Key Points
Active infection pretransplant adversely impacts survival (81% in patients with active infection vs 95% in infection-free patients; P = .009).
Preparative chemotherapy improved 1-year post-HCT median CD4 counts (P = .02) and freedom from IV immunoglobulin (P < .001).
Abstract
The Primary Immune Deficiency Treatment Consortium (PIDTC) is enrolling children with severe combined immunodeficiency (SCID) to a prospective natural history study. We analyzed patients treated with allogeneic hematopoietic cell transplantation (HCT) from 2010 to 2014, including 68 patients with typical SCID and 32 with leaky SCID, Omenn syndrome, or reticular dysgenesis. Most (59%) patients were diagnosed by newborn screening or family history. The 2-year overall survival was 90%, but was 95% for those who were infection-free at HCT vs 81% for those with active infection (P = .009). Other factors, including the diagnosis of typical vs leaky SCID/Omenn syndrome, diagnosis via family history or newborn screening, use of preparative chemotherapy, or the type of donor used, did not impact survival. Although 1-year post-HCT median CD4 counts and freedom from IV immunoglobulin were improved after the use of preparative chemotherapy, other immunologic reconstitution parameters were not affected, and the potential for late sequelae in extremely young infants requires additional evaluation. After a T-cell–replete graft, landmark analysis at day +100 post-HCT revealed that CD3 < 300 cells/μL, CD8 < 50 cells/μL, CD45RA < 10%, or a restricted Vβ T-cell receptor repertoire (<13 of 24 families) were associated with the need for a second HCT or death. In the modern era, active infection continues to pose the greatest threat to survival for SCID patients. Although newborn screening has been effective in diagnosing SCID patients early in life, there is an urgent need to identify validated approaches through prospective trials to ensure that patients proceed to HCT infection free. The trial was registered at www.clinicaltrials.gov as #NCT01186913.
Introduction
The Primary Immune Deficiency Treatment Consortium (PIDTC) is a collaboration of pediatric immunology and hematopoietic cell transplantation (HCT) centers in the United States and Canada.1 It was organized to facilitate multi-institutional research to improve the diagnosis and definitive therapy of primary immunodeficiencies and to direct current and future approaches for management of these conditions.2 Typical SCID, associated with a near absence of T cells, is among the most severe forms of primary immunodeficiency. Patients generally do not survive beyond the first year of life without HCT, gene therapy (GT), or enzyme replacement therapy (ERT).3 Hypomorphic mutations, allowing some T-cell development, cause leaky SCID, which is characterized by severe infections and high rates of autoimmune phenomena.4 Omenn syndrome is characterized by autoreactive T cells causing a severe inflammatory state.5 Reticular dysgenesis (RD) is characterized by T-cell lymphopenia and poor function with severe neutropenia as well as sensorineural deafness.6 Until recently, SCID was diagnosed after the development of infectious complications or by prenatal/newborn testing in families with a previously affected child. In 2008, the initiation of newborn screening (NBS) for SCID allowed population-based detection of the disease with the hope of diagnosis and treatment before the development of life-threatening complications. As of 2017, 90% of US newborns were screened.7,8 HCT has been used as the treatment of SCID since 19689 and is considered the standard of care. However, most prospective North American reports of outcomes after HCT for SCID have been single-center experiences.10-12 Questions remain regarding the role of conditioning regimens, optimal alternative donor selection (when a matched sibling is unavailable), and which biomarkers in the early post-HCT period are prognostic. Since 2010, the PIDTC has enrolled patients diagnosed and treated for SCID with HCT, GT (adenosine deaminase [ADA]-SCID and X-linked SCID/IL2RG), or ERT (ADA-SCID) on the prospective Protocol 6901. We report a comprehensive evaluation of clinical and immunologic outcomes of 100 patients treated with HCT.
Methods
Patients
PIDTC 6901 is a prospective study approved by the institutional review boards of each center and performed in accordance with the Declaration of Helsinki. The trial is registered at www.clinicaltrials.gov as NCT01186913 and opened for enrollment in August 2010. Patients identified to have typical or atypical SCID underwent diagnostic evaluation and therapy per local center practice, including choice of donor source and conditioning. After signed informed consent was obtained, the pretransplant eligibility form was submitted to the PIDTC eligibility review panel, comprised of 12 clinical immunologists and transplant specialists, with 3 reviewers specifically tasked to monitor pathogenicity of RAG, IL2RG, and DCLRE1C mutations. PIDTC published definitions for typical and atypical SCID were used to characterize each patient. Briefly, patients in stratum A had typical SCID (CD3 < 300/μL or maternal engraftment present and response to phytohemagglutinin (PHA) <10% of the lower limit of normal); patients in stratum B had either: (1) leaky SCID (CD3 < 1000/μL if <2 years of age, CD3 <800/μL if 2-4 years of age, or CD3 < 600/μL if >4 years of age, without maternal engraftment, and either response to PHA 10-30% of the lower limit of normal, absent a response to Candida or post-immunization tetanus toxoid, or a pathologic mutation in a known SCID gene); (2) Omenn syndrome (generalized skin rash, PHA response <30% of the lower limit of normal, >80% T cells with the CD45RO phenotype and the absence of maternal engraftment or a genotype consistent with Omenn); or (3) RD (CD3 < 300/μL, PHA response <10% of the lower limit of normal, sensorineural deafness, and either neutrophils < 200/μL despite administration of granulocyte-colony stimulating factor or a mutation in AK2).13,14 Due to important biological differences, patients with RD were not included in the main analyses for this manuscript and are reported only descriptively. Patients who received ERT or GT were not analyzed in this report. Patients enrolled and treated with HCT at 25 centers from August 2010 through March 2014 are included in this report to allow ≥1 year of follow-up for surviving patients.
Clinical and laboratory diagnostic and post-HCT investigations
The patients’ clinical history and laboratory findings were evaluated for the presence of opportunistic infections, autoimmunity, failure to thrive, and other organ dysfunction. The history was documented at baseline, and outcomes at post-transplant day 100 and 6, 12, and 24 months were subsequently obtained. As part of the standard diagnostic work-up, patients had genotyping performed at a clinical or research laboratory. Data reports were reviewed centrally to confirm the pathologic nature of the mutation. The diagnostic and posttransplant laboratory evaluations recommended to be performed at the local centers at the time points outlined above included: lymphocyte phenotyping (CD3, CD4, CD8, CD4/CD45RA [naive T cell], CD4/CD45RO [memory T cell], CD19/20, and CD16/56), in vitro lymphocyte proliferation to PHA, and quantitative serum immunoglobulins. Transplacental maternal engraftment at baseline and posttransplant chimerism (unenriched, and T-, B-, NK, and myeloid cell lineage specific) were evaluated using short tandem repeats per local site protocol. The Center for International Blood and Marrow Transplant (CIBMTR) and PIDTC study-specific case report forms were used for data collection (supplemental Figure 1, available on the Blood Web site). Data were stored in the Data Management Coordinating Center of the Rare Disease Clinical Research Network and the CIBMTR databases.
Research laboratory investigations
Testing for T-cell receptor rearrangement excision circles (TRECs) and T-cell receptor spectratyping was performed at a PIDTC core laboratory at the same time points using methods described previously.13 Spectratyping results were scored for the number of peaks observed in each of 24 Vβ families and defined as absent, oligoclonal (1–4 peaks), or polyclonal (≥5 peaks), with normal being ≥20 polyclonal families.
Treatment details
Data on HCT, including donor type, stem cell source and processing, conditioning, and graft-versus-host disease (GVHD) prophylaxis, were recorded. Donor types included matched related donors (MRDs), T-cell–depleted mismatched-related donors (MMRDs), adult unrelated donors (URDs), and unrelated umbilical cord blood (UCB). Allele-level typing at HLA-A, -B, -C, and -DRB1 was used for URD, antigen-level typing at HLA-A and -B and allele-level typing at HLA-DRB1 were used for UCB. For analysis, the conditioning regimens were separated into 4 categories: none, immunosuppression (IS), which included serotheraphy alone or in combination with fludarabine or cyclophosphamide, reduced-intensity conditioning (RIC), and myeloablative conditioning (MAC), as defined by CIBMTR guidelines,15 with the exception that MAC regimens were defined as a busulfan dose of ≥12 mg per kg (supplemental Table 1).
Statistical analysis
Demographic, disease-related, and transplant-related variables were described with the use of frequencies for categorical variables and medians and ranges for quantitative variables. The association between variables was assessed with the use of the χ2 test or the Fisher’s exact test for categorical variables. Probabilities of OS after HCT were calculated with the use of the Kaplan-Meier estimator; data from children who were alive at the last follow-up were censored on that date. The probabilities of a second HCT (excluding nonconditioned infusions of donor cells, ie, “boosts”), acute GVHD (aGVHD), and chronic GVHD (cGVHD) were summarized with the use of a cumulative incidence method, with death considered to be a competing event. Confidence intervals (CIs) were calculated with the use of a log transformation. Univariate comparisons of survival or competing risks outcomes were conducted using the log-rank test. Immune reconstitution at 100 days and 6 and 12 months was analyzed in the group of children who were alive without a second HCT by the corresponding time point. The medians and ranges of each continuous measurement were described and compared between groups in a univariate analysis with the Kruskal-Wallis test. The log-rank test, using a landmark analysis at 100 days, was used to assess the impact of various immune reconstitution biomarkers on the risk of subsequent death or second transplant.
Results
Demographics
A total of 118 patients were enrolled in PIDTC 6901 at the time of data cutoff. Of these, 100 patients were treated with HCT, including 68 with typical SCID, 30 with leaky SCID/Omenn syndrome (Table 1), and 2 with RD (neither of whom survived). The remaining 18 patients (not in this report) included 11 treated with ERT, 6 treated with GT, and 1 SCID (with a 22q11.2 deletion syndrome) who was treated with a thymus transplant before HCT.
Demographics
| . | Overall (N = 100), n . | Stratum A, typical SCID (N = 68), n (% or range) . | Stratum B, atypical SCID (N = 32), n, (% or range) . | P . |
|---|---|---|---|---|
| Sex | <.001* | |||
| Male | 61 | 49 (72) | 12 (38) | |
| Female | 39 | 19 (28) | 20 (62) | |
| Race/ethnicity | .050* | |||
| White/non-Hispanic | 43 | 25 (37) | 18 (56) | |
| White/Hispanic | 20 | 17 (25) | 3 (9) | |
| Asian | 11 | 5 (7) | 6 (19) | |
| African American | 9 | 8 (12) | 1 (3) | |
| Other | 17 | 13 (19) | 4 (13) | |
| Age at diagnosis, d | 27 (0-1513) | 43 (0-4916) | .453‡ | |
| Diagnosis trigger | .219† | |||
| NBS/FH | 60 | 42 (62) | 18 (56) | |
| Infection | 32 | 23 (34) | 9 (28) | |
| Other Clinical Signs | 8 | 3 (4) | 5 (16) | |
| Genotype | <.001† | |||
| IL2Rg | 33 | 31 (46) | 2 (6) | |
| JAK3 | 5 | 4 (6) | 1 (3) | |
| RAG 1, 2 | 25 | 7 (11) | 18 (57) | |
| Artemis | 3 | 1 (1) | 2 (6) | |
| LIG4 | 1 | 1 (1) | 0 | |
| IL7R | 9 | 9 (14) | 0 | |
| CD3d | 3 | 3 (4) | 0 | |
| ADA | 3 | 3 (4) | 0 | |
| TTC7R | 1 | 1 (1) | 0 | |
| RMRP | 1 | 0 | 1 (3) | |
| Zap70 | 1 | 0 | 1 (3) | |
| AK2 | 2 | 0 | 2 (6) | |
| Unknown | 13 | 8 (12) | 5 (16) | |
| Baseline immunity | ||||
| Median TRECs | 65† | 0 (0-200) | 0 (0-90) | .791‡ |
| Median polyclonal Vβ families, n/24 | 41† | 1 (0-20) | 10 (2-24) | <.001‡ |
| . | Overall (N = 100), n . | Stratum A, typical SCID (N = 68), n (% or range) . | Stratum B, atypical SCID (N = 32), n, (% or range) . | P . |
|---|---|---|---|---|
| Sex | <.001* | |||
| Male | 61 | 49 (72) | 12 (38) | |
| Female | 39 | 19 (28) | 20 (62) | |
| Race/ethnicity | .050* | |||
| White/non-Hispanic | 43 | 25 (37) | 18 (56) | |
| White/Hispanic | 20 | 17 (25) | 3 (9) | |
| Asian | 11 | 5 (7) | 6 (19) | |
| African American | 9 | 8 (12) | 1 (3) | |
| Other | 17 | 13 (19) | 4 (13) | |
| Age at diagnosis, d | 27 (0-1513) | 43 (0-4916) | .453‡ | |
| Diagnosis trigger | .219† | |||
| NBS/FH | 60 | 42 (62) | 18 (56) | |
| Infection | 32 | 23 (34) | 9 (28) | |
| Other Clinical Signs | 8 | 3 (4) | 5 (16) | |
| Genotype | <.001† | |||
| IL2Rg | 33 | 31 (46) | 2 (6) | |
| JAK3 | 5 | 4 (6) | 1 (3) | |
| RAG 1, 2 | 25 | 7 (11) | 18 (57) | |
| Artemis | 3 | 1 (1) | 2 (6) | |
| LIG4 | 1 | 1 (1) | 0 | |
| IL7R | 9 | 9 (14) | 0 | |
| CD3d | 3 | 3 (4) | 0 | |
| ADA | 3 | 3 (4) | 0 | |
| TTC7R | 1 | 1 (1) | 0 | |
| RMRP | 1 | 0 | 1 (3) | |
| Zap70 | 1 | 0 | 1 (3) | |
| AK2 | 2 | 0 | 2 (6) | |
| Unknown | 13 | 8 (12) | 5 (16) | |
| Baseline immunity | ||||
| Median TRECs | 65† | 0 (0-200) | 0 (0-90) | .791‡ |
| Median polyclonal Vβ families, n/24 | 41† | 1 (0-20) | 10 (2-24) | <.001‡ |
Bold indicates statistically significant.
χ2 test.
Number of patients with a sample available for central testing.
Kruskal-Wallis test.
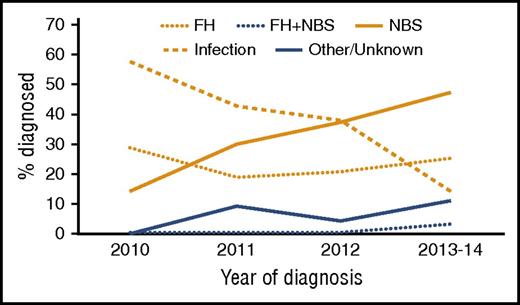
Strata A and B patients were diagnosed at median ages of 27 and 46 days, respectively (P = .337; Table 1). In the overall cohort of patients treated with HCT, 61% were male, and 43% were non-Hispanic white. A majority (59%) were diagnosed by family history and/or newborn screening (FH/NBS), rather than clinical signs (Figure 1). The median time from diagnosis to HCT was 63 days for patients diagnosed by NBS/FH (range, 14-245 days) vs 45 days (range, 4-221 days) for those diagnosed by clinical signs (p = .209). This resulted in a median age at HCT of 78 days (range, 16-251 days) vs 239 days (range, 57-5137 days) for those diagnosed by clinical signs (P < .001).
An increasing percentage of SCID patients in North America are being diagnosed due to NBS. Percentage of SCID patients diagnosed via NBS increased dramatically during the study period, while SCID diagnosis triggered by infection decreased. Rates of diagnosis due to family history or other/known factors were stable for the duration of the study period.
An increasing percentage of SCID patients in North America are being diagnosed due to NBS. Percentage of SCID patients diagnosed via NBS increased dramatically during the study period, while SCID diagnosis triggered by infection decreased. Rates of diagnosis due to family history or other/known factors were stable for the duration of the study period.
Sixty-one patients experienced pretransplant infection, and 35 of these patients had >1 infecting organism (Table 2). Similar percentages of patients presented with infection in strata A and B (34% vs 28%, P = .219). The median time from diagnosis to HCT was similar in infected patients (59 days; range, 4-245 days) vs noninfected patients (59 days; range, 16-169 days; P = .855). Almost all patients (97%) diagnosed by clinical signs had ≥1 infection before HCT, whereas 42% of those diagnosed via FH/NBS experienced ≥1 infection before HCT (P < .001), and 76% of infections seen in the FH/NBS subgroup were identified after confirmation of the SCID diagnosis, with the others already present by the time the NBS test returned positive. For patients diagnosed by FH/NBS, there was no significant difference in the median time between diagnosis and HCT in those who developed infections (77 days; range, 14-245 days) vs those without infection (60 days, range, 16-169 days) before HCT (P = .369).
Infections present before transplant
| . | Patients infected at any time before HCT, n (%) . | Patients with active infection at time of transplant, n (%) . | ||
|---|---|---|---|---|
| ≥1 Infection in all subjects (N = 98)* | 61 (62) | 37 (38) | ||
| ≥1 Infection in subgroups | ||||
| Diagnosed by NBS/FH (N = 59) | 25 (42) | 16 (27) | ||
| Diagnosed by clinical signs (N = 39) | 36 (92) | 21 (54) | ||
| <3.5 mo at time of HCT (N = 42) | 16 (38) | 7 (17) | ||
| >3.5 mo at time of HCT (N = 56) | 45 (80) | 30 (54) | ||
| Organism category | Clinical signs subgroup (N = 39), n (%) | NBS/FH subgroup (N = 59), n (%) | Clinical signs subgroup (N = 39), n (%) | NBS/FH subgroup (N = 59), n (%) |
| Staphylococcal infections | 6 (15) | 7 (12) | 1 (3) | 1 (2) |
| Gram-negative bacterial infections (Klebsiella, Pseudomonas, Escherichia, Enterobacter, Acinetobacter, Aeromonas, Branhamella/Moraxcella, Elizabethkingia, Haemophilus, Legionella, Neisseria, Salmonella, Stenotrophomonas) | 16 (41) | 4 (7) | 1 (3) | 0 |
| Clostridium difficile infection | 3 (8) | 4 (7) | 2 (5) | 3 (5) |
| Other bacterial infection | 14 (36) | 1 (2) | 1 (3) | 0 |
| Mycobacterial infection | 2 (5) | 0 | 1 (3) | 0 |
| Candida species infections | 6 (15) | 5 (8) | 0 | 0 |
| PJP Infections | 11 (28) | 0 | 1 (3) | 0 |
| Respiratory viral infections (rhinovirus, RSV, parainfluenza, influenza) | 22 (56) | 9 (15) | 10 (26) | 5 (8) |
| Double-stranded DNA viral infections (CMV, varicella, EBV, herpes simplex virus, adenovirus | 11 (28) | 10 (17) | 7 (18) | 7 (12) |
| Rotavirus infection | 1 (3) | 1 (2) | 1 (3) | 0 |
| Hepatitis B | 1 (3) | 0 | 0 | 0 |
| Parvovirus | 1 (3) | 0 | 0 | 0 |
| No organism identified, clinical signs present | 4 (10) | 0 | 0 | 0 |
| . | Patients infected at any time before HCT, n (%) . | Patients with active infection at time of transplant, n (%) . | ||
|---|---|---|---|---|
| ≥1 Infection in all subjects (N = 98)* | 61 (62) | 37 (38) | ||
| ≥1 Infection in subgroups | ||||
| Diagnosed by NBS/FH (N = 59) | 25 (42) | 16 (27) | ||
| Diagnosed by clinical signs (N = 39) | 36 (92) | 21 (54) | ||
| <3.5 mo at time of HCT (N = 42) | 16 (38) | 7 (17) | ||
| >3.5 mo at time of HCT (N = 56) | 45 (80) | 30 (54) | ||
| Organism category | Clinical signs subgroup (N = 39), n (%) | NBS/FH subgroup (N = 59), n (%) | Clinical signs subgroup (N = 39), n (%) | NBS/FH subgroup (N = 59), n (%) |
| Staphylococcal infections | 6 (15) | 7 (12) | 1 (3) | 1 (2) |
| Gram-negative bacterial infections (Klebsiella, Pseudomonas, Escherichia, Enterobacter, Acinetobacter, Aeromonas, Branhamella/Moraxcella, Elizabethkingia, Haemophilus, Legionella, Neisseria, Salmonella, Stenotrophomonas) | 16 (41) | 4 (7) | 1 (3) | 0 |
| Clostridium difficile infection | 3 (8) | 4 (7) | 2 (5) | 3 (5) |
| Other bacterial infection | 14 (36) | 1 (2) | 1 (3) | 0 |
| Mycobacterial infection | 2 (5) | 0 | 1 (3) | 0 |
| Candida species infections | 6 (15) | 5 (8) | 0 | 0 |
| PJP Infections | 11 (28) | 0 | 1 (3) | 0 |
| Respiratory viral infections (rhinovirus, RSV, parainfluenza, influenza) | 22 (56) | 9 (15) | 10 (26) | 5 (8) |
| Double-stranded DNA viral infections (CMV, varicella, EBV, herpes simplex virus, adenovirus | 11 (28) | 10 (17) | 7 (18) | 7 (12) |
| Rotavirus infection | 1 (3) | 1 (2) | 1 (3) | 0 |
| Hepatitis B | 1 (3) | 0 | 0 | 0 |
| Parvovirus | 1 (3) | 0 | 0 | 0 |
| No organism identified, clinical signs present | 4 (10) | 0 | 0 | 0 |
Patients with RD excluded.
The most common infectious complications were respiratory viral infections (n = 31), gram-negative infections (n = 20), and double-stranded DNA viral infections (n = 21) (Table 2). Many patients with double-stranded DNA (67%) or respiratory (48%) viral infections proceeded to HCT with unresolved active infection. Patients transplanted at <3.5 months of age were more commonly without active infection (66%) compared with those transplanted at >3.5 months of age (46%; P = < .001).
Autoimmunity was seen more commonly in stratum B than stratum A (27% vs 7%; P = .01) and affected 12 patients pretransplant. This included neutropenia (n = 4), thrombocytopenia (n = 2), hemolytic anemia (n = 2), hepatitis (n = 2), vitiligo (n = 1), and alopecia (n = 1). Autoimmunity was seen in 10.2% of patients diagnosed by NBS/FH vs 18.4% of patients diagnosed clinically (P = .244).
Genotypes and baseline immunity
Most patients (87%) had a genetic diagnosis (Table 1). In stratum A, IL2RG defect (46%) was the most common genetic etiology. RAG-1/2 defects were most common in stratum B (57%). Median CD3 counts were significantly higher in stratum B (405 cells/μL; range, 9–10 164 cells/μL) than stratum A (17.5 cells/μL; range, 0-8898 cells/μL; P < .001). Lymphocyte proliferation to PHA, represented as the percentage of the lower limit of normal, was also higher in stratum B (median, 17.5%; range, 0.3-100%) vs stratum A (median, 0.3%; range, 0-44%; P < .001). Strata A and B patients typically had nearly undetectable TREC levels at diagnosis (P = .791). Stratum A patients typically had absent to oligoclonal Vβ peaks (median = 1/24), whereas stratum B patients had more polyclonal T-cell receptor families (median, 10/24; P < .001).
Donor source and conditioning
Stratum A patients underwent HCT at a median age of 103 days (range, 16-1630 days) compared with stratum B patients (median age, 142 days; range, 30-5137 days; P = .037; Table 3). Only 11 patients had an MRD. In stratum A, there was an even distribution of MMRDs (29%), URDs (28%), and UCB (31%), whereas in stratum B, 80% of patients received unrelated grafts (URD, 47%; UCB, 33%), and 10% received MMRD grafts (P = .132; Table 1). The median time from diagnosis to HCT was less for recipients of either MRD (22 days; range, 4-109 days) or MMRD (30 days; range, 16-145 days) transplants compared with URD (77 days; range, 30-221 days) or UCB (62 days; range, 14-245 days) transplants (P < .001). The vast majority of stratum B patients received some degree of conditioning (MAC, 40%; RIC, 43%), compared with 53% of stratum A patients (MAC, 25%, RIC, 28%; P = .027; Table 1). The most common MAC therapy (busulfan/fludarabine/antithymocyte globulin) was used in 48% of patients; of those receiving RIC, the most common therapies (busulfan/fludarabine/antithymocyte globulin, and busulfan/cyclophosphamide) were each used in 19% of patients. All conditioning regimens, including the use of serotherapy (used in 53% of HCTs), are summarized in supplemental Table 1.
Transplant characteristics
| . | Stratum A, typical SCID (N = 68) . | Stratum B, atypical SCID (n = 30)* . | P . |
|---|---|---|---|
| Active infection at time of HCT, n (%) | 23 (34) | 14 (47) | .444 |
| Age at HCT, d, n (range) | 103 (16-1630) | 142 (30-5137) | .037 |
| Donor, n (%) | .132 | ||
| MRD | 8 (12) | 3 (10) | |
| Bone marrow | 7 (10) | 3 (10) | |
| Peripheral stem cells | 1 (2) | 0 | |
| MMRD | 20 (29) | 3 (10) | |
| Bone marrow | 9 (13) | 2 (7) | |
| Peripheral stem cells | 11 (16) | 1 (3) | |
| Adult URD | 19 (28) | 14 (47) | |
| Bone marrow | 17 (25) | 12 (40) | |
| Peripheral stem cells | 2 (3) | 2 (7) | |
| UCB | 21 (31) | 10 (33) | |
| Median stem cell dose in TNC × 107/kg (range; n) | |||
| MRD | 79.8 (27-130; 6) | 50.3 (14.4-111.7; 3) | |
| MMRD | 3.7 (1.3-233.3; 17) | 2.7 (1.9-3.6; 3) | |
| MMRD: CD34+ selection | 3.1 (2.0-4.8; 9) | 1.92 (1.92-1.92; 1) | |
| MMRD: T-cell depletion | 14.6 (1.3-233.3; 8) | 3.1 (2.7-3.6; 2) | |
| MUD | 48.2 (0.5-226; 16) | 66 (1.7-146.7; 11) | |
| UCB | 13.6 (7.8-42.1; 18) | 20 (15.9-24.8; 10) | |
| Conditioning, n (%) | .027 | ||
| None | 22 (32) | 2 (7) | |
| Immunosuppression (serotherapy) only | 10 (15) | 3 (10) | |
| RIC | 19 (28) | 13 (43) | |
| MAC (busulfan > 12 mg/kg) | 17 (25) | 12 (40) | |
| GVHD prophylaxis, n (%) | .020 | ||
| None | 5 (7) | 0 | |
| Ex vivo T-cell depletion | 23 (34) | 4 (13) | |
| Calcineurin inhibitor with or without other agents | 40 (59) | 26 (87) |
| . | Stratum A, typical SCID (N = 68) . | Stratum B, atypical SCID (n = 30)* . | P . |
|---|---|---|---|
| Active infection at time of HCT, n (%) | 23 (34) | 14 (47) | .444 |
| Age at HCT, d, n (range) | 103 (16-1630) | 142 (30-5137) | .037 |
| Donor, n (%) | .132 | ||
| MRD | 8 (12) | 3 (10) | |
| Bone marrow | 7 (10) | 3 (10) | |
| Peripheral stem cells | 1 (2) | 0 | |
| MMRD | 20 (29) | 3 (10) | |
| Bone marrow | 9 (13) | 2 (7) | |
| Peripheral stem cells | 11 (16) | 1 (3) | |
| Adult URD | 19 (28) | 14 (47) | |
| Bone marrow | 17 (25) | 12 (40) | |
| Peripheral stem cells | 2 (3) | 2 (7) | |
| UCB | 21 (31) | 10 (33) | |
| Median stem cell dose in TNC × 107/kg (range; n) | |||
| MRD | 79.8 (27-130; 6) | 50.3 (14.4-111.7; 3) | |
| MMRD | 3.7 (1.3-233.3; 17) | 2.7 (1.9-3.6; 3) | |
| MMRD: CD34+ selection | 3.1 (2.0-4.8; 9) | 1.92 (1.92-1.92; 1) | |
| MMRD: T-cell depletion | 14.6 (1.3-233.3; 8) | 3.1 (2.7-3.6; 2) | |
| MUD | 48.2 (0.5-226; 16) | 66 (1.7-146.7; 11) | |
| UCB | 13.6 (7.8-42.1; 18) | 20 (15.9-24.8; 10) | |
| Conditioning, n (%) | .027 | ||
| None | 22 (32) | 2 (7) | |
| Immunosuppression (serotherapy) only | 10 (15) | 3 (10) | |
| RIC | 19 (28) | 13 (43) | |
| MAC (busulfan > 12 mg/kg) | 17 (25) | 12 (40) | |
| GVHD prophylaxis, n (%) | .020 | ||
| None | 5 (7) | 0 | |
| Ex vivo T-cell depletion | 23 (34) | 4 (13) | |
| Calcineurin inhibitor with or without other agents | 40 (59) | 26 (87) |
Bold indicates statistically significant.
Patients with RD excluded.
Survival
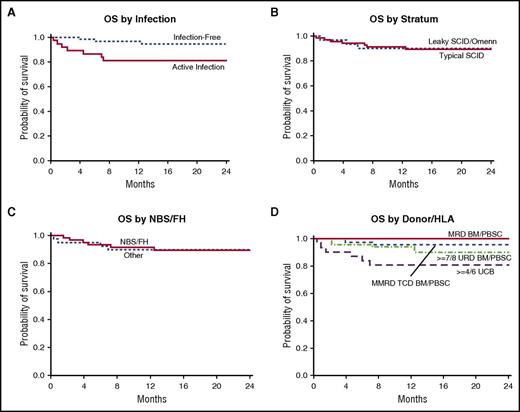
The 2-year OS of the entire cohort was 90% (95% CI, 80-95%) with a median follow-up of 25 months (range, 10–51 months). One patient died more than 2 years after HCT of unexplained encephalopathy. Infection impacted survival. Infection-free transplant patients had a 2-year OS of 95% (95% CI, 84-98%) vs 81% (95% CI, 64-90%) for transplant patients with an active infection (P = .009; Figure 2A). Nine of 11 deaths occurred in patients who had an infection present before HCT; 8 of these had an active infection at the time of HCT. Patients transplanted at <3.5 months of age (regardless of infection status) had a 2-year OS of 92% (95% CI, 78-97%) compared with 96% (95% CI, 76-99%) for patients transplanted at >3.5 months of age and infection-free at HCT, and 80% (95% CI, 61-90%) for transplant patients >3.5 months of age with an active infection (P = .036). The 2-year OS was similar for strata A and B at 89% (95% CI, 82-97%) and 90% (95% CI, 80-100%) (P = .66; Figure 2B), respectively, and for patients diagnosed by NBS/FH vs those diagnosed clinically (P = .67; Figure 2C). Survival was similar across donor sources; 2-year OS was 100% for MRD recipients, 96% for MMRD recipients, 90% for URD recipients, and 81% for UCB recipients (P = .17; Figure 2D). The use of RIC/MAC (90%; 95% CI, 79-95%) compared with no conditioning/IS (89%; 95% CI, 73-96%; P = .895) did not impact 2-year OS. Multivariate analysis of these risk factors was limited by sample size.
Survival by stratum, donor type, and infection at the time of transplant. (A) OS was significantly better in patients transplanted without infection (OS, 95%) compared with those with active infection at the time of transplant (OS, 81%; P = .009). (B) OS was similar in those presenting with typical SCID and assigned to stratum A (OS, 89%) compared with those presenting with leaky SCID or Omenn syndrome and assigned to stratum B (OS, 90%; P = .66). (C) OS was similar in those diagnosed via NBS/FH (OS, 90%) compared with those diagnosed by other means (OS, 90%; P = .67). (D) OS was not significantly different by donor type: 100% for MRD; 96% for MMRD; 90% for URD; and 81% for UCB (P = .17).
Survival by stratum, donor type, and infection at the time of transplant. (A) OS was significantly better in patients transplanted without infection (OS, 95%) compared with those with active infection at the time of transplant (OS, 81%; P = .009). (B) OS was similar in those presenting with typical SCID and assigned to stratum A (OS, 89%) compared with those presenting with leaky SCID or Omenn syndrome and assigned to stratum B (OS, 90%; P = .66). (C) OS was similar in those diagnosed via NBS/FH (OS, 90%) compared with those diagnosed by other means (OS, 90%; P = .67). (D) OS was not significantly different by donor type: 100% for MRD; 96% for MMRD; 90% for URD; and 81% for UCB (P = .17).
Risk factors for the 11 deaths in the cohort were examined (Table 4). Genotype did not significantly affect mortality, with deaths occurring in 13% of patients (5 of 38) with IL2RG/JAK3 mutations, 8% of patients (2 of 25) with RAG-1/2 mutations, 1 patient each with RMRP and LIG4 mutations, and 15% of patients (2 of 13) with unknown genotypes (P = .47). Three patients experienced aGVHD before death, and no patient experienced cGVHD. Six of 11 deaths occurred in those receiving a UCB transplant, however, comparison of survival for UCB recipients with those who received transplants from all other donor sources was not significant (P = .102). Nine of the 11 (82%) patients who died received either IS conditioning, RIC, or MAC (Table 4). For the 2 patients who died after no conditioning, the causes of death were aGVHD with respiratory failure (n = 1) and cytomegalovirus (CMV) (n = 1). Of the 9 patients who died post–IS conditioning, -RIC, or -MAC, mortality was attributable to multiple causes, including infection (n = 4; CMV, Epstein-Barr virus [EBV], respiratory syncytial virus [RSV], Pneumocystis jirovic pneumonia (PJP), Aspergillus, and polymicrobial sepsis), GVHD (n = 2), hemophagocytic lymphohistiocytosis (n = 1), unexplained encephalopathy (n = 1), and conditioning-related toxicities, such as sinusoidal obstruction syndrome (SOS; n = 2).
Clinical characteristics observed in deceased patients
| ID no. . | Genotype . | Stratum . | Time from diagnosis to HCT, d . | Age at HCT, d . | Time from HCT to death, mo . | Donor type . | Conditioning . | Infection status at time of HCT . | Post-HCT infection . | GVHD . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosed by family history or NBS | |||||||||||
| 1 | IL2RG | A | 30 | 30 | 12.4 | URD | None | Never infected | None | aGVHD | Respiratory failure |
| 2 | Unknown | A | 21 | 49 | 2.3 | MMRD | None | Active (CMV) | CMV | None | CMV |
| 3 | RAG1 | A | 83 | 124 | 1.5 | UCB | MAC | Active (rhinovirus) | Rhinovirus, adenovirus | None | SOS (with pulmonary hemorrhage, liver failure) |
| 4 | IL2RG | A | 69 | 69 | 3.9 | URD | RIC | Never infected | EBV PTLD, polymicrobial sepsis | None | EBV, sepsis, multiorgan Failure |
| 5 | RMRP | B | 85 | 113 | 4.5 | UCB | MAC | Active (VRE) | Unknown | None | SOS (respiratory failure) |
| 6 | IL2RG | A | 77 | 109 | 7.2 | URD | MAC | Active (CMV); resolved (Staphylococcus, Candida) | CMV, EBV, aspergillosis | None | Multiple infections (CMV, EBV, aspergillosis) |
| Diagnosed by infection or other clinical signs | |||||||||||
| 7 | IL2RG | A | 25 | 220 | 0.3 | UCB | IS | Active (RSV, PJP) | RSV, PJP | None | RSV, PJP |
| 8 | Unknown | B | 123 | 182 | 5.9 | UCB | RIC | Resolved (Staphylococcus, Candida) | Enterococcus faecalis sepsis | aGVHD | GVHD, sepsis |
| 9 | RAG1 | B | 62 | 114 | 0.9 | UCB | RIC | Active (CMV) | CMV | None | CMV |
| 10 | JAK3 | B | 45 | 764 | 26.5 | URD | MAC | Active (CMV) | Unknown | None | Encephalopathy |
| 11 | LIG4 | A | 117 | 1630 | 6.9 | UCB | IS | Active (Pseudomonas); resolved (Enterococcus, Candida) | Unknown | aGVHD | GVHD, HLH |
| ID no. . | Genotype . | Stratum . | Time from diagnosis to HCT, d . | Age at HCT, d . | Time from HCT to death, mo . | Donor type . | Conditioning . | Infection status at time of HCT . | Post-HCT infection . | GVHD . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosed by family history or NBS | |||||||||||
| 1 | IL2RG | A | 30 | 30 | 12.4 | URD | None | Never infected | None | aGVHD | Respiratory failure |
| 2 | Unknown | A | 21 | 49 | 2.3 | MMRD | None | Active (CMV) | CMV | None | CMV |
| 3 | RAG1 | A | 83 | 124 | 1.5 | UCB | MAC | Active (rhinovirus) | Rhinovirus, adenovirus | None | SOS (with pulmonary hemorrhage, liver failure) |
| 4 | IL2RG | A | 69 | 69 | 3.9 | URD | RIC | Never infected | EBV PTLD, polymicrobial sepsis | None | EBV, sepsis, multiorgan Failure |
| 5 | RMRP | B | 85 | 113 | 4.5 | UCB | MAC | Active (VRE) | Unknown | None | SOS (respiratory failure) |
| 6 | IL2RG | A | 77 | 109 | 7.2 | URD | MAC | Active (CMV); resolved (Staphylococcus, Candida) | CMV, EBV, aspergillosis | None | Multiple infections (CMV, EBV, aspergillosis) |
| Diagnosed by infection or other clinical signs | |||||||||||
| 7 | IL2RG | A | 25 | 220 | 0.3 | UCB | IS | Active (RSV, PJP) | RSV, PJP | None | RSV, PJP |
| 8 | Unknown | B | 123 | 182 | 5.9 | UCB | RIC | Resolved (Staphylococcus, Candida) | Enterococcus faecalis sepsis | aGVHD | GVHD, sepsis |
| 9 | RAG1 | B | 62 | 114 | 0.9 | UCB | RIC | Active (CMV) | CMV | None | CMV |
| 10 | JAK3 | B | 45 | 764 | 26.5 | URD | MAC | Active (CMV) | Unknown | None | Encephalopathy |
| 11 | LIG4 | A | 117 | 1630 | 6.9 | UCB | IS | Active (Pseudomonas); resolved (Enterococcus, Candida) | Unknown | aGVHD | GVHD, HLH |
HLH, Hemophagocytic lymphohistiocytosis; PTLD, posttransplant lymphoproliferative disease; VRE, vancomycin-resistant enterococcus.
GVHD
GVHD prophylaxis was used in the majority of patients, including ex vivo T-cell depletion alone (stratum A, 34%; stratum B, 13%) and calcineurin inhibitor with or without other therapies (stratum A, 59%; stratum B, 87%; Table 3). Grade 2 through 4 and grade 3 through 4 aGVHD occurred by day 100 in 19% (95% CI, 12-28%) and 8% (95% CI, 4-15%) of patients, respectively. cGVHD was seen in 16% (95% CI, 9-24%) of patients by 2 years and was extensive in 44% of those affected, and 94% of patients required immunosuppression to treat their cGVHD. There was no significant difference in aGVHD or cGVHD rates based on donor type (aGVHD across donor types, P = .751; cGVHD across donor types, P = .236), or conditioning (aGVHD comparing no conditioning/IS conditioning vs RIC/MAC, P = .698 and cGVHD comparing no conditioning/IS conditioning vs RIC/MAC, P = .776).
Second transplant
Nine patients required a second HCT due to graft failure/rejection (n = 7), persistent T-cell lymphopenia (n = 1), or immune dysregulation (n = 1). Seven patients survived (median follow-up, 29 months), and 2 patients died of infection. From the initial HCT, the 2-year cumulative incidence of a second HCT was lower in patients who were initially received RIC or MAC than in those who initially received no conditioning or IS conditioning (3%; 95% CI, 0-12% vs 25%; 95% CI, 11-42%; P < .001). In contrast, the donor source did not impact the rate of second transplant by 2 years: MMRD, 13% (95% CI, 3-30%); URD, 19% (95% CI, 6-36%); MRD, 12% (95% CI, 0-44%); and UCB, 0% (95% CI, not defined) (P = .14).
Immune reconstitution over the first year after HCT
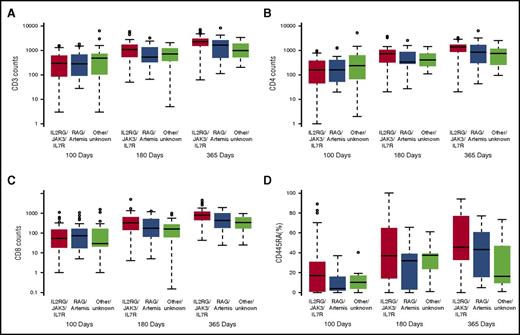
Regarding genotype influence on immune reconstitution kinetics, patients with IL2RG, JAK3, and IL7R SCID mutations had significantly higher T-cell counts and a significantly higher percentage of naive T cells (CD3, P = .013; CD4, P = .047; CD8, P = .007; CD45RA %, P = .031) at 1 year post-HCT compared with all other genotypes (Figure 3A-D). B-cell counts were lower in the RAG1/RAG2/DCLRE1C group compared with other genotypes (P = .001). Genotype did not impact on immunoglobulin independence at 1 year post-HCT, although the numbers were small.
Immune reconstitution by genotype. Patients with IL2RG, JAK3, and IL7R SCID mutations had significantly higher mean T-cell counts and percentages of naive T cells at 1 year post-HCT compared with RAG1/RAG2/Artemis or other mutations. (A) CD3, 2291 cells/μL vs 1646 cells/μL vs 982 cells/μL (P = .013). (B) CD4: 1366 cells/μL vs 826 cells/μL vs 754 cells/μL (P = .047). (C) CD8: 814 cells/μL vs 434 cells/μL vs 339 cells/μL (P = .007). (D) CD45RA: 30% vs 16% vs 12% (P = .031)
Immune reconstitution by genotype. Patients with IL2RG, JAK3, and IL7R SCID mutations had significantly higher mean T-cell counts and percentages of naive T cells at 1 year post-HCT compared with RAG1/RAG2/Artemis or other mutations. (A) CD3, 2291 cells/μL vs 1646 cells/μL vs 982 cells/μL (P = .013). (B) CD4: 1366 cells/μL vs 826 cells/μL vs 754 cells/μL (P = .047). (C) CD8: 814 cells/μL vs 434 cells/μL vs 339 cells/μL (P = .007). (D) CD45RA: 30% vs 16% vs 12% (P = .031)
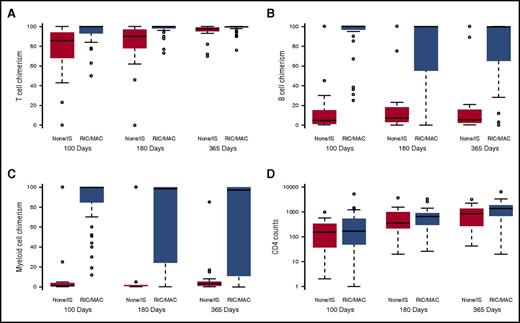
Patients who received RIC/MAC conditioning attained significantly higher levels of T-, B-, and myeloid cell (all P < .001) chimerism (Figure 4A-C) as early as 100 days post-HCT, which persisted to 1 year post-HCT. At 1 year post-HCT, conditioned patients had higher median CD4 cell counts (Figure 4D) and were more likely to be off IV immunoglobulin therapy than unconditioned patients (47% vs 14%; P = .003). Conditioning did not impact the levels of naive T cells, TRECs, or polyclonal Vβ families at 1 year post-HCT (supplemental Tables 2-4).
Immune reconstitution by conditioning regimen. (A) Patients who received conditioning (RIC or MAC) attained higher levels of T-cell chimerism at all time points measured (P ≤ .001 at 100 days, P < .001 at 180 days, and P < .001 at 365 days, respectively). (B) Patients who received conditioning (RIC or MAC) attained higher levels of B-cell chimerism at all time points measured (P ≤ .001 at 100 days, P < .001 at 180 days, P < .001 at 365 days, respectively). (C) Patients who received conditioning (RIC or MAC) attained higher levels of myeloid cell chimerism at all time points measured (P ≤ .001 at 100 days, P < .001 at 180 days, P < .001 at 365 days, respectively). (D) At 1 year post-HCT, conditioned vs nonconditioned patients had higher median CD4 cell counts (1352 cells/μL vs 855 cells/μL; P = .02).
Immune reconstitution by conditioning regimen. (A) Patients who received conditioning (RIC or MAC) attained higher levels of T-cell chimerism at all time points measured (P ≤ .001 at 100 days, P < .001 at 180 days, and P < .001 at 365 days, respectively). (B) Patients who received conditioning (RIC or MAC) attained higher levels of B-cell chimerism at all time points measured (P ≤ .001 at 100 days, P < .001 at 180 days, P < .001 at 365 days, respectively). (C) Patients who received conditioning (RIC or MAC) attained higher levels of myeloid cell chimerism at all time points measured (P ≤ .001 at 100 days, P < .001 at 180 days, P < .001 at 365 days, respectively). (D) At 1 year post-HCT, conditioned vs nonconditioned patients had higher median CD4 cell counts (1352 cells/μL vs 855 cells/μL; P = .02).
With respect to the effect of donor type on T-cell function (supplemental Tables 2-4), there were no significant differences that persisted to 1 year post-HCT. Regarding B-cell reconstitution, recipients of grafts from MRDs, adult URDs, and UCB were more likely to have donor B-cell engraftment (P < .001) and be immunoglobulin independent (P = .013) at 1 year post-HCT than MMRD graft recipients (supplemental Tables 2-4). The use of either MAC or RIC was more common in recipients of URD or UCB grafts (83%) compared with recipients of MMRD grafts (22%; P < .001), which might account for some of the difference in B-cell engraftment and immunoglobulin independence.
Finally, in a landmark analysis, we considered the predictive effect of various immunologic parameters at 100 days after T-cell–replete HCT for the risk of subsequent death or need for a second HCT. The following factors were each associated with at least a twofold increased risk of death or the need for a second HCT by 2 years post-HCT: CD3 < 300 cells/μL (25% vs 9%; P = .053), CD8 < 50 cells/μL (25% vs 9%; P = .051), CD45RA < 10% (20% vs 0%; P = .034), and polyclonal Vβ families < 13 of 24 (33% vs 11%; P = .048). There was >75% agreement between any 2 of these markers. The lack of detectable TRECs at 100 days was not associated with an increased risk of second HCT or death.
Discussion
We demonstrate that in a contemporary North American cohort, the 2-year OS rate after HCT for SCID is very good (90%). Active infection at the time of HCT continues to negatively impact survival, with a rate of 80% OS for those >3.5 months of age with an active infection at the time of HCT. However, this represents a significant improvement compared with the 50% survival rate for patients with similar characteristics from the PIDTC retrospective study of 240 SCID patients transplanted from 2000 to 2009.16 Supportive care up to and through HCT continues to improve and may partly explain the similar survival rates for those patients whose disease was detected clinically compared with those who were diagnosed via NBS/FH as well as the greatly improved survival rate of patients treated at >3.5 months of age with an active infection.
Although patients diagnosed via NBS were less likely to have an infection before HCT, 42% still developed infections before HCT. This indicates an essential need to evaluate and optimize the management of infants with suspected SCID to minimize infection exposure before HCT, which should impact the occurrence of infection. Pre-HCT eradication of viral infections remains challenging in patients with significantly compromised cellular immunity. Serial polymerase chain reaction–based monitoring for CMV may be useful before HCT, because CMV was among the most common infections seen in this subset (possibly due to maternal transmission), and although antiviral therapy is available, it is not always effective without an immune system. Pneumocystis and Candida species infections were seen less commonly in patients diagnosed via NBS/FH, suggesting that these infections are being prevented by earlier diagnosis via NBS and early administration of prophylaxis.
The time from diagnosis to treatment was similar in patients with and without active infection, however, an OS rate of 92% was seen in patients treated a <3.5 months of age, irrespective of infection status. In addition, the median time from diagnosis to transplant in the clinically diagnosed group occurred at 45 days vs 63 days in the FH/NBS group, which may be indicative of a delay in treatment, possibly while alternative donor sources were being identified or due to a reluctance to give chemotherapy before a certain age. These data suggest that early HCT remains critically important. As the prospective study continues and more patients are analyzed, it will be important to reassess the survival of patients diagnosed by NBS.
In addition, OS has limitations as an end point for patients with SCID because it does not capture quality of life or event-free survival, and additional analyses will need to be performed to determine if diagnosis by NBS helps prevents late complications, such as pulmonary, neurologic, or other sequelae from severe pre-HCT infections. Finally, one of the purposes of NBS is to detect patients who otherwise may have died of overwhelming infection without a diagnosis of SCID. With >90% of babies in the United States now being screened via NBS for SCID, there is a higher estimated population incidence of SCID.7 The rate of diagnosis via NBS improved dramatically during the study period.
The genetic identification of patients with SCID continues to improve, with 87% of patients in this cohort having a molecular diagnosis vs 69% from 2000 to 2009.16 Although patients in both strata A and B were typically diagnosed early in life, had similar rates of infection leading to HCT, and had similar 2-year OS, there were significant differences in their treatment. Stratum A patients were transplanted early in life, with a median age of <3.5 months, and there was no preferred alternative donor type, nor more common use of conditioning. In comparison, stratum B patients were transplanted at a slightly older age, more commonly received URD or UCB stem cells, and typically received some form of chemotherapy-based conditioning. It will be important to continue to assess these groups for differences in their outcomes with varying HCT donor sources and conditioning regimens, particularly because increasing numbers of stratum B patients appear to be diagnosed in the era of NBS (30% of SCID cases) compared with the 2000-2009 cohort (13% of SCID cases).16
MSD recipients have the best clinical outcomes for SCID. However, there has been considerable debate regarding optimal alternative donor sources. Our data show good survival and immune reconstitution for all alternative donor recipients; however, 6 of 11 patients who died received UCB transplants (Table 4). GVHD remains a significant issue; grade 2 to 4 aGVHD was seen in 19% of patients by day 100 and cGVHD was seen in 17% of patients by 2 years. Additional studies are needed to determine the best strategies to prevent the morbidity and mortality of GVHD in this patient population.
In our cohort, there was no significant difference in the short-term survival of patients who received chemotherapy-based conditioning (RIC/MAC) compared with those transplanted without conditioning or with IS conditioning. Nevertheless, 9 of 11 (82%) patients who died received IS conditioning, RIC, or MAC (Table 4). The use of RIC or MAC was associated with a decreased need for a second HCT and an increased likelihood of independence from immunoglobulin replacement, similar to our previous retrospective analysis.16 Some patients who underwent RIC or MAC died not only of infection, but also of GVHD and SOS, suggesting that these benefits come with potential risk. Whether lower doses of conditioning might achieve the same benefits with less toxicity should be tested in prospective trials. Careful targeting of busulfan exposure, while identifying the lowest possible effective exposure, is essential to decrease morbidity in very young infants.17,18 Consideration of genotype in the decision process regarding chemotherapy is practical now that a molecular diagnosis can be made in the majority of patients. This may lead to improved outcomes given that prior data support improved sustained immune reconstitution with the use of conditioning in certain SCID genotypes, including RAG1/RAG2/DCLRE1C and other forms of B-cell SCID.16,19 This must be tempered with the increased late effects seen in association with the conditioning used for Artemis-SCID.20 Although 1-year follow-up did not demonstrate higher CD4/CD45RA cells, TRECs, or polyclonal V-β families after the use of RIC or MAC in our overall cohort, additional long-term study is needed to see if this changes over time, because patients who received RIC or MAC did have higher CD4 counts and T-cell chimerism.
Finally, we found that patients who received a T-cell–replete graft had day 100 counts of CD3 < 300 cells/μL, CD8 < 50 cells/μL, CD45RA < 10%, and <13 of 24 TCR V-β polyclonal families were associated with a twofold higher risk of death or the need for a second HCT by 2 years post-HCT. Careful consideration of these biomarkers at day 100 may therefore identify patients who are predicted to experience poor outcomes and who may benefit from earlier preemptive consideration of a repeat HCT.
The PIDTC 6901 study has been prospectively collecting data in a standardized fashion. However, wide variation in terms of donor source selection, conditioning, GVHD prophylaxis, and pre- and post-HCT supportive care complicates analysis, because these variables can impact OS. Furthermore, although the study calls for standardized immunologic laboratory assessment, not every center obtained every recommended analysis. This somewhat limited our ability to evaluate immunologic parameters of interest at each of the identified post-HCT time points. Moreover, follow-up of these recently treated patients remains short (median, 25 months), limiting the assessment of the impact of various treatment strategies on late immunologic function and long-term sequelae. Finally, with a larger study population, the power will be greater to detect differences between genotype, graft sources, and conditioning regimens on outcomes. Future directions of the 6901 protocol include continued enrollment of newly diagnosed SCID patients, longer-term immunologic follow-up, and evaluation of late effects after HCT. Building on our current foundation of knowledge, prospective interventional trials with modified pretransplant conditioning regimens are under development to achieve the highest levels of immune function while minimizing transplant-related morbidity and mortality.
In conclusion, active infection and older age at the time of transplant continue to negatively impact survival for SCID patients, but the survival of older, actively infected patients has improved from earlier decades. Patients with both typical and leaky SCID who undergo HCT have excellent survival rates regardless of donor type. More work remains to be done to approach 100% survival.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases (NIAID), Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Public Health Service grant/cooperative agreements U54-AI082973 (principal investigator [PI]: M.J.C.), U54-NS064808 and U01-TR001263 (PI: J. P. Krischer), and R13-AI094943 (M.J.C.), and the Laboratory of Host Defenses, Division of Intramural Research, NIAID, NIH. The PIDTC is a part of the Rare Diseases Clinical Research Network of ORDR, NCATS. Collaborative work of the PIDTC with the Pediatric Blood and Marrow Transplant Consortium is supported by the U54 grants mentioned above along with support from the Pediatric Blood and Marrow Transplant Consortium Operations Center by the St. Baldrick’s Foundation and grant/cooperative agreement U10HL069254 (PI: M.A.P.) from both the National Heart, Lung and Blood Institute, and the National Cancer Institute, NIH. The collaborative work of the PIDTC with the CIBMTR is supported by: grant/cooperative agreement U24-CA76518 (PI: M.M. Horowitz) from the National Cancer Institute, National Heart, Lung and Blood Institute, and the NIAID; grant/cooperative agreement U01HL069294 from the National Heart, Lung and Blood Institute; National Cancer Institute contracts HHSH250201200016C and HHSH234200637015C with the Health Resources and Services Administration, US Department of Health and Human Services; and grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research.
The content and opinions expressed are solely the responsibility of the authors and do not represent the official policy or position of the NIAID, ORDR, NCATS, NIH, Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: J.H., B.R.L., and C.C.D. wrote the manuscript; B.R.L. and Z.Y. performed the statistical analysis; M.J.C., L.D.N., L.M.G., J.M.P., D.B.K., M.A.P., W.T.S., T.F., R.H.B., and C.C.D. developed the study as the senior leadership for PIDTC; J.H., B.R.L., M.J.C., L.D.N., L.M.G., J.M.P., S.-Y.P., R.H.B., and C.C.D. were on the Protocol 6901 Steering Committee; and J.H., M.J.C., L.D.N., L.M.G., J.M.P., D.B.K., M.A.P., S.P., C.M., N.K., R.O., M.B., S.-Y.P., F.G., L.B., S.C., M.K., M.T., J.C., G.C., B.J.D.S., E.S., A.K., K.E.S., K.D., A.G., E.H., A.P., T.Q., A.R.S., E.S., W.T.S., R.H.B., and C.C.D. contributed patients to the study and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer Heimall, Children's Hospital of Philadelphia, 34th St and Civic Center Blvd, Philadelphia, PA 19104; e-mail: heimallj@e-mail.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal