Key Points
Resistance to glucocorticoid treatment in pediatric T-ALL can be reversed by LCK inhibitors in vitro and in vivo.
IL-4 overexpression contributes to LCK-induced glucocorticoid resistance.
Abstract
Pediatric T-acute lymphoblastic leukemia (T-ALL) patients often display resistance to glucocorticoid (GC) treatment. These patients, classified as prednisone poor responders (PPR), have poorer outcome than do the other pediatric T-ALL patients receiving a high-risk adapted therapy. Because glucocorticoids are administered to ALL patients during all the different phases of therapy, GC resistance represents an important challenge to improving the outcome for these patients. Mechanisms underlying resistance are not yet fully unraveled; thus our research focused on the identification of deregulated signaling pathways to point out new targeted approaches. We first identified, by reverse-phase protein arrays, the lymphocyte cell-specific protein-tyrosine kinase (LCK) as aberrantly activated in PPR patients. We showed that LCK inhibitors, such as dasatinib, bosutinib, nintedanib, and WH-4-023, are able to induce cell death in GC-resistant T-ALL cells, and remarkably, cotreatment with dexamethasone is able to reverse GC resistance, even at therapeutic drug concentrations. This was confirmed by specific LCK gene silencing and ex vivo combined treatment of cells from PPR patient-derived xenografts. Moreover, we observed that LCK hyperactivation in PPR patients upregulates the calcineurin/nuclear factor of activated T cells signaling triggering to interleukin-4 (IL-4) overexpression. GC-sensitive cells cultured with IL-4 display an increased resistance to dexamethasone, whereas the inhibition of IL-4 signaling could increase GC-induced apoptosis in resistant cells. Treatment with dexamethasone and dasatinib also impaired engraftment of leukemia cells in vivo. Our results suggest a quickly actionable approach to supporting conventional therapies and overcoming GC resistance in pediatric T-ALL patients.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy of childhood that may be of B or T lineage (B-ALL or T-ALL). T-ALL accounts for approximately the 10% to 15% of cases,1 and it is historically associated with an inferior outcome in comparison with B-ALL, mainly because of an increased relapse risk, with relapsed T-ALL patients having a particularly dismal outcome.2 With contemporary risk-adapted therapy protocols, the outcome for pediatric T-ALL improved, approaching cure rates of 70%,3 but the aggressive high-dose multiagent treatment regimens are often associated with severe acute toxicities and long-term side effects.4
Pediatric T-ALL patients frequently experience chemotherapy resistance, showing a lower clearance of leukemic blasts during induction therapy than do B-ALL patients and a higher proportion of no response to the first 7 days of glucocorticoid (GC) treatment.5,6 The Associazione Italiana di Emato-Oncologia Pediatrica (AIEOP) Berlin-Frankfurt-Münster (BFM) group has traditionally used the peripheral blood (PB) blast cell count after this 7-day GC prephase to classify patients as prednisone good responders (PGR; <1 × 109/L blasts on day 8) or prednisone poor responders (PPR; ≥1 × 109/L blasts), stratifying all PPR patients to the high-risk (HR) arm of treatment protocols. Although PPR T-ALL patients are assigned to the latter protocol, they tend to have a worse prognosis than do the other pediatric T-ALL HR patients.6 Importantly, glucocorticoids are administered to ALL patients during all the different phases of therapy, making GC resistance an important challenge to be addressed to improve the outcome for these patients. Because mechanisms underlying GC resistance are not yet fully elucidated, our research focused on the identification of deregulated signaling pathways, with the aim of developing new approaches and improving therapy efficacy in GC-resistant patients.
To this end, here we performed a phosphoproteomic profiling of newly diagnosed pediatric T-ALL patients by using reverse-phase protein arrays (RPPA)7-9 and identified the lymphocyte cell-specific protein-tyrosine kinase (LCK) as hyperactivated in PPR patients. LCK is a nonreceptor protein-tyrosine kinase belonging to the Src family mostly expressed in T cells, but also in B cells, where it plays an essential role in activation and development.10,11 In this article we functionally validated LCK as a new therapeutic target to reverse GC resistance in PPR T-ALL patients by treatment, both in vitro and in vivo, with LCK small-molecule inhibitors. Our findings also revealed that LCK in these cells controls the activation of the calcineurin/nuclear factor of activated T cells (NFAT) pathway, thus triggering interleukin-4 (IL-4) overexpression, which contributes to increased GC resistance. Our results suggest a quickly translationable approach to support conventional therapy protocols, overcoming GC resistance and ameliorating the outcome of these poor-prognosis patients.
Materials and methods
Patients and cell lines
Bone marrow (BM) samples of 87 newly diagnosed pediatric T-ALL patients were analyzed in this study.12 Diagnosis was made according to standard cytomorphology, cytochemistry, and immunophenotypic criteria.13,14 Samples were collected at the Pediatric Oncohematology Laboratory (University of Padova, Italy) between 1995 and 2006 and were enrolled in the AIEOP-ALL 95 and AIEOP-BFM ALL 2000 therapy protocols. This study was approved by the local ethical committee according to institutional guidelines and Declaration of Helsinki principles. Patients’ parents or their legal guardians provided written informed consent. BM mononuclear cells were transported at room temperature in NaC anticoagulant, separated by Ficoll-Hypaque technique (Pharmacia, Sigma-Aldrich, St. Louis, MO), and stored in the BioBank in liquid nitrogen in fetal bovine serum FBS (Gibco, Thermo Fisher Scientific, Waltham, MA) + 10% dimethyl sulphoxide (DMSO) (Mylan, Canonsburg, PA). After thawing, the cell viability of blood blast cells was evaluated by Trypan blue cell count, and only samples with a viability of ≥70% were considered. The blood blast cells percentage for all samples was between 80% and 98%. The median age of patients was 8 years old; 35.7% were early-T, 31% cortical, 20.7% cortical/mature, and 12.6% mature T-ALL. Fifty-four patients (62%) were defined as PGR and 33 (38%) as PPR.
Human leukemia cell lines CCRF-CEM, Jurkat, and TALL-1 were purchased from DSMZ (Braunschweig, Germany). ALL-SIL, P12-ICHIKAWA, and KOPT-K1 cells were the kind gift of S. Indraccolo and team, who routinely authenticate cell lines by short tandem repeat profiling (Promega Corporation, Fitchburg, WI). Cells were cultured in RPMI 1640 (Gibco) with 10% (CCRF-CEM and Jurkat) or 20% (P12-ICHIKAWA, ALL-SIL, TALL-1, and KOPT-K1) FBS (Gibco), glutamine (2 mM/L; Gibco), penicillin (100 U/mL; Gibco), and streptomycin (100 mg/mL; Gibco) and were maintained at 37°C in a humidified atmosphere with 5% CO2. All cell lines were periodically tested for mycoplasma infection.
Primary xenograft (PDX) cells were cultured in minimum essential medium α (Gibco) with 10% FBS, 10% human serum (Gibco), penicillin (100 U/mL), human IL-7 (10 ng/mL; Peprotech), human stem cell factor (50 ng/mL; Peprotech, Rocky Hill, NJ), human FLT3-ligand (20 ng/mL; Peprotech), and insulin (20 nM; Sigma-Aldrich).
RPPA
In vitro treatments
T-ALL cell lines and primary samples were treated with dasatinib, bosutinib, nintedanib, and WH-4-023 (Selleckchem, Houston, TX) dissolved in DMSO. Control cells were treated with DMSO only. Dasatinib is a second-generation BCR-ABL inhibitor, and bosutinib is a third- generation BCR-ABL inhibitor that also targets LCK and Src family kinases.18-20 The triple angiokinase inhibitor nintedanib was recently clinically approved for idiopathic pulmonary fibrosis treatment and targets LCK.21,22 WH-4-023 is a high-specific and orally active LCK inhibitor.23 For 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT) and apoptosis tests, they were used at the specified concentrations alone or in combination with dexamethasone (Dex; Sigma-Aldrich).
P12-ICHIKAWA cells were treated for 1 hr with 0.01 μM of WH-4-023, the most specific LCK inhibitor, followed by 1 hr of anti-CD3 monoclonal antibody (BioLegend, San Diego, CA) and Dex for 48 hr. In a set of experiments (see Figure 5E), P12-ICHIKAWA were stimulated with anti-CD3 for 1 min after 24 hr of cell starvation and then treated for 48 hr with Dex. Mouse immunoglobulin G2A isotype (Thermo Fisher Scientific) was used as control. We could not use the other PGR cell line KOPT-K1 in these experiments, because these cells do not express surface membrane CD3.
P12-ICHIKAWA and KOPT-K1 cells were treated with 10 ng/mL of IL-4 (Peprotech) for 16 hr and then treated with Dex for 72 hr. ALL-SIL, TALL-1, and CCRF-CEM were pretreated with 100 ng/mL of anti-IL-4 neutralizing antibody (Cell Signaling) for 16 hr and then treated with Dex (10 μM for ALL-SIL and 1 μM for TALL-1 and CCRF-CEM) for 48 hr.
WB, MTT assay, annexin V/propidium iodide (PI) staining, Sybr Green Real Time PCR, and LCK specific gene silencing are described in the supplemental Methods section.
Animal experiments
Primary T-ALL cells (PD-TALL) were previously obtained from the BM of newly diagnosed pediatric patients, according to the guidelines of the local ethics committees. Xenografts' establishment and their genetic characterization are reported elsewhere.24 NSG mice were purchased from Charles River (Wilmington, MA). Procedures involving animals and their care conformed with institutional guidelines that comply with national and international laws and policies (European Economic Community Council Directive 86/609, OJ L 358, December 12, 1987) and were authorized by the Italian Ministry of Health (permission 227/2017-PR). To test the therapeutic effects on leukemia cells in vivo, we intraperitoneally injected NSG mice with Dex (5 mg/kg) or treated them by oral gavage with dasatinib PEG400/H2O (35 mg/kg) 18 days after IV injection of 5 × 106 PD-TALL40 and 11 days after IV injection of 5 × 106 PD-TALL18 leukemic cells (n = 6 mice/group). PD-TALL40 and PD-TALL18 cells derive from a 12-year-old patient and a 6-year-old patient, respectively, at diagnosis, both affected by cortical T-ALL and PPR at day 8. They also both retained high levels of minimal residual disease at day 15.25 Dasatinib, Dex, and the combination of the 2 were subsequently administered 5 days a week. Human CD7 was used to track leukemia engraftment. In all experiments, mice were inspected twice weekly to detect early signs and symptoms of leukemia, and blood was drawn to measure T-ALL cell engraftment.
Statistics
All experiments were performed at least 3 times, and data represent mean ± SEM. Statistical analyses were performed using Prism 7 (GraphPad Software, Inc., La Jolla, CA). Identification of differentially activated or expressed proteins between PGR and PPR patients was obtained through a 2-sided Mann-Whitney test or an unpaired t test with Welch’s correction when variances were unequal. Spearman’s correlation coefficient was used to determine the relationship between the variables. To determine the synergistic, additive, or antagonistic effects of the drug combinations from MTT experiments, we used CalcuSyn software, which is based on the method of the combination index (CI) of Chou and Talalay.26 To calculate these effects from annexin V/PI staining assays, we used the method “Response Additivity” described by Foucquier and Guedj.27 Synergy, additivity, and antagonism were defined by CI < 1, CI = 1, and CI > 1, respectively. The difference between untreated and treated cells was calculated using paired t test.
Results
LCK is less inhibited in PPR patients
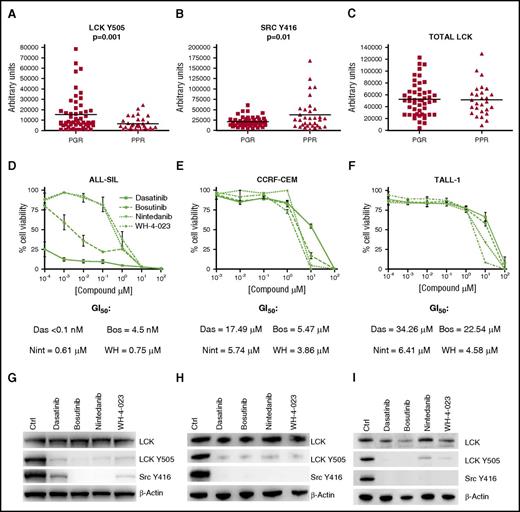
From the analysis of RPPA data, we found a higher expression of LCK phosphorylated in Y505 in PGR patients than in PPR patients (Figure 1A). The phosphorylation in Y505 brings to inhibition of the enzymatic activity of LCK, and we verified its activation through the Src family Y416 antibody staining, which was higher in PPR patients than in PGR patients (Figure 1B). Total LCK (Figure 1C) and LCK messenger RNA (mRNA) (supplemental Figure 1A) are not differentially expressed in the 2 subgroups of patients. We also looked at the glucocorticoid receptor (GR), and it is not differentially expressed between PGR and PPR patients (supplemental Figure 1B). These data suggest that in PPR patients, LCK could be aberrantly activated and thus play a role in GC resistance. To validate these findings and assess the potential role of LCK in cell survival and GC resistance, we proceeded with in vitro and in vivo experiments, as are further described.
LCK activation in PPR patients and efficacy of LCK inhibitors in decreasing cell viability of GC-resistant T-ALL cell lines. (A) LCK Y505 is downregulated in PPR (n = 33, mean 6346 ± 1089) in comparison with PGR (n = 54, mean 15 291 ± 2370) patients (Mann-Whitney t test, P = .001). (B) The active form of LCK, Src Y416, is upregulated in PPR (n = 33, mean 38 430 ± 6733) in comparison with PGR (n = 54, mean 21 120 ± 1623) patients (Mann-Whitney t test, P = .01). (C) Total form of LCK is not different between PPR (n = 27, mean 51 469 ± 5191) and PGR (n = 49, mean 52 463 ± 3977) patients. Results are presented as mean ± SD. (D-F) Cell viability in ALL-SIL, CCRF-CEM, and TALL-1 cells after 48 hr of treatment with dasatinib, bosutinib, nintedanib, and WH-4-023, measured by MTT assay (n = 3 for all experiments). Results are presented as means ± SEM. (G-I) WB analysis of total LCK, LCK Y505, and Src Y416 expression in ALL-SIL, CCRF-CEM, and TALL-1. Cells were treated with DMSO only (Ctrl), dasatinib, bosutinib, nintedanib, or WH-4-023 overnight at the compound concentrations corresponding to the GI50 at 48 hr. Bos, bosutinib; Das, dasatinib; Nint, nintedanib; WH, WH-4-023.
LCK activation in PPR patients and efficacy of LCK inhibitors in decreasing cell viability of GC-resistant T-ALL cell lines. (A) LCK Y505 is downregulated in PPR (n = 33, mean 6346 ± 1089) in comparison with PGR (n = 54, mean 15 291 ± 2370) patients (Mann-Whitney t test, P = .001). (B) The active form of LCK, Src Y416, is upregulated in PPR (n = 33, mean 38 430 ± 6733) in comparison with PGR (n = 54, mean 21 120 ± 1623) patients (Mann-Whitney t test, P = .01). (C) Total form of LCK is not different between PPR (n = 27, mean 51 469 ± 5191) and PGR (n = 49, mean 52 463 ± 3977) patients. Results are presented as mean ± SD. (D-F) Cell viability in ALL-SIL, CCRF-CEM, and TALL-1 cells after 48 hr of treatment with dasatinib, bosutinib, nintedanib, and WH-4-023, measured by MTT assay (n = 3 for all experiments). Results are presented as means ± SEM. (G-I) WB analysis of total LCK, LCK Y505, and Src Y416 expression in ALL-SIL, CCRF-CEM, and TALL-1. Cells were treated with DMSO only (Ctrl), dasatinib, bosutinib, nintedanib, or WH-4-023 overnight at the compound concentrations corresponding to the GI50 at 48 hr. Bos, bosutinib; Das, dasatinib; Nint, nintedanib; WH, WH-4-023.
LCK inhibitors alone can decrease viability in GC-resistant T-ALL cell lines
Aiming to first validate the potential role of LCK on T-ALL cell survival, we evaluated the effects of LCK inhibition in the 3 T-ALL cell lines CCRF-CEM, ALL-SIL, and TALL-1. These cells were selected because they demonstrated resistance to Dex treatment28 (supplemental Figure 2A) and had low levels of inhibited LCK (supplemental Figure 2B). Of note, GR expression did not differ between sensitive and resistant cell lines (supplemental Figure 2C). Cells were treated with the 4 LCK inhibitors dasatinib, bosutinib, nintedanib, and WH-4-023, and as is shown in Figure 1D-F, all the inhibitors were able to significantly decrease cell viability in the 3 cell lines. In particular, dasatinib and bosutinib have a strong antiproliferative activity, even at nanomolar concentrations, in ALL-SIL cells. LCK was always efficiently dephosphorylated, according to the ATP-competitive nature of the 4 tested compounds, without affecting the expression of total LCK (Figure 1G-I). The effect of the 4 LCK inhibitors was also tested on cell viability of PB mononuclear cells (PBMCs) from 4 healthy volunteers (supplemental Figure 2D). As demostrated in supplemental Figure 2E, all compounds were less toxic for healthy cells than for leukemia cells, with GI50 values higher in PBMCs than in T-ALL cell lines, apart from bosutinib, which had higher GI50 in TALL-1 cells than in PBMCs. Of note, nintedanib and WH-4-023 showed GI50 values that were about 10 times higher in PBMCs than in leukemic cell lines.
LCK inhibition reverses GC resistance in T-ALL cells
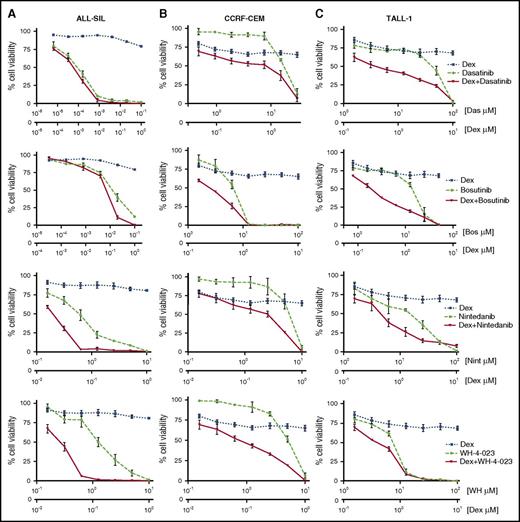
To assess whether LCK inhibition could reverse GC resistance in T-ALL cells, we treated the 3 selected cell lines with LCK inhibitors in association with Dex. Cell viability was strongly reduced by combined treatments, as is shown in Figure 2, even if the effect size of drug combinations varied depending on the cell line and the LCK inhibitor used. The synergistic activity of the 4 LCK inhibitors with Dex was confirmed by CI values that are always <1 (supplemental Table 2). The ability of LCK inhibition to reverse GC resistance was confirmed by annexin V/PI staining (Figure 3A-C; supplemental Table 3). These results strongly suggest that LCK plays a key role in inducing GC resistance in T-ALL cells, and its activity can be targeted to sensitize these cells to GC treatment.
Pharmacological inhibition of LCK reverses GC resistance in PPR T-ALL cells. (A) ALL-SIL cells were treated at the molar ratio (drug: Dex) of 0.1:1 for dasatinib, 0.1:1 for bosutinib, 10:1 for nintedanib, and 10:1 for WH-4-023. (B) CCRF-CEM cells were treated at the molar ratio (drug: Dex) of 30:1 for dasatinib, 100:1 for bosutinib, 10:1 for nintedanib, and 10:1 for WH-4-023. (C) TALL-1 cells were treated at the molar ratio (drug: Dex) of 100:1 for dasatinib, 50:1 for bosutinib, 100:1 for nintedanib, and 100:1 for WH-4-023. Cell viability was determined by MTT test after 48 hr of treatment (n = at least 3 for all experiments). Results are presented as means ± SEM.
Pharmacological inhibition of LCK reverses GC resistance in PPR T-ALL cells. (A) ALL-SIL cells were treated at the molar ratio (drug: Dex) of 0.1:1 for dasatinib, 0.1:1 for bosutinib, 10:1 for nintedanib, and 10:1 for WH-4-023. (B) CCRF-CEM cells were treated at the molar ratio (drug: Dex) of 30:1 for dasatinib, 100:1 for bosutinib, 10:1 for nintedanib, and 10:1 for WH-4-023. (C) TALL-1 cells were treated at the molar ratio (drug: Dex) of 100:1 for dasatinib, 50:1 for bosutinib, 100:1 for nintedanib, and 100:1 for WH-4-023. Cell viability was determined by MTT test after 48 hr of treatment (n = at least 3 for all experiments). Results are presented as means ± SEM.
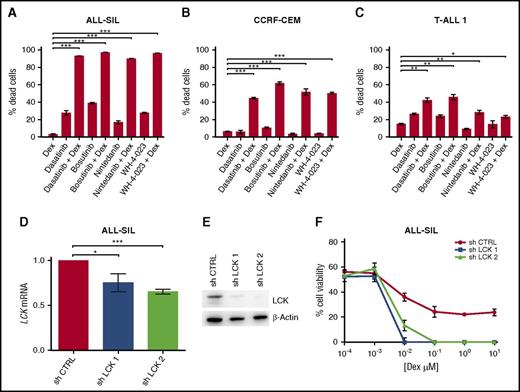
Pharmacological inhibition and specific gene silencing of LCK increases Dex-induced cell death in PPR T-ALL cells. (A-C) ALL-SIL, CCRF-CEM, and TALL-1 cells treated with Dex, dasatinib, bosutinib, nintedanib, and WH-4-023, alone or in combination. ALL-SIL cells were treated with Dex (0.1 μM), dasatinib (0.5 nM), bosutinib (20 nM), nintedanib (0.6 μM), or WH-4-023 (1.25 μM); CCRF-CEM cells were treated with Dex (0.1 μM), dasatinib (10 μM), bosutinib (6.25 μM), nintedanib (5 μM), or WH-4-023 (5 μM); T-ALL1 cells were treated with Dex (60 nM), dasatinib (25 μM), bosutinib (6.25 μM), nintedanib (12.5 μM), or WH-4-023 (6.25 μM). Cell mortality was determined by flow cytometry and annexin V/PI staining after 48 hr of treatment. The percentage of dead cells was established after normalizing cells on DMSO-treated cells. Paired t test; n = 3 for all experiments. Results are presented as means ± SEM. Dex and LCK inhibitor concentrations used in these experiments were selected on the basis of MTT test results, by choosing the ones most able to reduce cell viability in combination. (D) Inhibition of LCK mRNA expression in ALL-SIL cells after 48 hr with sh LCK 1 and sh LCK 2. LCK mRNA expression in control cells was arbitrarily set at 1. sh CTRL vs sh LCK 1, paired t test P = .016; sh CTRL vs sh LCK 2, paired t test P = .001; n = 3 for all experiments. Results are presented as means ± SEM. (E) Inhibition of LCK protein expression was examined by WB after 72 hr in control (sh CTRL) and silenced (sh LCK 1, sh LCK 2) ALL-SIL cells. (F) Cell viability after LCK silencing and 48 hr of Dex treatment in ALL-SIL cells was evaluated by MTT assay (n = 3). Results are presented as means ± SEM. *P < .05; **P < .01; ***P < .001.
Pharmacological inhibition and specific gene silencing of LCK increases Dex-induced cell death in PPR T-ALL cells. (A-C) ALL-SIL, CCRF-CEM, and TALL-1 cells treated with Dex, dasatinib, bosutinib, nintedanib, and WH-4-023, alone or in combination. ALL-SIL cells were treated with Dex (0.1 μM), dasatinib (0.5 nM), bosutinib (20 nM), nintedanib (0.6 μM), or WH-4-023 (1.25 μM); CCRF-CEM cells were treated with Dex (0.1 μM), dasatinib (10 μM), bosutinib (6.25 μM), nintedanib (5 μM), or WH-4-023 (5 μM); T-ALL1 cells were treated with Dex (60 nM), dasatinib (25 μM), bosutinib (6.25 μM), nintedanib (12.5 μM), or WH-4-023 (6.25 μM). Cell mortality was determined by flow cytometry and annexin V/PI staining after 48 hr of treatment. The percentage of dead cells was established after normalizing cells on DMSO-treated cells. Paired t test; n = 3 for all experiments. Results are presented as means ± SEM. Dex and LCK inhibitor concentrations used in these experiments were selected on the basis of MTT test results, by choosing the ones most able to reduce cell viability in combination. (D) Inhibition of LCK mRNA expression in ALL-SIL cells after 48 hr with sh LCK 1 and sh LCK 2. LCK mRNA expression in control cells was arbitrarily set at 1. sh CTRL vs sh LCK 1, paired t test P = .016; sh CTRL vs sh LCK 2, paired t test P = .001; n = 3 for all experiments. Results are presented as means ± SEM. (E) Inhibition of LCK protein expression was examined by WB after 72 hr in control (sh CTRL) and silenced (sh LCK 1, sh LCK 2) ALL-SIL cells. (F) Cell viability after LCK silencing and 48 hr of Dex treatment in ALL-SIL cells was evaluated by MTT assay (n = 3). Results are presented as means ± SEM. *P < .05; **P < .01; ***P < .001.
We also explored whether LCK inhibitors alone could affect GR expression and cellular localization. As is reported in supplemental Figure 3A, a weak but insignificant increase of GR mRNA was observed only after treatment with dasatinib, and GR translocation to the nucleus was not affected by treatment with the 4 LCK inhibitors (supplemental Figure 3B-D), thus indicating that these compounds do not directly act on GR. Moreover, overnight treatment with Dex alone did not modify LCK phosphorylation levels (supplemental Figure 3E).
To validate the specific association between LCK inhibition and GC sensitization, we suppressed LCK expression using 2 different specific short hairpin RNAs (shRNAs; sh LCK 1 and 2) in ALL-SIL cells. LCK mRNA and protein expression were efficiently reduced after lentiviral vector transduction (Figure 3D-E). As was expected, silenced ALL-SIL cells underwent more cell death than did control cells (sh CTRL) when treated with Dex only (Figure 3F). Because of resistance to lentiviral infection of CCRF-CEM cells, we performed in this cell line a transient gene silencing, introducing a specific small interfering RNA for LCK by electroporation. LCK mRNA and protein expression were efficiently reduced (supplemental Figure 4A-B), and again silenced cells showed a decrease in cell viability in comparison with control cells after Dex treatment (supplemental Figure 4C). Thus, LCK silencing sensitizes PPR cells to GC similarly to treatment with LCK inhibitors; therefore the observed effect of Dex combined with the 4 compounds can be mainly attributed to LCK deactivation.
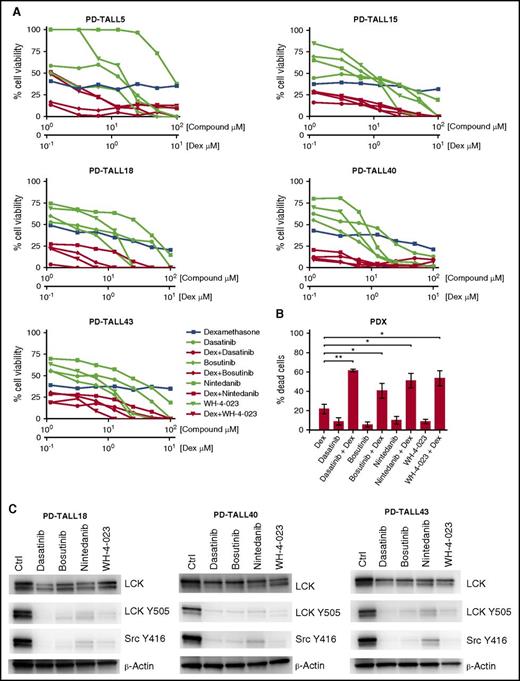
Finally, we tested the effects of dasatinib, bosutinib, nintedanib, and WH-4-023 alone or in combination with Dex in cells from 8 T-ALL patient-derived xenografts (PDXs) (5 PPR and 3 PGR) treated ex vivo. As was expected, viability of PGR PDX-derived cells was already strongly reduced by treatment with Dex alone (supplemental Figure 5A), whereas PPR PDX-derived cells were sensitized to GC treatment by the cotreatment with LCK inhibitors (Figure 4A-B; supplemental Figure 5B). Calculated CIs from both MTT (supplemental Table 4) and annexin V/PI experiments (supplemental Table 5) again demonstrated synergy between the 4 compounds and Dex. LCK dephosphorylation after treatment was verified by WB (Figure 4C).
Treatment of PPR PDX cells with LCK inhibitors and Dex. (A) Primary cells from 5 different GC-resistant PDX were isolated from mice spleen and treated ex vivo for 48 hr with the indicated concentrations of drugs in the presence of Dex at the molar ratio of 10:1. Cell viability was determined by MTT assay. (B) Primary cells isolated from 3 different PPR PDX were treated with Dex (0.1 μM) and LCK inhibitors (1 μM) alone or in combination for 48 hr. Cell mortality was determined by flow cytometry and annexin V/PI staining. The percentage of dead cells was established after normalizing cells on DMSO-treated cells. Paired t test; *P < .05, **P < .01; n = 3 for all experiments. (C) WB analysis of LCK, LCK Y505, and Src Y416 in PPR PDX cells. Cells were treated with DMSO only (Ctrl), 10 μM of dasatinib, 5 μM of bosutinib, 10 μM of nintedanib, or 10 μM of WH-4-023 for 1 hr.
Treatment of PPR PDX cells with LCK inhibitors and Dex. (A) Primary cells from 5 different GC-resistant PDX were isolated from mice spleen and treated ex vivo for 48 hr with the indicated concentrations of drugs in the presence of Dex at the molar ratio of 10:1. Cell viability was determined by MTT assay. (B) Primary cells isolated from 3 different PPR PDX were treated with Dex (0.1 μM) and LCK inhibitors (1 μM) alone or in combination for 48 hr. Cell mortality was determined by flow cytometry and annexin V/PI staining. The percentage of dead cells was established after normalizing cells on DMSO-treated cells. Paired t test; *P < .05, **P < .01; n = 3 for all experiments. (C) WB analysis of LCK, LCK Y505, and Src Y416 in PPR PDX cells. Cells were treated with DMSO only (Ctrl), 10 μM of dasatinib, 5 μM of bosutinib, 10 μM of nintedanib, or 10 μM of WH-4-023 for 1 hr.
Taken together, these data demonstrate that inhibition of LCK kinase activity reverses glucocorticoid resistance in T-ALL cells, pointing out the essential role played by LCK in GC resistance and the potential use of LCK inhibitors as an additional therapeutic strategy for PPR T-ALL pediatric patients.
LCK regulates calcineurin/NFAT signaling in PPR T-ALL cells
We next questioned which LCK downstream pathway could affect GC response. LCK can control the activation of several downstream effectors, but because we did not observe a different activation between PGR and PPR patients from RPPA analysis of RAS/MAPK, SAPK/JNK, PKC, and AKT/mTOR (data not shown), we focused our studies on the calcineurin/NFAT signaling as the critical mediator of GC resistance induced by LCK.29
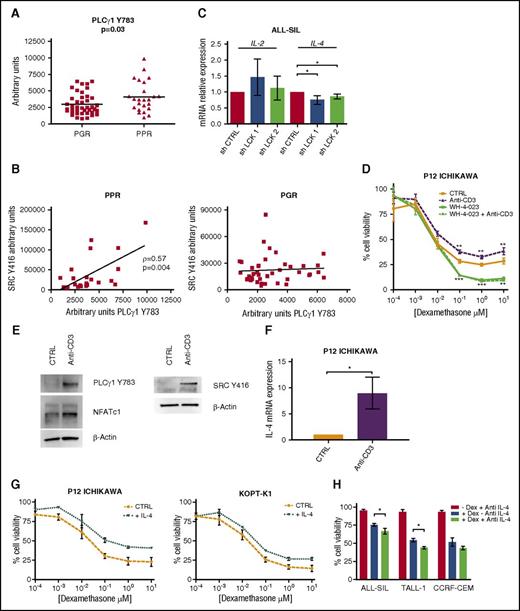
To evaluate the activation status of this pathway, we first measured by RPPA PLCγ1 phosphorylated at Y783, which was higher in PPR patients than in PGR patients (Figure 5A). We also observed a positive correlation between Src Y416 and PLCγ1 Y783 (Figure 5B, left panel) in PPR patients, whereas the activation of these 2 proteins is not correlated in PGR patients (Figure 5B, right panel). Moreover, we had available gene expression data of 73 patients,30 and we performed a gene set enrichment analysis (GSEA) that revealed a positive enrichment of NFAT pathway genes in PPR patients (supplemental Figure 6A).
The calcineurin/NFAT pathway is more active in PPR patients than in PGR patients. (A) PLCγ1 is more phosphorylated in Y783 in PPR (n = 24; mean = 4134 ± 470.7) than in PGR (n = 41; mean = 2992 ± 253.4) T-ALL patients (Mann-Whitney t test, P = .03). Results are presented as means ± SD. (B) Positive correlation (Spearman’s ρ = 0.57, P = .004) between active Src Y416 and PLCγ1 Y783 in PPR patients (left). In PGR patients, Src Y416 and PLCγ1 Y783 are not correlated (right). (C) Expression analysis by quantitative reverse transcription polymerase chain reaction (qRT-PCR) of the NFAT target genes IL-2 and IL-4 after LCK gene silencing in ALL-SIL cells. IL-4 mRNA decreases after LCK silencing (n = 3; paired t test, P = .01). Results are presented as means ± SEM. (D-F) Rescue of LCK activity in GC-sensitive T-ALL cells. (D) P12-ICHIKAWA cell viability was determined by MTT test after treatment with Dex only (48 hr), with anti-CD3 (1 hr) followed by Dex (48 hr), with WH-4-023 (0.01 μM, 1 hr) followed by Dex (48 hr), and with WH-4-023 (0.01 μM, 1 hr) followed by anti-CD3 (1 hr) and by Dex (48 hr). Cell viability of control cells was set to 100%. Results are presented as means ± SEM (n = 3). Anti-CD3 stimulation induced GC resistance in comparison with DMSO only, whereas WH-4-023 pretreatment was able to completely abrogate the anti-CD3 effect (paired t test). (E) WB analysis shows the hyperactivation of Src at Y416, PLC-γ1 at Y783, and NFATc1 in P12-ICHIKAWA cells after anti-CD3 stimulation. (F) qRT-PCR analysis of IL-4 mRNA expression in P12-ICHIKAWA cells after 48 hr of stimulation with anti-CD3 (n = 3, paired t test, P = .04). Unstimulated cells were set at 1. Results are presented as means ± SEM. (G-H) IL-4 affects GC response in T-ALL cells. (G) Increased cell viability measured by MTT assay of P12-ICHIKAWA (right) and KOPT-K1 (left) cells treated or not (CTRL) for 16 hr with IL-4 followed by Dex for 48 hr (n = 3). Cell viability of control cells was set to 100%. Results are presented as means ± SEM. (H) The anti-IL-4-neutralizing antibody increases GC-induced cell death in PPR T-ALL cell lines. Cells were treated with 100 ng/mL of blocking antibody for 16 hr prior to Dex treatment (10 μM for ALL-SIL and 1 μM for TALL-1 and CCRF-CEM, for 48 hr), and cell viability was evaluated by MTT assay, at least in triplicate. Cell viability of control cells was set to 100%. Results are presented as means ± SEM. *P < .05; **P < .01; ***P < .001.
The calcineurin/NFAT pathway is more active in PPR patients than in PGR patients. (A) PLCγ1 is more phosphorylated in Y783 in PPR (n = 24; mean = 4134 ± 470.7) than in PGR (n = 41; mean = 2992 ± 253.4) T-ALL patients (Mann-Whitney t test, P = .03). Results are presented as means ± SD. (B) Positive correlation (Spearman’s ρ = 0.57, P = .004) between active Src Y416 and PLCγ1 Y783 in PPR patients (left). In PGR patients, Src Y416 and PLCγ1 Y783 are not correlated (right). (C) Expression analysis by quantitative reverse transcription polymerase chain reaction (qRT-PCR) of the NFAT target genes IL-2 and IL-4 after LCK gene silencing in ALL-SIL cells. IL-4 mRNA decreases after LCK silencing (n = 3; paired t test, P = .01). Results are presented as means ± SEM. (D-F) Rescue of LCK activity in GC-sensitive T-ALL cells. (D) P12-ICHIKAWA cell viability was determined by MTT test after treatment with Dex only (48 hr), with anti-CD3 (1 hr) followed by Dex (48 hr), with WH-4-023 (0.01 μM, 1 hr) followed by Dex (48 hr), and with WH-4-023 (0.01 μM, 1 hr) followed by anti-CD3 (1 hr) and by Dex (48 hr). Cell viability of control cells was set to 100%. Results are presented as means ± SEM (n = 3). Anti-CD3 stimulation induced GC resistance in comparison with DMSO only, whereas WH-4-023 pretreatment was able to completely abrogate the anti-CD3 effect (paired t test). (E) WB analysis shows the hyperactivation of Src at Y416, PLC-γ1 at Y783, and NFATc1 in P12-ICHIKAWA cells after anti-CD3 stimulation. (F) qRT-PCR analysis of IL-4 mRNA expression in P12-ICHIKAWA cells after 48 hr of stimulation with anti-CD3 (n = 3, paired t test, P = .04). Unstimulated cells were set at 1. Results are presented as means ± SEM. (G-H) IL-4 affects GC response in T-ALL cells. (G) Increased cell viability measured by MTT assay of P12-ICHIKAWA (right) and KOPT-K1 (left) cells treated or not (CTRL) for 16 hr with IL-4 followed by Dex for 48 hr (n = 3). Cell viability of control cells was set to 100%. Results are presented as means ± SEM. (H) The anti-IL-4-neutralizing antibody increases GC-induced cell death in PPR T-ALL cell lines. Cells were treated with 100 ng/mL of blocking antibody for 16 hr prior to Dex treatment (10 μM for ALL-SIL and 1 μM for TALL-1 and CCRF-CEM, for 48 hr), and cell viability was evaluated by MTT assay, at least in triplicate. Cell viability of control cells was set to 100%. Results are presented as means ± SEM. *P < .05; **P < .01; ***P < .001.
Among genes regulated by NFAT activity, cytokines, chemokines, and cell surface receptors are important for T-cell activation and survival.31 Therefore, we investigated changes, after LCK specific silencing in ALL-SIL and CCRF-CEM cells, in transcriptional levels of the 2 NFAT target genes previously implicated in GC resistance: IL-2 and IL-4.32-35 We observed a significant decrease of IL-4 transcript after LCK silencing (Figure 5C for ALL-SIL; supplemental Figure 4D for CCRF-CEM), thus pointing out LCK as a critical regulator of NFAT-mediated IL-4 transcription. We also measured IL-4 mRNA expression in a cohort of 82 pediatric T-ALL patients and observed that it tends to be higher in PPR patients than in PGR patients (supplemental Figure 6B).
Hyperactivation of LCK can induce GC resistance in Dex-sensitive T-ALL cells
To confirm the key role played by LCK in GC resistance of T-ALL cells, we induced LCK hyperactivation in P12-ICHIKAWA GC-sensitive cells by T-cell receptor (TCR) stimulation with soluble anti-CD3. Cell viability was higher in anti-CD3 stimulated cells than in unstimulated cells after Dex treatment (Figure 5D). Of note, when LCK activity was inhibited with WH-4-023 prior to CD3 stimulation, the increased GC resistance observed after anti-CD3 stimulation was abrogated, demonstrating the specific contribution of LCK activation to GC resistance (Figure 5D; experimental setting described in supplemental Figure 7A-C). Moreover, LCK activation was associated with an increase in PLCγ1 Y783 levels, followed by an augmentation of the dephosphorylated active form of NFATc1 (Figure 5E) and by an increase of IL-4 transcription (Figure 5F). Thus, these results confirm the pivotal role played by LCK in inducing GC resistance and support our idea that LCK-induced GC resistance is accompanied by the activation of the calcineurin/NFAT pathway.
IL-4 affects GC response in T-ALL cells
To investigate the contribution of IL-4 overexpression in increasing GC resistance, we treated the 2 GC-sensitive T-ALL cell lines P12-ICHIKAWA and KOPT-K1 with IL-4, followed by Dex. MTT tests (Figure 5G) revealed an increased resistance to Dex in both IL-4-treated cell lines in comparison with controls, meaning that higher levels of IL-4 contribute to a diminished response to glucocorticoids in these cells. We also evaluated the effects of an anti-IL-4 neutralizing antibody on ALL-SIL, TALL-1, and CCRF-CEM viability after Dex treatment. As is shown in Figure 5I, cell death is higher when IL-4 signaling is blocked (paired t test, for ALL-SIL and TALL-1 cells, P = .01).
The important role of IL-4 in GC resistance was already reported in other cell models,32-35 and our results indicate that this interleukin is also involved in T-ALL lymphoblasts in inducing a lower response to Dex treatment. Nevertheless, the potential contribution of other LCK/calcineurin/NFAT downstream effectors to GC resistance cannot be excluded at this time.
LCK inhibition with dasatinib sensitizes PPR PDX mice to Dex treatment
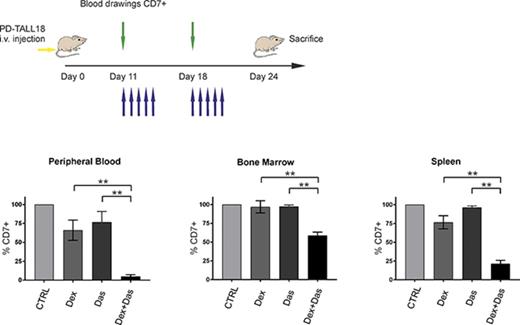
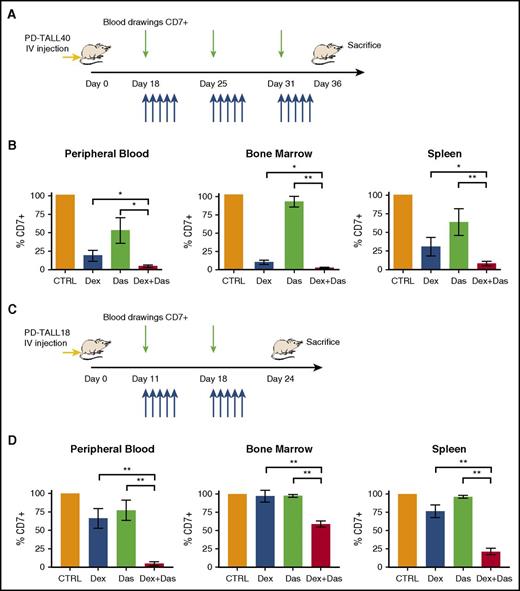
To determine in vivo the potential of LCK inhibition to reverse GC resistance, we treated NSG mice engrafted with PD-TALL40 or PD-TALL18 cells with dasatinib and Dex alone or in combination. Dasatinib was chosen because of its wide clinical use and its specificity for LCK. Administration of the drugs started when organs and blood displayed a moderate infiltration by T-ALL cells (supplemental Figure 8A-B). Mice treatments are illustrated in Figure 6A,C. Combined treatment of Dex and dasatinib significantly reduced leukemia cells in PB, BM, and spleen (Figure 6B,D) in comparison with Dex alone, thus confirming in vitro results. The effects of dasatinib in combination with Dex can be particularly appreciated in PD-TALL18 mice that are strongly resistant to Dex, and CI values revealed a clear synergy of the 2 drugs. These observations indicate that dasatinib could represent a valuable therapeutic option to reverse GC resistance in PPR T-ALL in vivo.
The pharmacological inhibition of LCK together with Dex treatment reduces T-ALL engraftment in GC-resistant PDX mice. (A) Outline of treatment with Dex and dasatinib alone and in combination, or control mice (vehicle). NSG mice (n = 6 per group) were treated intraperitoneally with Dex (5 mg/kg), by oral gavage with dasatinib (35 mg/kg), or by vehicle 18 days after IV injection of PD-TALL40 cells (5 × 106 cells/mouse). Compounds were subsequently administered daily for 3 weeks (blue arrows). Leukemia engraftment was tracked by serial blood drawings and flow cytometric analysis (green arrows). (B) Quantification of leukemia cells (CD7+) in the PB, BM, and spleen at sacrifice (day 36 from injection) by flow cytometry and human CD7 staining. Mann-Whitney t test. The average percentage of CD7+ cells in control mice was set to 100%. Results are presented as means ± SEM. (C) Outline of treatment, as described in panel A, 11 days after IV injection of PD-TALL18 cells (5 × 106 cells/mouse). Compounds were subsequently administered daily for 2 weeks (blue arrows). Leukemia engraftment was tracked by serial blood drawings and flow cytometric analysis (green arrows). (D) Quantification of leukemia cells (CD7+) in the PB, BM, and spleen at sacrifice (day 24 from injection) by flow cytometry and human CD7 staining. Mann-Whitney t test. The average percentage of CD7+ cells in control mice was set to 100%. Results are presented as means ± SEM. CIs are 0.6 (PB), 0.14 (BM), and 0.34 (spleen). *P < .05; **P < .01.
The pharmacological inhibition of LCK together with Dex treatment reduces T-ALL engraftment in GC-resistant PDX mice. (A) Outline of treatment with Dex and dasatinib alone and in combination, or control mice (vehicle). NSG mice (n = 6 per group) were treated intraperitoneally with Dex (5 mg/kg), by oral gavage with dasatinib (35 mg/kg), or by vehicle 18 days after IV injection of PD-TALL40 cells (5 × 106 cells/mouse). Compounds were subsequently administered daily for 3 weeks (blue arrows). Leukemia engraftment was tracked by serial blood drawings and flow cytometric analysis (green arrows). (B) Quantification of leukemia cells (CD7+) in the PB, BM, and spleen at sacrifice (day 36 from injection) by flow cytometry and human CD7 staining. Mann-Whitney t test. The average percentage of CD7+ cells in control mice was set to 100%. Results are presented as means ± SEM. (C) Outline of treatment, as described in panel A, 11 days after IV injection of PD-TALL18 cells (5 × 106 cells/mouse). Compounds were subsequently administered daily for 2 weeks (blue arrows). Leukemia engraftment was tracked by serial blood drawings and flow cytometric analysis (green arrows). (D) Quantification of leukemia cells (CD7+) in the PB, BM, and spleen at sacrifice (day 24 from injection) by flow cytometry and human CD7 staining. Mann-Whitney t test. The average percentage of CD7+ cells in control mice was set to 100%. Results are presented as means ± SEM. CIs are 0.6 (PB), 0.14 (BM), and 0.34 (spleen). *P < .05; **P < .01.
Discussion
T-ALL is an aggressive malignancy that accounts for about 15% of childhood ALL.1 Pediatric T-ALL patients show a dismal outcome in comparison with B-ALL, with a 25% to 30% of cases not able to ever achieve a complete remission and thus experience relapse.2 To overcome drug resistance, possibly avoiding high-dose ineffective overtreatment, we urgently need new, more specific targeted therapies. One of the first signs of drug resistance during pediatric ALL treatment following AIEOP-BFM protocols is the PB blast count after the first 7 days of steroid prephase. Regardless of a very aggressive chemotherapy that includes GC administration throughout the therapeutic regimen anyway, PPR T-ALL patients show an inferior outcome in comparison with the other HR T-ALL patients.6 In recent years, several efforts have been made to understand the basis of GC resistance in T-ALL. Beesley and colleagues36,37 reported that GC resistance is directly associated with a glycolytic phenotype, and the hyperactivation of the PI3K/AKT pathway was observed in GC-resistant T-ALL cells.38 Despite this, precise mechanisms that lead to poor response to GC in T-ALL patients are not yet fully understood.
To this aim, we performed an RPPA analysis comparing protein activation/expression in a cohort of pediatric T-ALL patients at diagnosis. Surprisingly, we did not observe an upregulation of the PI3K/AKT signaling pathway in our cohort, but we identified the hyperactivation of the nonreceptor protein-tyrosine kinase LCK in the majority of PPR patients. LCK belongs to the Src family, and it is mostly expressed in T cells where it is bound to the cytoplasmic domains of the TCR coreceptors CD4 and CD8. Following TCR engagement, LCK promotes T-cell activation, proliferation, and differentiation. Downstream activated signaling branches to several different pathways, including RAS/MAPK, JNK, AKT, and PLCγ1.39,40 LCK is positively regulated by the phosphorylation of a tyrosine (Y394) in the catalytic domain, which stabilizes an open conformation and, conversely, is negatively regulated by phosphorylation of a tyrosine residue (Y505) in the carboxy-terminal domain, which results in a closed conformation.41,42 Very interesting is that in normal T lymphocytes, LCK is part of a TCR-linked multiprotein complex together with FYN, HSP90, and GR. In the absence of the ligand, GR sustains this complex and therefore TCR signaling. After treatment with Dex this protein complex is disrupted, LCK phosphorylation and enzymatic activity reduced, and downstream signaling inhibited, thus leading to cell death. These data demonstrate that in normal T lymphocytes, LCK is the target of a recently reported “nongenomic” GR activity,43-45 contributing to immunosuppressive effects of GC treatment in T cells. In this light, in leukemia cells not responding to GC treatment, an abnormally hyperactivated LCK could sustain downstream signals and cell survival regardless of GC/GR activity. Of note, the aberrant phosphorylation of LCK was already observed in GC- resistant pediatric B-ALL patients,15 suggesting the intriguing idea of a shared mechanism responsible for GC resistance in ALL that will be worthy of further studies. Moreover, we also recently reported LCK hyperactivation in pediatric early T-cell precursor-ALL,16 a cohort of patients known to display a slow response to initial treatment, with many subjects failing to respond to GC therapy.46
To first investigate whether LCK activity is key to sustaining cell proliferation and survival in PPR T-ALL cells, we treated 3 resistant cell lines with the LCK inhibitors dasatinib, bosutinib, nintedanib, and WH-4-023. All the compounds were able to induce a marked dephosphorylation of LCK and a concomitant strong decrease of cell viability. In ALL-SIL cells, in particular, dasatinib and bosutinib were able to induce cell death, even at nanomolar concentrations. This could be explained by the presence of the NUP214-ABL1 fusion in these cells, which determines the sensitivity to ABL1 inhibitors, including dasatinib and bosutinib, strengthened by the fact that LCK kinase activity is required for proliferation and survival of NUP214-ABL1 T-ALL cells.47 These results demonstrate that LCK activation is required to sustain viability in PPR T-ALL cells. Next, to verify our hypothesis that LCK plays a crucial role in inducing GC resistance, we treated cells with the 4 LCK inhibitors together with dexamethasone. Remarkably, inhibition of LCK was able to synergize with Dex and to reverse GC resistance in all treated cell lines. Even though the combination of LCK inhibitors with Dex was always synergic in inducing the death of leukemic cells, the effect size is dependent on both cell line and LCK inhibitor administered. The ability of LCK inhibition to reverse GC resistance was confirmed by treatment of primary cells derived from 5 PPR T-ALL PDX and by specific gene-silencing experiments. Moreover, supporting our observations, the activation of LCK through TCR engagement was able to counteract GC sensitivity in PGR P12-ICHIKAWA cells.
We next questioned which pathway downstream LCK could be involved in conferring GC resistance to PPR T-ALL cells. LCK controls the activation of several downstream signaling pathways important for T-cell differentiation and survival, such as RAS/MAPK, SAPK/JNK, PKC, and AKT/mTOR,10 but none of these was found to be differentially activated in PPR T-ALL patients from our RPPA analysis. We thus focused our attention on another pathway controlled by LCK, the calcineurin/NFAT signaling, which cannot be studied by RPPA because the only way to assess calcineurin activity is to evaluate the ratio between the different forms of phosphorylated NFAT by WB. This pathway is crucial for many aspects of the immune response and is reported to be critical for the maintenance of the T-ALL phenotype.48,49 Briefly, following TCR engagement and LCK activation, recruitment of PLCγ1 mediates the induction of the calcium/calmodulin-dependent activation of calcineurin.50 Calcineurin is a serine/threonine phosphatase that is able to dephosphorylate the NFAT transcription factors, thus inducing their rapid nuclear import during which they bind specific DNA elements and regulate transcription of several target genes.51,52 Thus, we first measured PLCγ1 Y783 by RPPA, which was found to be hyperactivated and correlated to Src Y416 only in PPR patients, corroborating our hypothesis. In addition, gene expression analysis of 73 T-ALL pediatric patients performed in our laboratory for other purposes,30 followed by GSEA, revealed a positive enrichment of NFAT pathway genes in PPR patients. These data prompted us to test in our model whether LCK inhibition and the resulting sensitization to GC treatment were accompanied by the downmodulation of the calcineurin/NFAT pathway. We first measured transcription levels of the 2 well-known NFAT target genes IL-2 and IL-4 after LCK-specific gene silencing, and we observed a decrease of IL-4 mRNA in both silenced ALL-SIL and CCRF-CEM cells. Moreover, we report the increased phosphorylation of PLCγ1, the dephosphorylation of NFATc1, and the strong increase of IL-4 transcription, accompanied by an increased GC resistance following LCK activation in P12- ICHIKAWA cells. We also show that exogenous IL-4 is able to counteract the effects of Dex treatment in GC-sensitive cells, whereas an anti-IL-4-blocking antibody was able to increase Dex-induced cell death in GC-resistant cells. IL-4 was already implicated in GC resistance of normal T lymphocytes controlling, together with IL-2, GR translocation to the nucleus and transcriptional activity.32-34,53 Also in activated eosinophils, treatment with IL-2 and IL-4 upregulates PP5 activity, thus impairing phosphorylation of GR and response to GC. The synergistic effect of IL-2 and IL-4 implies that inhibiting 1 of the 2 may abrogate synergy between them and exert a therapeutic effect.35 Moreover, IL-4 resulted one of the most upregulated genes in the GC-resistant T-ALL cell line CUTLL1, and its overexpression was maintained also after Dex treatment.54 It is thus tempting to speculate that, in GC-resistant T-ALL cells, an aberrant activation of LCK brings to calcineurin/NFAT-mediated overexpression of IL-4 which, in turn, contributes to inhibition of GR function and response.
To assess the potential therapeutic role of LCK inhibition to treat PPR T-ALL patients, we generated NSG mice engrafted with T-ALL cells from 2 pediatric PPR patients and treated them with Dex and dasatinib alone or in combination. In line with what we observed in vitro, the combination of dexamethasone + dasatinib in vivo also significantly reduced leukemia engraftment, thus sensitizing these cells to GC treatment. Of note, the effect of combined treatment can be most appreciated in PD-TALL18 mice, which are highly resistant to Dex alone.
Our results give new insight into the biology of pediatric T-ALL, even if this mechanism could not be shared by all PPR patients and IL-4 could not be the only downstream effector of the LCK/NFAT pathway accounting for GC resistance.
Moreover, why LCK is hyperactivated in PPR T-ALL cells and how IL-4 contributes to impair GC response remain open questions and will be the subject of further studies.
Our study demonstrates, however, that LCK plays a pivotal role in inducing resistance to GC therapy in pediatric T-ALL patients, and this resistance can be reversed through treatment with LCK inhibitors already approved for clinical use such as dasatinib. Resistance to GC appears to be mediated downstream of LCK by the activation of the calcineurin/NFAT pathway that triggers the upregulation of IL-4. Classical immunosuppressive drugs such as cyclosporine A and FK506 target the calcineurin/NFAT pathway and could have therapeutic benefits in T-ALL treatment49,55,56 ; however, they show severe toxic effects, thus limiting their application in T-ALL therapy.52 Our research points out a new targeted therapy option using LCK inhibitors, which could be easily translated into clinical practice to overcome GC resistance and improve the outcome of poor-responder T-ALL pediatric patients.
Presented in part at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank E. Giarin (Istituto di Ricerca Pediatrica 'Città della Speranza') for assistance with the BioBank and patients’ data management. The authors are also grateful to G. Viola for constructive comments on the manuscript.
This work was supported by grants from Fondazione CARIPARO-Istituto di Ricerca Pediatrica Città della Speranza (grant 13/05) and from Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG 19186 to G.B., and IG 18803 to S.I.) and by fellowships from Fondazione Italiana per la Ricerca sul Cancro (16616) (V.S.), AIRC (18232) (M.P.), and Fondazione Umberto Veronesi (1142) (E.P.).
Authorship
Contribution: V.S. designed and performed experiments and wrote the manuscript; G.C., R.B., and E.P. performed in vitro experiments; G.M. performed RPPA experiments; S.A.M. and M.P. performed in vivo experiments and lentiviral transduction; C.F. sorted cells; S.B. performed gene expression analysis; S.I. guided in vivo experiments and reviewed the data and the manuscript; G.B. provided patient samples and clinical data, guided the research, and reviewed the data and the manuscript; B.A. designed and guided the research, analyzed and reviewed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for G.M. is the Center for Medical Genetics, Ghent University, Ghent, Belgium.
Correspondence: Benedetta Accordi, Department of Woman’s and Child’s Health, University of Padova, via Giustiniani 3, 35128 Padova, Italy; e-mail: benedetta.accordi@unipd.it.
References
Author notes
G.B. and B.A. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal