Abstract
In less than 2 decades, major clinical advances have been made in various areas of hematologic malignancies. Clinicians and patients now frequently face challenging choices regarding various treatments that are often similar in regard to safety or clinical effectiveness; hence, medical decision making has grown in complexity. For example, several novel drugs have been developed as oral agents, introducing an additional challenge in patient management, such as ensuring an optimal adherence to therapy in order to maximize drug effectiveness. This rapidly changing scenario provides a rationale for a more systematic collection of patient-reported outcomes (PRO) in clinical research and routine care. In the former case, PRO may help to better understand overall treatment effectiveness of a new drug being tested. In the latter case, it may aid in making more informed, individualized treatment decisions in daily practice by obtaining more accurate information on the actual symptom burden experienced by the patient. In any case, evaluating PRO requires making several, and often challenging, decisions depending, for example, on the population being studied and the specific setting. Therefore, if PROs are to fulfill their potential of generating clinically meaningful data that robustly inform patient care, special attention should be given to methodological rigor. We outline the value of a more systematic and rigorous implementation of PRO assessment in the current hematology arena, by providing some real world examples of how PRO data have contributed in better understanding the value of new therapies. We also discuss practical considerations in PRO assessment in clinical research.
Background
Hematologists have traditionally focused on laboratory research and have greatly contributed to cancer advances by developing new effective therapies that translate into better clinical outcomes for many patients. In less than 2 decades, remarkable progress has been made in various areas of hematologic diseases. The outlook for the future is encouraging with improved diagnostic methods and several novel drugs under development that hold the promise of further improving health care quality.
However, clinicians and patients now frequently face challenging choices regarding treatments that are often similar in regard to safety or clinical effectiveness, and medical decision making has become more complex. For example, several novel drugs have been developed as oral agents, introducing an additional challenge in patient management, such as ensuring optimal adherence to therapy in order to maximize clinical efficacy. Within this rapidly evolving scenario, a more systematic and methodologically sound implementation of patient-reported outcomes (PRO) assessment offers the opportunity to facilitate transition to a more patient-centered approach and to further raise standards of health care delivery.
The US Food and Drug Administration (FDA) defines PRO as a measurement of any aspect of a patient’s health status that comes directly from the patient, without the interpretation of the patient’s responses by a clinician or anyone else.1 Assessment of quality of life (QoL) and symptom burden, as reported by patients themselves, are well-known examples of key PRO frequently used in oncology.
Overview on stakeholders’ perspective on PRO
Stakeholders now highly value the role of the patients’ perspective into health care research. Major efforts have been put forth to foster a more patient-centered, developmental drug approach, in recognition of the added value that PRO information can offer to better inform clinical decisions.2 The establishment of the Patient-Centered Outcomes Research Institute (PCORI) as part of the US Patient Protection and Affordable Care Act of 20103 is an excellent example of the importance of promoting patient-centeredness in health care research. Its mission is to fund research that offers patients and caregivers the information they need to make important health care decisions.4 PCORI has issued important standards that emphasize the value of involving patients throughout the research continuum that should also guide investigators when considering funding requests.5 To illustrate, the full PCORI Methodology Report5 includes a chapter on Patient-Centeredness in which a number of standards are listed; these standards include the following: (1) Engage people representing the population of interest and other relevant stakeholders in ways that are appropriate and necessary in a given research context; (2) Identify, select, recruit, and retain study participants representative of the spectrum of the population of interest and ensure that data are collected thoroughly and systematically from all study participants; (3) Use PRO when patients or people at risk of a condition are the best source of information for outcomes of interest; and (4) Support dissemination and implementation of study results.
Improvement of patients’ QoL is now included among the strategic goals of major cancer organizations, both in Europe6 and in the United States,7 and several initiatives are ongoing at an international level to enhance PRO implementation in clinical research and routine practice. Over the last decade, for example, the US National Institutes of Health (NIH) has substantially invested in the development a various PRO measurement systems that are now available to be used across a wide spectrum of diseases.8
In the hematology area, the NIH also convened a “Patient-Centered Outcome Working Group” to specifically focus on the needs of hematopoietic stem cell transplantation (HCT) survivors that recently summarized evidence-based data to guide investigators when designing future studies in this area.9
However, the value of incorporating PRO into cancer research is fully endorsed not only by several funding agencies and research organizations but also by regulatory agencies such as the FDA,1 in the United States, or the European Medicines Agency (EMA), in Europe.10 In 2009, the FDA issued a guidance on the use of PRO in drug development and labeling,1 also addressing the importance of methodological rigor in the development of PRO instruments. More recently, the FDA also formally included PRO, in the list of Clinical Outcome Assessments that can be used to determine whether a drug has demonstrated a treatment benefit.11 The EMA also highly considers the value of PRO and has recently issued an important guidance document that covers general aspects of the use of PRO endpoints in oncology studies. This document has been included as an Appendix of the EMA Guideline on the evaluation of anticancer medicinal products in man.10 Some differences in the approach of PRO data exist between these 2 regulatory agencies, with regard to PRO labeling. For example, although the FDA is more likely to grant a PRO claim on symptoms, the EMA has often considered more general aspects such as functioning or QoL.12
Recent examples of PRO implementation in clinical trials of lymphoid malignancies
Chronic lymphocytic leukemia (CLL) is a good model for clarifying the profound impact that innovative treatments have had on patients’ outcome and survival.13,14 Despite being the most frequent leukemia in adults, CLL treatments and outcomes have seen few advances for many decades. This scenario has changed drastically during the last few years and continues to change at a sustained pace, thanks to the integration of better understanding of CLL genetics and availability of new compounds.15
There are several examples of PRO studies conducted in CLL patients that have been very informative and have contributed to better understand the burden of disease and treatment from a patient’s perspective.16-18 To illustrate, a recent randomized controlled trial (RCT) in untreated patients with CLL showed that the addition of ofatumumab to chlorambucil led to clinical improvement (progression-free survival) vs monotherapy with chlorambucil. Nevertheless, more patients in the chlorambucil plus ofatumumab group had adverse events (AEs) of any grade and serious AEs.19 In this study, QoL was used as a secondary endpoint, and results obtained were of particular value in understanding that worse toxicity did not actually translate into a worse QoL for patients treated with the addition of ofatumumab.20 The QoL analysis of this trial indicated no significant differences between arms over time, allowing the conclusion that the beneficial clinical effects of ofatumumab plus chlorambucil could be obtained without detrimental effects on patients’ QoL.
There is also recent evidence showing that PRO can be successfully implemented in patients with acute leukemia treated with novel drugs. The added value of PRO in this area has been demonstrated in a recent pivotal RCT demonstrating better overall survival (OS) for patients treated with blinatumomab over standard chemotherapy.21 In this RCT, QoL was included as a secondary endpoint, using a well-validated cancer generic questionnaire, which demonstrated better outcomes for several symptoms and functional aspects for patients treated with blinatumomab over those treated with standard chemotherapy.22 This finding is important because it adds novel information from the patients’ standpoint which, taken together with efficacy and safety findings,21 further supports the use of this drug in acute lymphoblastic leukemia patients.
Another important area in lymphoproliferative disorders where major progress has been achieved is multiple myeloma (MM). These advances mainly result from the introduction of new drugs, such as bortezomib and lenalidomide, as first-line treatments leading to improved response rates and survival outcomes.23 The number of available treatment options, also for patients with advanced disease, has substantially increased over the last few years, making treatment decisions highly challenging.24,25 Also in this area, we have seen recent important studies that are contributing to a better understanding of the QoL effects on several new treatments in patients with newly diagnosed diseases26,27 as well as in relapsed patients.28,29 For example, a recent RCT was conducted to determine the effects of carfilzomib, lenalidomide, and dexamethasone (KRD) vs lenalidomide and dexamethasone in patients with relapsed MM where QoL was included as a secondary endpoint.28 In the publication reporting clinical and efficacy data,28 progression-free survival (primary endpoint) was better for patients treated with KRD, but these patients also reported a higher rate of common AEs compared with the control group. The full QoL data analysis was presented in a separate report,29 showing that this higher rate found in some common AEs for patients treated with KRD did not result in worse patient-reported QoL outcomes. Rather, global QoL was better in patients treated with KRD, and there was no difference in patient-reported symptom severity between treatment arms.29
Recent examples of PRO implementation in clinical trials of myeloproliferative disorders
Important advances have also been achieved in the area of myeloproliferative disorders. For example, although intensive chemotherapy remains a mainstay for acute myeloid leukemia, new treatment paradigms have been recently established and others will likely emerge soon. Perhaps the most remarkable progress made in acute leukemia in recent years is the established notion that low-risk acute promyelocytic leukemia (APL) is now curable by targeted agents only (ie, without any or with minimal chemotherapy). Two large independent RCTs showed that a regimen combining arsenic trioxide (ATO) and all-trans retinoic acid is superior to standard all-trans retinoic acid and chemotherapy, resulting in better event-free survival and inferior risk of relapse. These results led to the extension of drug labeling in Europe, by the EMA, for the use of ATO in newly diagnosed, low- to intermediate-risk APL.30-32 Notably, PRO data favoring patients treated with ATO postinduction therapy in one of these trials31 were also used in the report of drug approval by the EMA.33
Myelofibrosis represents an area in which the contribution of PRO data has been most critical in determining the most effective strategies for these patients. By using a disease-specific PRO measure (ie, Myelofibrosis Symptom Assessment Form) in an RCT setting comparing ruxolitinib vs placebo, Mesa and colleagues34 demonstrated improvement over time in key symptoms, including fatigue, relative to baseline. Remarkably, these improvements were also noted in those patients who did not have a reduction of 35% of spleen volume (ie, the primary endpoint of the study).34,35 Conversely, patients treated with placebo reported worsening of myelofibrosis-related symptoms and other PRO symptoms over time. This better PRO was also supported in another trial comparing ruxolitinib vs best supportive therapy.36 The FDA approval of ruxolitinib was therefore granted, based not only on a biologic endpoint (ie, reduction in splenic volume) but also on a PRO endpoint (ie, reduction in patient-reported symptoms).37
We note that inclusion of PROs can still provide helpful information if results are negative and do not meet the initial hypothesis. For example, a recent RCT in polycythemia vera patients who had been receiving hydroxyurea tested the value of switching to ruxolitinib therapy to possibly reduce symptom burden.38 Contrary to the study hypothesis, no statistically significant difference was found between arms in the primary patient-reported symptom endpoint. Nonetheless, because this was the first study in patients with myeloproliferative neoplasms using patient-reported symptoms as primary endpoint, the authors concluded that findings provided important insights into how to best design future studies.
Outstanding clinical advances have also been made for patients with chronic myeloid leukemia (CML) since the introduction of tyrosine kinase inhibitors (TKIs). Survival of many CML patients is now approaching that of the general population.39,40 Notably, PRO data of the RCT, which led to the approval of the first TKI developed for this population (ie, imatinib), were used by the FDA in the support of drug approval.41 Since then, newer TKIs were developed, and clinical decision making has considerably grown in complexity. For example, 3 drugs (ie, imatinib, dasatinib, and nilotinib) with similar survival outcomes are available as first-line therapy, and dasatinib and nilotinib, plus another 2 (ie, bosutinb and ponatinib) can be used as second or further lines of therapy.42 However, for the large majority of CML patients, treatment is lifelong, and even low-grade AEs can substantially affect patients’ QoL and therefore influence adherence to therapy and clinical response.43
The role of PRO in AEs documentation: a paradigm shift
Traditionally, we have assessed the safety of anticancer drugs by using the National Cancer Institute’s (NCI’s) Common Terminology Criteria for Adverse Events (CTCAE) reporting system. Although the large majority of AEs in the CTCAE mirror clinical and laboratory abnormal values, such as anemia, thrombocytopenia, or neutropenia, some others AEs are meant to describe symptomatic toxicities of the patients, such as fatigue, pain, or headache. Being rather objective data, the former category of AEs is clearly independent of the person who is measuring it. However, the way the latter category of AEs is typically assessed and documented in medical files has been questioned in several empirical studies, which have indicated that clinicians may miss a large portion of patients’ symptomatic AEs in clinical trials and also may underestimate actual symptoms experienced by patients.44
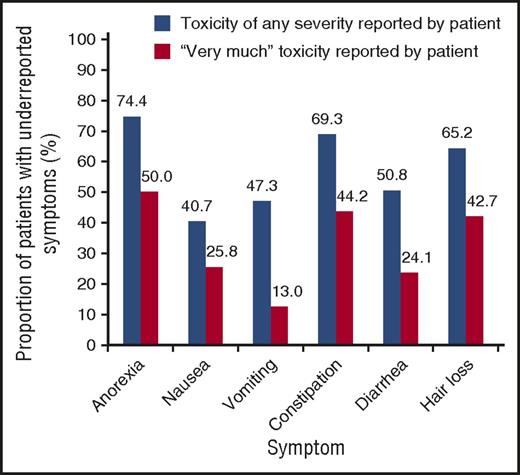
To illustrate, Figure 1 depicts the extent of underreporting treatment toxicities by clinicians in 3 large RCTs. Underestimation ranged from 74% for appetite loss to 41% for nausea.45 Data reported in Figure 1 are not stand-alone evidence and rather are a part of a wider literature documenting underreporting of patient’s symptoms by physicians.46-48
Underreporting of treatment-related toxicities by physicians, relative to patients with either advanced stage lung cancer or early-stage breast cancer. Underreporting of toxicities is defined as the proportion of patients with self-reported treatment-associated toxicities in any of the treatment cycles that were not reported at all by their physician. Data are from 3 RCT.45 Reprinted from Di Maio et al44 with permission.
Underreporting of treatment-related toxicities by physicians, relative to patients with either advanced stage lung cancer or early-stage breast cancer. Underreporting of toxicities is defined as the proportion of patients with self-reported treatment-associated toxicities in any of the treatment cycles that were not reported at all by their physician. Data are from 3 RCT.45 Reprinted from Di Maio et al44 with permission.
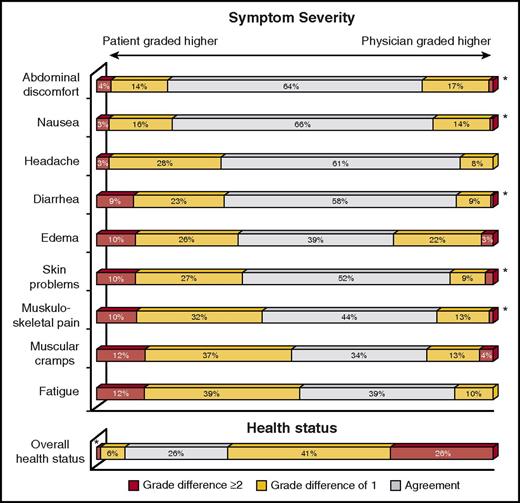
Importantly, there is also evidence indicating that physicians who have been treating the same patients for years may have a poor judgment on their patients’ symptoms and health status. Figure 2 reports the level of concordance between CML patients receiving lifelong therapy with TKIs and their treating physicians on a core set of treatment-related symptoms.49 In this real-life study, 83% of patients were in treatment with the same physician for 3 or more years. Patients reported higher severity more often than their physicians did for all symptoms considered. The 3 symptoms whose severity was most frequently underestimated by physicians were fatigue (51%), muscular cramps (49%), and musculoskeletal pain (42%). Also, health status was overestimated by physicians in 67% of the cases (Figure 2).
Level of concordance on symptoms and health status between CML patients and their treating physicians. The respondents were asked to report the severity of each symptom with the following possible answers: “not at all,” “a little,” “quite a bit,” and “very much.” Possible answers to the last issue on overall health status were as follows: “excellent,” “very good,” “good,” “fair,” and “poor.” *≤2% of cases. Obtained from the Haematologica Journal Web site (http://www.haematologica.org) and reprinted from Ferrata Storti Foundation (Pavia, Italy) with permission. Originally published in Efficace et al.49
Level of concordance on symptoms and health status between CML patients and their treating physicians. The respondents were asked to report the severity of each symptom with the following possible answers: “not at all,” “a little,” “quite a bit,” and “very much.” Possible answers to the last issue on overall health status were as follows: “excellent,” “very good,” “good,” “fair,” and “poor.” *≤2% of cases. Obtained from the Haematologica Journal Web site (http://www.haematologica.org) and reprinted from Ferrata Storti Foundation (Pavia, Italy) with permission. Originally published in Efficace et al.49
Based on compelling evidence on the discrepancy in reporting symptomatic toxicities, the US NCI initiated ∼10 years ago a large-scale project to develop a new way of recording AEs that would also incorporate the patient’s direct input.50 This massive effort has recently been finalized with the development and recent validation of the PRO-CTCAE measurement system.51
The PRO-CTACE currently consists of a library of 78 symptoms (https://healthcaredelivery.cancer.gov/pro-ctcae/item-library.pdf) and is already available in English, Danish, German, Japanese, and Spanish; several other translations will be available soon. The main goal of this measurement system, developed as a companion to the standard CTCAE, is that of increasing validity, reliability, and precision where symptomatic adverse effects are evaluated in cancer clinical trials.52 The development of the PRO-CTCAE by the NCI is a major paradigm shift in AEs documentation, because it clearly points out the need to move toward a more patient-centered approach in drug development. In a recent feasibility study53 reporting the use of this system across 9 NCI-sponsored trials, an overall compliance of 93.9% with use of the PRO-CTCAE by patients was documented, along with a 100% completion rate at baseline. The most common reason for missing PRO was institutional error (eg, staff forgetting to bring tablets to patients at visits). The large majority of patients (234 [93.2%]) found this system easy to use, and investigators also thought this approach was useful in monitoring toxicities (133 [94.3%]). Overall, this study demonstrated the feasibility of self-reporting AEs in multicenter cancer clinical trials.53
Feasibility of electronic administration of PRO-CTCAE was also demonstrated in a study of patients undergoing HCT, where the following 3 cohorts were considered: (1) Patients undergoing planned autologous HCT, (2) Patients undergoing full-intensity conditioning allogeneic HCT, and (3) Patients undergoing reduced-intensity conditioning allogeneic HCT.54 They were all given the possibility of completing an electronic version of the survey and completed it at the time of enrollment (baseline), on the first day of conditioning chemotherapy and weekly to day 100 after stem cell infusion. Weekly electronic completion of the 34 PRO-CTCAE items used required a median of 4.3 minutes to complete it. Patients in the full-intensity conditioning allogeneic HCT reported the greatest median overall symptom severity. Considering the recent development of the PRO-CTCAE, it is likely that we will see this approach more frequently implemented in the hematology arena over the coming years, to increase accuracy of documentation of drug toxicities.
Practical considerations in implementing PRO in clinical research
Inclusion of PRO in hematology is feasible and desirable in a wide range of research settings, including clinical trials,41 population-based registry studies,55 as well as survivorship research.56
However, if PROs are to fulfill their potential of generating clinically meaningful data that facilitate treatment decisions, special attention should be given to methodological rigor. A naive approach to PRO assessment will unlikely provide data that robustly informs patient care.10,57-60 Therefore, a multidisciplinary approach to PRO implementation is desirable, and specific skills might be needed. It is important that hematologists/oncologists wishing to incorporate PRO assessments in clinical studies rely on the expertise of health outcomes or PRO experts to ensure that their methodology is optimized.
Evaluating PROs in clinical research requires making several, and often challenging, decisions. Some of these decisions need to be considered at the time of protocol writing, whereas others are more relevant at the time of the final PRO analysis and outcome reporting. Many studies, mainly in the past, may have failed simply because PRO evaluation was an afterthought in the overall study design. It is not uncommon to see, even in large phase 3 RCTs, that only a few generic sentences are dedicated to PRO assessment methodology in the study protocol. This practice may seriously undermine the overall quality of PRO data collection and eventually jeopardize a study’s findings. Clearly, in some hematologic malignancies, such as acute leukemia, PRO data collection may be more challenging because of the acute course of the disease and the debilitating health conditions of patients.61,62 This challenging situation, for example, may have prevented inclusion of PRO endpoints in clinical studies, especially in those without adequate resources or lacking a logistic infrastructure able to ensure high quality of PRO data collection. Indeed, out of all the potential sources of missing data, staff oversights or other logistical/administrative failures have frequently been noted to be the main causes of poor compliance.53,63 However, more recent studies in patients with acute lymphoblastic leukemia or APL suggest that satisfactory compliance with PRO questionnaires can also be obtained from this patient population.22,31 There are also other examples in the hematology literature indicating that compliance with QoL questionnaires can be optimal in debilitated patients such as those with relapsed MM.29
Several high-quality guidelines have been published over the last 2 decades, including the newly developed CONSORT PRO recommendations,64-68 which may have helped the development of higher-quality studies in hematology in recent years. For example, PCORI has issued specific methodological standards that could help investigators when setting up patient-centered outcomes in research studies.5,69
Also, the International Society for Quality of Life Research has published recommended standards for PRO measures used in patient-centered outcomes as well as comparative effectiveness research,64,70 which can guide the development of high-quality studies in this area. Although it is beyond the scope of this article to comprehensively address all the methodological issues that point to the success of PRO studies, we note that one of the most commonly reported themes across all guidelines for the conduct and reporting of PRO studies is the problem of missing data. Indeed, they all emphasize the importance of preventing, appropriately documenting, and adequately considering statistical analyses in order to draw meaningful conclusions.
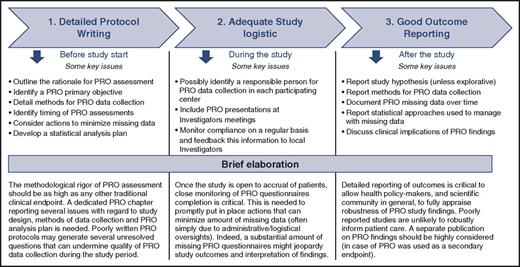
In light of the progress made in the science of PRO measurement, we advocate that specific methodological difficulties in PRO implementation should no longer be seen as overwhelming barriers by investigators wishing to include PRO in hematologic trials. Of course, other hurdles may exist (eg, lack of administrative resources, budget restrictions, logistic challenges, etc), but these are more general concerns that apply to the implementation of any other clinical or laboratory study outcome, and not specifically to PRO assessment. For illustrative purposes, and based on the above reported guidelines, in Figure 3 we outline 3 important steps that should be considered essential for the success of studies, including PRO. Failure in any of these 3 sequential steps may undermine the ultimate goal of PRO inclusion in clinical research, that is, to generate evidence that facilitates clinical decision making.
Important steps to increase likelihood that PRO findings from clinical research robustly inform patient care. This figure is not meant to provide a comprehensive list of all issues that are to be carefully considered when planning a study including patient-reported outcomes (PRO). Rather, it briefly outlines some specific issues that are to be mainly considered at different steps: from protocol writing to final publication.
Important steps to increase likelihood that PRO findings from clinical research robustly inform patient care. This figure is not meant to provide a comprehensive list of all issues that are to be carefully considered when planning a study including patient-reported outcomes (PRO). Rather, it briefly outlines some specific issues that are to be mainly considered at different steps: from protocol writing to final publication.
PRO assessment in routine practice to facilitate individual treatment decisions: an outlook on the future
Another important area of PRO application, although this represents a relatively novel area of PRO implementation, is the use of standardized questionnaires in routine care. Indeed, obtaining symptom information is a critical issue to make informed decisions in daily practice. For example, improvement in some symptoms might indicate that the therapy is effective, whereas the contrary might suggest disease progression. In both cases, some actions might be necessary to change, or temporarily discontinue, therapy. However, this information is typically collected in a nonsystematic way, and if patients do not inform us during their visits (or if we do not explicitly ask him/her), we may miss the real symptom burdens experienced by the patient.71 Also, although one might argue that clinicians can accurately capture patient’s symptoms, there is ample evidence demonstrating that patients detect symptoms sooner and with a higher severity than clinicians do.47,72 For example, one of the most notable finding in cancer research has been the evidence that PRO predicts survival outcomes independent of other known traditional prognostic markers. This evidence has been replicated across several cancer populations,73,74 including various hematologic malignancies, such as myelodysplastic syndromes,75 MM,76 lymphoma,77 or in mixed hematologic diagnoses treated for chronic graft-versus-host disease.78 These data provide a further rationale for systematically collecting PRO information in routine practice, because they might provide an early indicator of disease progression.
Therefore, there has been increasing interest over recent years in the use of PRO instruments in clinical practice. Nowadays, thanks to major progress in technology and the widespread use of the Web and Internet-connected computers, it is possible to easily implement PRO surveys in an electronic format. In many cancer institutions, electronic PRO (ePRO) systems are now typically used in clinical care and have been well described in other reports.79 For example, when patients complete ePRO questionnaires in the waiting room, easily interpretable survey results are automatically displayed in real time on the clinician’s computer, and therefore, are readily available when the patient starts the visit. This electronic administration of PRO surveys is a major advantage because it can save time during consultation, by enabling clinicians to directly focus on relevant symptoms (or other health status issues) in need of special attention. Also, this information can easily be collected via Web between visits to alert clinicians of acute needs of symptom management.79,80
Ideally, PRO data should be collected in a prospective fashion during routine visits and possibly integrated into other medical information. A substantial body of evidence has been accumulated in this area showing that integration of such tools into routine care might have several benefits, which are briefly summarized in Table 1.79,81-86
Overview of benefits found in scientific literature regarding the use of PRO data collection into routine cancer care
| Potential benefits of inclusion of PRO instruments in routine practice . |
|---|
| • Improve accuracy of symptom assessment and symptom control |
| • Improve patient-physician communication |
| • Improve patient’s QoL and satisfaction with care |
| • Save time during clinical visits |
| • Facilitate shared medical decision making |
| • Easily linking PRO information with medical data for research purposes |
| • Timely referral to supportive or palliative care services |
| Potential benefits of inclusion of PRO instruments in routine practice . |
|---|
| • Improve accuracy of symptom assessment and symptom control |
| • Improve patient-physician communication |
| • Improve patient’s QoL and satisfaction with care |
| • Save time during clinical visits |
| • Facilitate shared medical decision making |
| • Easily linking PRO information with medical data for research purposes |
| • Timely referral to supportive or palliative care services |
For example, a recent RCT in 766 patients with metastatic (solid) cancer receiving outpatient chemotherapy well illustrates the value of routine assessment of PRO in daily care.87 This study, conducted at Memorial Sloan Kettering Cancer Center in New York, compared a Web-based self-reporting of symptoms approach (experimental arm) vs usual care (control arm). In the electronic reporting system, severity was graded on a 5-point scale from 0 (not present) to 4 (disabling). Whenever a patient in the experimental group reported a worsened symptom by ≥2 or reached an absolute grade ≥3, an e-mail alert was sent to nurses. Patients randomized to use a Web-based symptom reporting system had better QoL outcomes over time, fewer emergency room visits, fewer hospitalizations, and a longer duration of palliation. Remarkably, a recent update of this RCT also demonstrated an OS advantage for ePRO patients vs those receiving usual care, with median OS being, respectively, 31.2 months (95% confidence interval, 24.5-39.6) and 26 months (95% confidence interval, 22.1-30.9), P = .03.88 As noted by the authors, 1 possible explanation for this OS difference is the early responsiveness to patient symptoms, preventing adverse downstream consequences.
Of course, challenges do exist to the successful implementation of ePRO instruments into clinical practices, and these are to be carefully considered. These challenges might relate to individual, structural, and organizational factors. For example, it is important to consider the specific organizational context where ePRO systems are to be implemented and to understand current practice. Also, it is important to consider the need of training for all parties involved, including clinical staff and patients.79,86 Robust, evidence-based data in hematology are still lacking, and this is probably due to the relatively novel implementation of PRO in this cancer area, as compared with solid tumors . Nonetheless, we advocate that time is ripe for a more systematic implementation into routine care practice. For example, many of the novel drugs are being developed as oral therapies, and considering that adherence with drug schedules is critical to maximize clinical efficacy,89,90 routine PRO assessments in daily care might be instrumental to improve our ability to early detect worrisome symptoms that could lead to non-medication-taking behaviors. Therefore, we expect to see in the near future more systematic implementations in patients with hematologic diseases to further improve patient management and outcome.
Acknowledgments
The authors are grateful to Laura Cannella and Lauren Mary Fitz for their editorial assistance.
Authorship
Contribution: All authors contributed equally to this work.
Conflict-of-interest disclosure: F.E. provided Consultancy for Bristol-Myers Squibb, Seattle Genetics, TEVA, and Incyte and received research funding from Lundbeck, TEVA, and Amgen; G.G. served on advisory boards for Jannsen, Gliead, Roche, Amgen, Morphosys, Abbvie, Karyopharm; and F.L.-C. received honoraria from Novartis, Lundbeck, and TEVA; served Membership on Board of Directors or advisory committees for Novartis and Lundbeck; and served Membership on an entity’s Board of Directors or advisory committees for TEVA.
Correspondence: Fabio Efficace, Health Outcomes Research Unit, Italian Group for Adult Hematologic Diseases (GIMEMA), GIMEMA Data Center and Health Outcomes Research Unit, Via Benevento 1, 00161 Rome, Italy; e-mail: f.efficace@gimema.it.