, which was 18.3 µg/mL for eculizumab. (E) AP activity measured by AP Wieslab assay. At a concentration of 1 µM ACH-4471 (0.58 µg/mL), complete inhibition was achieved.
Effect of complement inhibitors on killing of Neisseria meningitidis by nonimmune blood from an unvaccinated adult
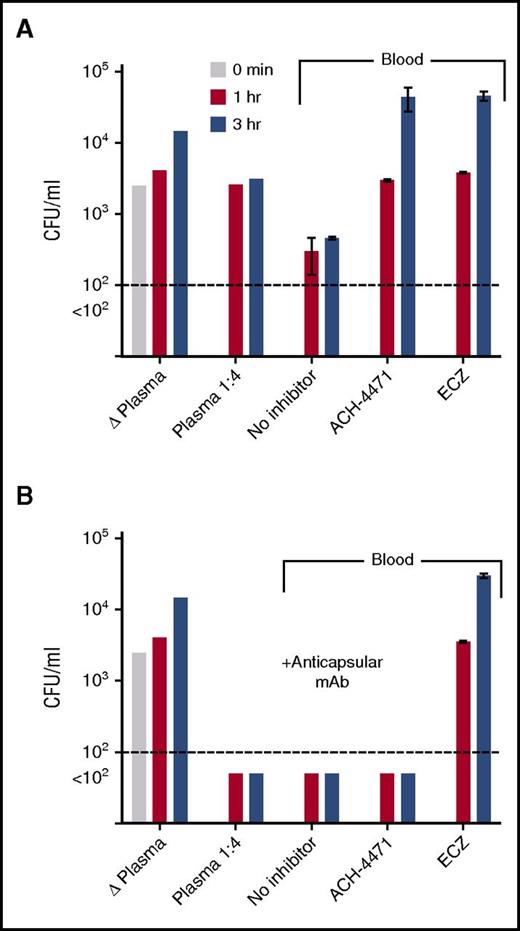
Serogroup B strain H44/76 was added to plasma or blood from a healthy adult who had not been immunized with a meningococcal serogroup B vaccine (Figure 3A). In the control plasma that had been heated to inactivate complement, or in fresh-frozen plasma diluted 1/4, there was no significant killing after 1 or 3 hours of incubation. With whole blood, there was an approximately eightfold decrease in CFUs per milliliter at both time points compared with time 0. Addition of ACH-4471 or eculizumab to whole blood completely abrogated killing with >10-fold increases in CFUs per milliliter at 3 hours compared with time 0. Thus, the meningococcal killing activity by blood from the unvaccinated donor was dependent on both the AP and terminal complement pathway.
Effect of complement inhibitors on survival of serogroup B meningococci in plasma or whole blood from an unvaccinated adult subject. At time 0, ∼4750 CFU/mL N meningitidis serogroup B strain H44/76 were added to plasma or whole blood containing no complement inhibitor or inhibitors indicated. CFUs per milliliter were quantified in aliquots of blood or plasma collected at 1 and 3 hours postincubation. (A) Test conditions without adding anticapsular antibody. (B) Test conditions with adding 25 µg/mL of a meningococcal serogroup B anticapsular mAb (SEAM 1227 ). Data are the mean CFU per milliliter (ranges) from replicate determinations. Δ Plasma, plasma heated for 30 minutes at 56°C to inactivate complement. The results were replicated in a second experiment with blood from the same donor. ACH-4471, factor D inhibitor of AP; ECZ, eculizumab.
Effect of complement inhibitors on survival of serogroup B meningococci in plasma or whole blood from an unvaccinated adult subject. At time 0, ∼4750 CFU/mL N meningitidis serogroup B strain H44/76 were added to plasma or whole blood containing no complement inhibitor or inhibitors indicated. CFUs per milliliter were quantified in aliquots of blood or plasma collected at 1 and 3 hours postincubation. (A) Test conditions without adding anticapsular antibody. (B) Test conditions with adding 25 µg/mL of a meningococcal serogroup B anticapsular mAb (SEAM 1227 ). Data are the mean CFU per milliliter (ranges) from replicate determinations. Δ Plasma, plasma heated for 30 minutes at 56°C to inactivate complement. The results were replicated in a second experiment with blood from the same donor. ACH-4471, factor D inhibitor of AP; ECZ, eculizumab.
The addition of 25 µg/mL of a meningococcal serogroup B anticapsular mAb27 to the subject’s plasma or whole blood resulted in sterile cultures (Figure 3B). Unexpectedly, eculizumab blocked bacterial killing by whole blood containing the anticapsular mAb; there was an ∼18-fold increase in CFUs per milliliter at 3 hours, compared with time 0, which suggested that not only C5b, which was required for MAC-dependent killing, but also C5a was required for effective opsonophagocytosis-dependent killing in the whole-blood assay. In contrast, ACH- 4471 showed no effect on bacterial killing by blood containing the anticapsular mAb, which suggested that killing of meningococci was independent of the AP, that is, opsonic activity and/or terminal pathway activation via CP alone was sufficient. Together, these data suggest that vaccination could be an effective strategy to mitigate the risk of meningococcal disease associated with a complement factor D inhibitor, whereas vaccination may provide limited to no protection with eculizumab or other C5 inhibitors that block formation of MAC as well as cleavage of C5.
Effect of complement inhibitors on killing of N meningitidis by blood from vaccinated adults
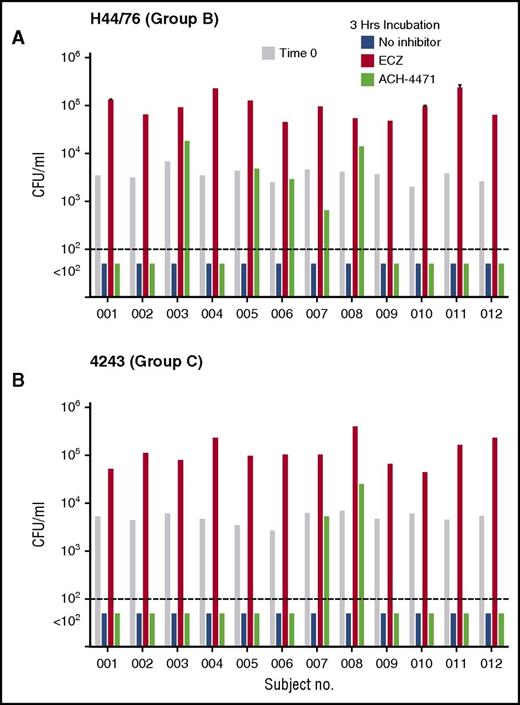
To evaluate the clinical relevance of our findings in vaccinated persons, we investigated the effect of eculizumab or ACH-4471 on killing of N meningitidis by anticoagulated blood samples from 12 health care or laboratory workers who had been immunized with a meningococcal serogroup B vaccine and an A, C, W, and Y polysaccharide or conjugate vaccine. The test strains were serogroup B strain H44/76 and serogroup C strain 4243. After 3 hours of incubation in the absence of an inhibitor, cultures of either strain were sterile (Figure 4 blue bars). The addition of eculizumab (Figure 4 orange bars) completely blocked killing of both strains. In contrast, the addition of ACH-4471 (Figure 4 green bars) had no effect on killing of the serogroup B strain in 7 of the 12 subjects (Figure 4A), or on the serogroup C strain in 10 of the 12 subjects (Figure 4B). In persons immunized with serogroup B vaccines, the principal antibody responsible for protection against serogroup B strain H44/76 is directed at FHbp.21,22 The 5 subjects whose killing of the serogroup B strain by whole blood was impaired after blocking the AP by the factor D inhibitor had, by ELISA, ∼10-fold lower serum IgG anti-FHbp titers (geometric mean, 1/273) than the 7 subjects whose whole blood did not require AP amplification to kill the strain (geometric mean, 1/2650; P < .001). These results are consistent with previously reported data that when serum anti-FHbp antibody titers are high and of good quality, sufficient antibody complex is formed on the surface of meningococci to activate the classical complement pathway and proceed to bacteriolysis without the need for amplification of complement activation by AP.28,29 In contrast, when antibody titers are low, AP amplification of C3b deposition is needed to proceed to bacteriolysis mediated by the terminal pathway. There were similar respective trends after inhibition of the AP in whole-blood killing of the serogroup C strain 4243. Both subjects whose whole-blood killing was dependent on an intact AP had lower serum IgG serogroup C anticapsular titers than the 10 subjects whose whole-blood killing was not impaired after blocking AP (respective median titers of 1/25 vs 1/125; P = .08 by Mann-Whitney U test).
Effect of complement inhibitors on survival of meningococci in whole blood of individual vaccinated adults. (A) Serogroup B strain H44/76. (B) Serogroup C strain 4243. At time 0, ∼3600 CFU/mL N meningitidis were added to blood (gray bars). In the absence of a complement inhibitor, blood from all 12 subjects had sterile cultures (blue bars) by 3 hours. In contrast, addition of eculizumab (ECZ; anti-C5, orange bars) completely blocked bacterial killing of both strains. ACH-4471 (complement factor D inhibitor, green bars) had no effect (sterile culture) on killing of the serogroup B strain H44/76 in 7 of 12 subjects, and no effect on killing of the serogroup C 4243 strain in 10 of 12 subjects (see “Results”).
Effect of complement inhibitors on survival of meningococci in whole blood of individual vaccinated adults. (A) Serogroup B strain H44/76. (B) Serogroup C strain 4243. At time 0, ∼3600 CFU/mL N meningitidis were added to blood (gray bars). In the absence of a complement inhibitor, blood from all 12 subjects had sterile cultures (blue bars) by 3 hours. In contrast, addition of eculizumab (ECZ; anti-C5, orange bars) completely blocked bacterial killing of both strains. ACH-4471 (complement factor D inhibitor, green bars) had no effect (sterile culture) on killing of the serogroup B strain H44/76 in 7 of 12 subjects, and no effect on killing of the serogroup C 4243 strain in 10 of 12 subjects (see “Results”).
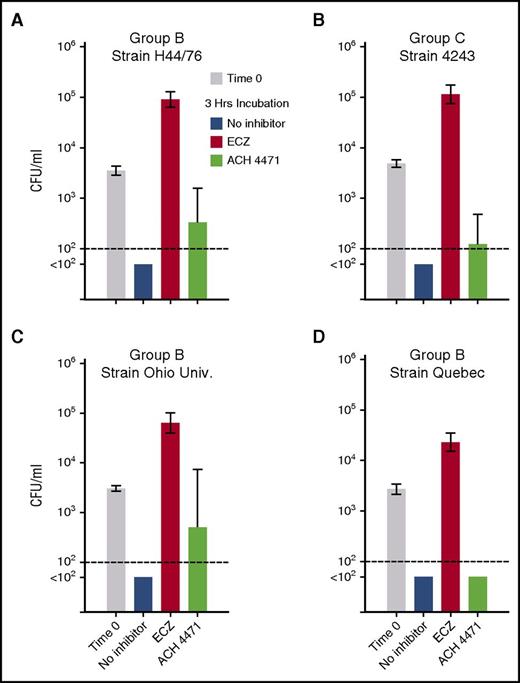
The geometric means of the CFUs per milliliter at the indicated end points were used to evaluate statistical significance across the data set (Figure 5A-B). For the serogroup B strain H44/76, the geometric mean CFUs per milliliter increased 25-fold after 3 hours of incubation of the bacteria and blood in the presence of eculizumab (from 3561 at time 0 to 90 597, P < .0001 by paired t test on log-transformed data; Figure 5A). In contrast, in the presence of ACH-4471, the geometric mean CFUs per milliliter of blood decreased at 3 hours compared with time 0 (from 3561 to 331, P < .0001). There were sterile cultures after 3 hours of incubation of serogroup B strain H44/76 in blood without inhibitor (geometric mean, <100 CFU/mL). At 3 hours, the geometric mean CFU per milliliter was >900-fold higher in blood with eculizumab than with no inhibitor (P < .0001 by Holm-Sidak multiple comparisons test), more than threefold higher with ACH-4471 than no inhibitor (P = .02), and 274-fold higher in blood with eculizumab than ACH-4471 (P < .0001).
Effect of complement inhibitors on survival of 4 N meningitidis strains in whole blood from vaccinated adult subjects. Geometric mean CFU per milliliter (95% confidence interval) after incubation of meningococci for 3 hours. (A-B) Data from 12 subjects tested against serogroup B strain H44/65 and serogroup C strain 4243, respectively. (C-D) Serogroup B strains Ohio University and Quebec tested with blood from 5 subjects immunized with MenB-4C. Irrespective of the strain tested, eculizumab blocked killing of bacteria and the geometric mean CFU per milliliter increased significantly at 3 hours compared with time 0 (P < .0001). In contrast, in the presence of the complement factor D inhibitor ACH-4471, the geometric mean CFU per milliliter of blood decreased at 3 hours compared with time 0 (P < .005 for strains H44/76, 4243, and Quebec; P = .13 for Ohio University). The P values for comparisons between the respective geometric mean CFUs per milliliter at 3 hours for the different treatment groups for each strain are provided in “Results.”
Effect of complement inhibitors on survival of 4 N meningitidis strains in whole blood from vaccinated adult subjects. Geometric mean CFU per milliliter (95% confidence interval) after incubation of meningococci for 3 hours. (A-B) Data from 12 subjects tested against serogroup B strain H44/65 and serogroup C strain 4243, respectively. (C-D) Serogroup B strains Ohio University and Quebec tested with blood from 5 subjects immunized with MenB-4C. Irrespective of the strain tested, eculizumab blocked killing of bacteria and the geometric mean CFU per milliliter increased significantly at 3 hours compared with time 0 (P < .0001). In contrast, in the presence of the complement factor D inhibitor ACH-4471, the geometric mean CFU per milliliter of blood decreased at 3 hours compared with time 0 (P < .005 for strains H44/76, 4243, and Quebec; P = .13 for Ohio University). The P values for comparisons between the respective geometric mean CFUs per milliliter at 3 hours for the different treatment groups for each strain are provided in “Results.”
Similar respective results were obtained for the serogroup C strain 4243. Compared with time 0, the geometric mean CFUs per milliliter increased 23-fold after 3 hours of incubation of the bacteria and blood in the presence of eculizumab (from 4903 to 114 683, P < .0001; Figure 5B). In contrast, in the presence of ACH-4471, the geometric mean CFUs per milliliter of blood decreased at 3 hours compared with time 0 (P < .0001). There were sterile cultures after 3 hours of incubation of serogroup C strain 4243 in blood without inhibitor (geometric mean, <100 CFU/mL). At 3 hours, the geometric mean CFUs per milliliter of the 12 subjects was >1000-fold higher in blood with eculizumab than no inhibitor (P ≤ .0001), and ∼1000-fold higher in blood with eculizumab than ACH-4471 (P < .0001). The difference between the geometric mean CFUs per milliliter in blood with ACH-4471 or no inhibitor (sterile cultures) was not significant (P = .17).
We then evaluated the effect of complement inhibitors on killing of 2 additional serogroup B strains; Ohio University (Figure 5C) and Quebec (Figure 5D) by whole blood from 5 of the subjects immunized with the MenB-4C vaccine. In the absence of inhibitor, sterile cultures were observed at 3 hours for both strains. Compared with CFUs per milliliter at time 0, the addition of eculizumab resulted in a 20-fold increase in CFUs per milliliter at 3 hours for the Ohio University strain (P < .0001), and an eightfold increase in CFUs per milliliter for the Quebec strain (P = .0001). In contrast, in the presence of ACH-4471, the geometric mean CFU per milliliter at 3 hours decreased sixfold for the Ohio University strain (P = .13 compared with time 0), and decreased 54-fold for the Quebec strain (P = .0001).
At 3 hours, the geometric mean CFUs per milliliter of the Ohio University strain was 1000-fold higher in blood with eculizumab than no inhibitor (P < .0001), and 100-fold higher in blood with eculizumab than ACH-4471 (P < .01). The difference between in the geometric means of the CFUs per milliliter with ACH-4471 and no inhibitor was not significant (P = .07). For the Quebec strain, after 3 hours of incubation the geometric mean CFUs per milliliter of 5 subjects was 450-fold higher in blood with eculizumab than no inhibitor (P ≤ .0001), and 450-fold higher in blood with eculizumab than ACH-4471 (P < .0001). In blood incubated with ACH-4471, or with no inhibitor, there was no bacteria detected after 3 hours.
Addition of C5aR antagonist augments anti-C7 inhibition of whole-blood killing of meningococci
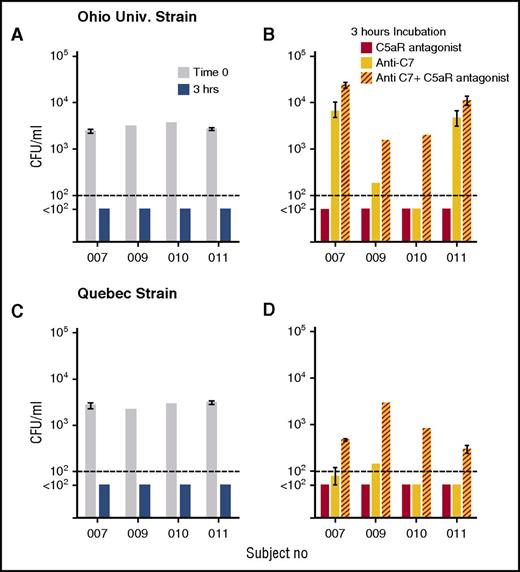
Blocking MAC formation by inhibition of the terminal pathway alone eliminates SBA but would not necessarily be expected to interfere with OPA activity (Figure 1), which is mediated by Fc (antibody) and depositions of C4 (such as C4b) and C3 fragments (such as C3b/iC3b) on the bacteria. Eculizumab, however, also inhibits cleavage of C5, and thereby inhibits release of C5a,30 which is a potent proinflammatory mediator reported to be needed for OPA killing of meningococci.4 To evaluate the contribution of C5a on bacterial killing by human blood when SBA was blocked, we used two additional inhibitors: an anti-C7 mAb which functionally blocks the terminal complement pathway and, thereby, MAC formation but has no effect on cleavage of C5 into C5a and C5b, and a C5aR antagonist, which blocks the effects of C5a. Figure 6 shows the effect of the inhibitors individually or in combination on killing of the Ohio University and Quebec strains by whole blood from 4 of the adults immunized with the MenB-4C vaccine. With either strain, after 3 hours of incubation of bacteria in blood in the absence of inhibitors, cultures were sterile (Figure 6A). For the Ohio University strain, inhibiting C7 blocked bacterial killing by blood from subjects 007 and 011, but not from subjects 009 or 010. The addition of the C5aR antagonist alone to blood had no effect on killing, but the addition of the C5aR antagonist in combination with the anti-C7mAb resulted in 4-, 8-, 40-, and 2-fold increases in CFU per milliliter compared with the anti-C7 mAb alone for subjects 007, 009, 010, and 011, respectively (P = .04 by paired t test). For the Quebec strain there were 6-, 20-, 16-, and 6-fold increases in CFU per milliliter in the presence of the combination of inhibitors in the blood from the respective subjects, as compared with the anti-C7 mAb alone (P = .005). These data demonstrate an important role for C5a as an additional protective mechanism for killing of meningococci by whole blood when SBA is blocked, and provide a mechanism for the lack of effective OPA killing of meningococci during eculizumab treatment.
C5aR antagonist augments anti-C7 inhibition of whole-blood killing of meningococci. Whole blood from 4 subjects vaccinated with MenB-4C was tested for killing activity against 2 serogroup B strains, Ohio University and Quebec, in the presence or absence of complement inhibitors. Data are geometric mean CFU per milliliter (ranges) from 2 experiments with blood from subjects 007 and 011, and CFU per milliliter from 1 experiment each for subjects 009 and 010. (A,C) CFU in blood at time 0 and after 3 hours of incubation in the absence of inhibitor. (B,D) CFU per milliliter in blood after 3-hour incubation with C5aR antagonist (red bars) or ant-C7 mAb (yellow bars), or anti-C7 + C5aR antagonist (red hatched yellow bars). The combination of the C5aR antagonist and anti-C7 mAb blocked killing of both strains to a greater extent than the anti-C7 mAb alone (P < .04 by paired t test on log-transformed data).
C5aR antagonist augments anti-C7 inhibition of whole-blood killing of meningococci. Whole blood from 4 subjects vaccinated with MenB-4C was tested for killing activity against 2 serogroup B strains, Ohio University and Quebec, in the presence or absence of complement inhibitors. Data are geometric mean CFU per milliliter (ranges) from 2 experiments with blood from subjects 007 and 011, and CFU per milliliter from 1 experiment each for subjects 009 and 010. (A,C) CFU in blood at time 0 and after 3 hours of incubation in the absence of inhibitor. (B,D) CFU per milliliter in blood after 3-hour incubation with C5aR antagonist (red bars) or ant-C7 mAb (yellow bars), or anti-C7 + C5aR antagonist (red hatched yellow bars). The combination of the C5aR antagonist and anti-C7 mAb blocked killing of both strains to a greater extent than the anti-C7 mAb alone (P < .04 by paired t test on log-transformed data).
Discussion
As of April 2014, 43 cases of meningococcal disease had been reported to the manufacturer in patients treated with eculizumab, with an estimated annual incidence rate of 330 per 100 000 (https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM423031.pdf). This rate is >2000-fold higher than the annual rate reported for the adult US population (0.15/100 000).31 Although nearly all of the eculizumab-associated cases were in patients who had been immunized with a serogroup A, C, W, Y vaccine, interpretation of the possible benefit or lack of benefit of vaccination is limited by incomplete information on the serogroups of the strains causing disease. Also, all 43 cases were from before 2015, when serogroup B vaccines first became available in the United States. A recent report, however, of serogroup B meningococcal disease in a patient with PNH being treated with eculizumab who had been immunized with MenB-4C underscores that serogroup B disease also can occur despite serogroup B vaccination.13 Collectively these cases raise concerns about whether vaccination confers the expected protection when C5 is blocked by eculizumab.
Recently, Alashkar et al reported that the majority of PNH patients immunized with a meningococcal serogroup A, C, W, Y vaccine because of eculizumab treatment had serologic evidence of protective SBA titers measured with rabbit complement.32 However, eculizumab specifically blocks human C5 and not rabbit C5 (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000791/WC500054212.pdf). The rationale for immunization of persons being treated with eculizumab who cannot mount protective SBA responses mediated by human complement is that the early components of complement, which are essential for opsonization of microorganisms, are preserved (Figure 1). There also is evidence that vaccine-induced opsonic antibodies can support phagocytic killing of meningococci,33-38 and that vaccination of persons with certain inherited complement deficiencies who cannot mount SBA responses, confers protection from developing meningococcal disease.39,40 However, our data indicate that blocking cleavage of C5 by eculizumab may be an exception to other complement deficiencies and that eculizumab-treated patients may remain at risk of meningococcal disease despite vaccination. Thus, anticoagulated blood from healthy immunized adults rapidly killed all four serogroup B or C test strains. The addition of eculizumab completely inhibited killing and, compared with CFUs per milliliter in blood at time 0, the CFUs per milliliter increased >20-fold during the 3 hours of incubation for all 4 strains.
Eculizumab is specific for C5 in the terminal complement pathway and blocks complement-mediated serum bactericidal activity, which requires MAC formation. The anti-C5 mAb would not be expected to affect C3 or C4 fragments’ deposition on bacteria, as the block is distal to C3 (Figure 1). Thus the defective OPA killing of meningococci observed in the whole blood assay in the presence of eculizumab involves blocking C5a, which is a pro-inflammatory peptide. Mollnes and colleagues have shown that blocking C5aR decreases Cd11b/CR3 expression by granulocytes and monocytes,17 and impairs CR3 expression, phagocytosis and oxidative burst in whole blood incubated with meningococci4 or Escherichia coli.18 In support of the importance of C5a in OPA killing of meningococci, in the present study we found that the addition of a C5aR antagonist to blood containing a functional anti-C7 mAb gave greater impairment of meningococcal killing compared with the anti-C7 mAb alone (Figure 6).
An important caveat is that although the whole blood sepsis model incorporates the most important mechanisms by which serum antibodies confer protection against meningococcal disease, the factors affecting bacterial growth in vivo are likely to be more complex. Thus, the effects of blocking different complement pathways on bactericidal and/or phagocytic killing of meningococci in the model may not be entirely comparable to those in vivo. Nevertheless, taken together with the high incidence of meningococcal disease in vaccinated patients treated with eculizumab, our results show that eculizumab profoundly interferes with the bactericidal and OPA-mediated protection elicited by meningococcal vaccines. In contrast, blocking the AP with a small-molecule factor D inhibitor, ACH-4471, which is in phase 2 clinical trials for PNH,20 had a significantly smaller effect on impairing killing of meningococci as sterile cultures were observed in the presence of ACH-4471 for the majority of subjects, with no increases or less than 10-fold increases in CFUs per milliliter for the remaining few subjects (supplemental Tables 1 and 2). Together, these data suggest that treatment with a complement factor D inhibitor, such as ACH-4471, is likely to carry a lower risk of acquiring invasive meningococcal disease than treatment with eculizumab under the current US immunization recommendations.12
In conclusion, although treatment with eculizumab provides substantial benefit to the quality of life of patients with PNH or aHUS, the annual risk of developing meningococcal disease is ∼330 per 100 000 population compared with 0.15 per 100 000 in the general population. Furthermore, the risk in patients treated with eculizumab does not appear to be mitigated by vaccination. Our data underscore the importance of educating patients to be evaluated immediately and treated at the earliest signs of meningococcal disease, and not rely on vaccination for protection. Consideration also should be given to use of prophylactic antibiotics since the cumulative risk of meningococcal disease over 5 to 10 years is substantial. For example, eculizumab also has been used in renal transplant recipients in whom immunizations are ineffective because of absent B cells or immunosuppression (reviewed in Struijk et al15 ). In this setting, antibiotic prophylaxis has been used successfully.41
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Flor Alicia Gowans (UCSF Benioff Children’s Hospital Oakland) provided expert technical assistance. Eduardo Lujan and Elizabeth Partridge (UCSF Benioff Children’s Hospital Oakland) helped enroll healthy volunteers and obtained the blood samples. Qin Liu (Wistar Institute, Philadelphia, PA) performed the statistical analyses of the repeated measurement 1-way analysis of variance with Holm-Sidak multiple comparisons. The authors are grateful to Jason Wiles, Avinash Phadke, and Venkat Gadhachanda (Achillion Pharmaceutical, Inc) for providing ACH-4471 (supplemental Figure 1). Sanjay Ram (University of Massachusetts School of Medicine, Worcester, MA) and Manuel Galvan and Mingjun Huang (Achillion Pharmaceutical Co, New Haven, CT) provided critical review of the manuscript.
This work was supported in part by a sponsored research grant to UCSF Benioff Children’s Hospital from Achillion Pharmaceuticals, Inc (principal investigator: D.M.G.). D.M.G. was also supported by research grants R01 AI046464 and R01 AI114701 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). The laboratory work was performed in a facility funded by Research Facilities Improvement Program grant C06 RR016226 from the National Center for Research Resources, NIH.
The authors were solely responsible for the study design, data analysis, and writing of the manuscript.
Authorship
Contribution: D.M.G. designed research, analyzed data, and wrote the paper; and M.K. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: D.M.G. is an inventor on patent applications or on issued patents in the area of meningococcal vaccines. Rights to these inventions have been assigned to UCSF Benioff Children’s Hospital Oakland. M.K. declares no competing financial interests.
Correspondence: Dan M. Granoff, Center for Immunobiology and Vaccine Development, UCSF Benioff Children’s Hospital Oakland, 5700 Martin Luther King Jr Way, Oakland, CA 94609; e-mail: dgranoff@chori.org.