Key Points
Patients with R/R ALCL who achieved CR with brentuximab vedotin had 79% OS and 57% PFS at 5 years, with median response duration not reached.
An estimated 91% of patients who experienced peripheral neuropathy with brentuximab vedotin reported resolution or improvement of symptoms.
Abstract
This pivotal phase 2 study evaluated the safety and efficacy of brentuximab vedotin in patients with relapsed or refractory (R/R) systemic anaplastic large cell lymphoma (ALCL). After a median observation period of approximately 6 years from first treatment, we examined the durability of remission, progression-free survival (PFS), overall survival (OS), and safety outcomes of patients treated on this trial. Among all enrolled patients (n = 58), no progressions were observed beyond 40 months, and median OS was not reached. Patients with a complete response (CR), as assessed by the investigator (38 of 58, 66%), continued to demonstrate improved outcomes with neither median OS nor PFS reached. Of the 38 CR patients, 16 received a consolidative stem cell transplant (SCT) with median PFS not reached. Among patients who were on-study and in remission at study closure, 16 patients had not received any new treatment after single-agent brentuximab vedotin other than consolidative SCT. Among this subset of 16 patients, 8 received SCT, and the remaining 8 patients (14% of all enrolled patients) remained in sustained remission without consolidative SCT or any new anticancer therapy. Thirty-three patients experienced peripheral neuropathy, among whom, the majority (30 of 33, 91%) had experienced resolution or improvement at their last assessment. These final results, which demonstrated a high rate of peripheral neuropathy resolution, and durable remissions in a subset of patients with relapsed or refractory systemic ALCL, provide evidence that single-agent brentuximab vedotin may be a potentially curative treatment option. This trial was registered at www.clinicaltrials.gov as #NCT00866047.
Introduction
Systemic anaplastic large cell lymphoma (ALCL) is a subtype of the peripheral T-cell lymphomas (PTCLs) characterized by the strong and uniform expression of CD30. Approximately 40% to 65% of patients with systemic ALCL experience recurrent disease after frontline treatment.1 Among patients who receive chemotherapy at first relapse, median progression-free survival (PFS) and overall survival (OS) are just 1.8 months and 3 months, respectively.2 High-dose chemotherapy with autologous stem cell transplantation (SCT) is standard therapy for patients with recurrent or refractory disease and is associated with a 5-year PFS rate of up to 56%.3,4 Considering the toxicity-related mortality rate is as high as 33%,5 the role of myeloablative allogeneic SCT is less clear.
Systemic ALCL is further classified by the expression of the anaplastic lymphoma kinase (ALK) protein on the surface of malignant T cells. In most studies, patients with ALK+ ALCL have fared better compared with patients with ALK– disease, with estimated 5-year OS rates of 79% and 38%, respectively. However, the majority of patients with ALK+ ALCL and a high International Prognostic Index score at diagnosis will experience relapse after initial therapies.6
Brentuximab vedotin is an antibody-drug conjugate composed of an anti-CD30 chimeric antibody conjugated to the microtubule-disrupting agent, monomethyl auristatin E. Targeted delivery of monomethyl auristatin E to CD30-expressing tumor cells is the primary mechanism of action, whereas additional proposed mechanisms of tumor cell killing that may contribute to the clinical activity of brentuximab vedotin include antibody-dependent cellular phagocytosis, immunogenic cell death, and the bystander effect.7-12
The objective response rate (ORR) on this pivotal trial among patients with relapsed or refractory systemic ALCL treated with single-agent brentuximab vedotin was 86% per independent review, with 57% of patients achieving a complete response (CR).13 Response rates according to the investigator were comparable, with 86% of patients experiencing an objective response and 66% achieving CR. In this final report, together with results regarding peripheral neuropathy incidence and resolution, we present end-of-study survival and durability outcomes after 5 years of long-term follow-up (LTFU) assessments per the investigator.
Patients and methods
Study design
This single-arm, open-label, multicenter, phase 2 study evaluated the efficacy and safety of brentuximab vedotin as a single agent in patients with relapsed or refractory systemic ALCL (#NCT00866047). Patients were enrolled between June 2009 and May 2010. Data presented in this report extend until study closure, which occurred approximately 5 years from the last patient’s end-of-treatment visit. The clinical trial was conducted at 22 sites in the United States, Canada, and Europe. The study was approved by the institutional review board at each study site, and all patients provided written informed consent. A complete description of the study design has been previously reported.13
Patients
Patients on this study had histologically confirmed CD30-expressing systemic ALCL with documented ALK status. Patients were required to have fluorodeoxyglucose-avid disease by positron emission tomography (PET) and measurable disease of at least 1.5 cm by spiral computed tomography (CT), and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. At US and Canadian sites, patients were 12 years or older and were at least 18 years old at European sites. Patients must not have received prior treatment with brentuximab vedotin or an allogeneic SCT.
Study treatment and assessments
Brentuximab vedotin (1.8 mg/kg) was administered to patients every 3 weeks as a 30-minute outpatient intravenous infusion for up to 16 cycles. CT scans were performed at baseline and at cycles 2, 4, 7, 10, 13, and 16; PET scans were done at baseline and at cycles 4 and 7. Tumor response and disease progression were assessed according to the Revised Response Criteria for Malignant Lymphoma.14 Follow-up for long-term survival and assessment of disease status after the end of treatment occurred every 3 months for 2 years, then every 6 months through 5 years, and annually thereafter. Patients who discontinued study drug for any response other than disease progression or initiation of a nonprotocol therapy for treatment of lymphoma were also assessed on this schedule for progression.
During LTFU, progression was assessed per independent central review of patient CT scans and clinically by the investigator. Evaluations occurred on this schedule until ∼2 years into the follow-up period, at which time the requirement for routine radiographic assessment of progression was removed from the study protocol. Central assessment of disease progression ended after 2 years, whereas investigator assessment of disease status continued throughout the follow-up period, with CT scans performed if progression were clinically suspected. Patients who experienced peripheral neuropathy were monitored for symptom improvement or resolution until ∼2 years into LTFU.
Statistical analysis
The primary endpoint of the study was the ORR per independent review, defined as the proportion of patients with CR or partial response (PR). Secondary endpoints included duration of response, CR rate, PFS per independent review, OS, and incidence and severity of adverse events. These endpoints have been previously described and reported.13 In this present report, we present investigator assessments of response duration and PFS, both prespecified exploratory analyses in the statistical analysis plan.
Response duration was calculated from the first objective tumor response (CR or PR) to tumor progression or death from any cause, and PFS was calculated from the start of treatment to tumor progression or death. Duration of response and PFS per investigator were censored either on the day after the date of the last radiological assessment of measured lesions documenting absence of progressive disease or the last clinical assessment where no progression was identified, whichever was later. Patients who received a new anticancer therapy before documentation of progression, with the exception of consolidative autologous or allogeneic SCT, were censored at their most recent prior assessment that documented the absence of progressive disease. OS was calculated from the start of study treatment to the date of death from any cause and was censored at the last date when the patient was known to be alive. We estimated duration of response, PFS, and OS using Kaplan-Meier methodology; we also calculated median and 5-year point estimates, as well as the corresponding 2-sided 95% confidence intervals (CIs).
We performed an analysis using the standard Medical Dictionary for Regulatory Activities (version 13.0) to identify peripheral neuropathy events. Grade of severity was determined per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.
Results
Survival and durability outcomes
Overall and by best response.
Baseline characteristics for this patient population have been previously summarized.13 In brief, 58 patients (33 men, 25 women) were enrolled with a median age of 52 years (age range, 14-76 years). Sixteen patients (28%) had ALK+ disease and 42 patients (72%) had ALK– disease. Relative to their most recent prior therapy, 29 patients (50%) experienced relapse, and 29 (50%) had refractory disease. Thirty-six patients (62%) had primary refractory ALCL (failure to achieve CR or relapse within 3 months of frontline therapy), and 13 patients (22%) never responded (achieved CR or PR) to prior therapies. A median of 16.8 months had passed from the time of initial diagnosis to patients’ first dose of brentuximab vedotin. Overall, patients were observed for a median of 71.4 months (range, 0.8-82.4 months), or nearly 6 years. Median follow-up time from end of treatment was 58.4 months (range, 0-78 months) overall. Median follow-up time for patients who achieved an objective response was 5.3 years and was the same among patients who achieved a CR (5.3 years).
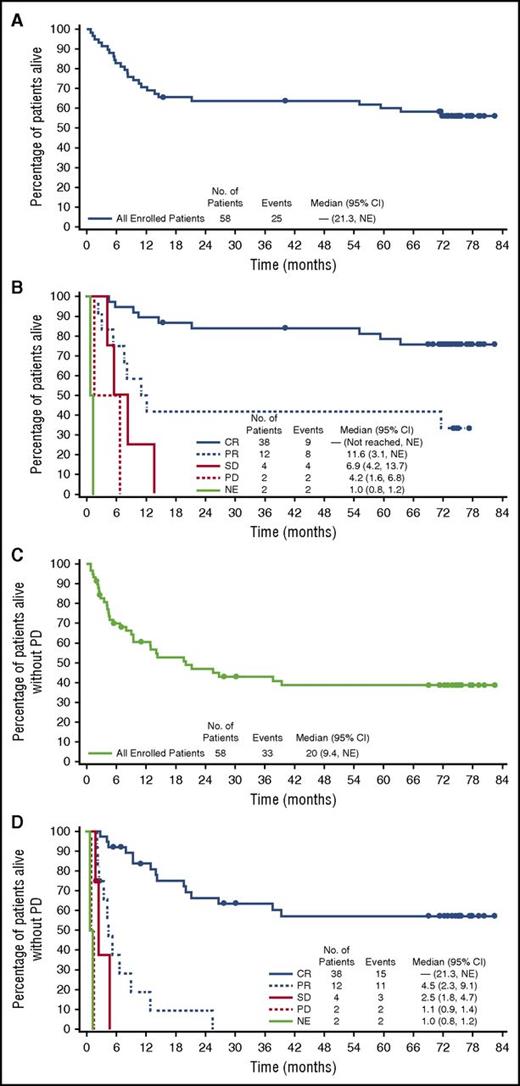
The estimated 5-year survival rate overall was 60% (95% CI, 47%-73%) with median OS not reached (95% CI, 21.3, not estimable [NE]) (Figure 1A). Patients who achieved a best response of CR (38 of 58, 66%) fared better with an estimated 5-year OS rate of 79% (95% CI, 65%-92%), with median OS not reached (endpoints of 95% CI not estimable) (Figure 1B). The 5-year OS rate for patients who did not achieve a CR was 25% (95% CI, 6%-44%). The estimated 5-year PFS rate for all enrolled patients was 39% (95% CI, 25%-52%), with a median of 20 months (95% CI, 9.4%, NE) (Figure 1C). Among CR patients, the median PFS rate was not reached (95% CI, 21.3%, NE); at 5 years, the estimated rate was 57% (95% CI, 41%-74%) (Figure 1D). Of the 50 patients with an objective response per the investigator, the median response duration was 25.6 months (95% CI, 11.8, NE [range, 0.9-79.7+]) and was not reached in the 38 patients with a CR (95% CI, 20.0, NE [range, 0.9-79.7+]). Investigator-assessed response rates, durability results, and OS are summarized in Table 1. Survival duration for all patients who achieved a CR is provided in supplemental Figure 1, available on the Blood Web site.
End-of-study OS and PFS in patients with relapsed or refractory systemic ALCL treated with brentuximab vedotin. OS and PFS per investigator assessment were analyzed using Kaplan-Meier methodology. Median observation time was 6 years from the start of treatment. Patients were observed for a median of 5 years after the end of treatment. (A) OS for all patients. (B) OS by best response. (C) PFS for all patients. (D) PFS by best response.
End-of-study OS and PFS in patients with relapsed or refractory systemic ALCL treated with brentuximab vedotin. OS and PFS per investigator assessment were analyzed using Kaplan-Meier methodology. Median observation time was 6 years from the start of treatment. Patients were observed for a median of 5 years after the end of treatment. (A) OS for all patients. (B) OS by best response. (C) PFS for all patients. (D) PFS by best response.
Overall response and durability results per investigator
| Results . | n = 58 . | 95% CI . |
|---|---|---|
| Objective response (%) | 86 | 74.6-93.9* |
| CR (%) | 66 | 51.9-77.5* |
| Partial response (%) | 21 | |
| Stable disease (%) | 7 | |
| Progressive disease (%) | 3 | |
| Not evaluable (%)† | 3 | |
| Median duration of objective response (months) | 25.6 | 11.8-NE‡ |
| Median duration of response in patients with CR (months) | Not reached | 20.0-NE‡ |
| Median PFS (months) | 20.0 | 9.4-NE‡ |
| Median OS (months) | Not reached | 21.3-NE‡ |
| Results . | n = 58 . | 95% CI . |
|---|---|---|
| Objective response (%) | 86 | 74.6-93.9* |
| CR (%) | 66 | 51.9-77.5* |
| Partial response (%) | 21 | |
| Stable disease (%) | 7 | |
| Progressive disease (%) | 3 | |
| Not evaluable (%)† | 3 | |
| Median duration of objective response (months) | 25.6 | 11.8-NE‡ |
| Median duration of response in patients with CR (months) | Not reached | 20.0-NE‡ |
| Median PFS (months) | 20.0 | 9.4-NE‡ |
| Median OS (months) | Not reached | 21.3-NE‡ |
At the time of study closure, 25 patients (43%) had died. Of the 25 deaths, 6 occurred during the adverse event–reporting period, within 30 days of last treatment. Of the 6 deaths, 4 were related to disease (3 patients died of ALCL and 1 died of respiratory failure due to spinal cord compression), 1 death was not related to ALCL (patient died of acute myocardial infarction and acute renal failure), and 1 death was attributed to “sudden death” (relatedness unknown). None of the fatal adverse events were considered related to brentuximab vedotin. The remaining 19 of 25 deaths occurred during the follow-up period (≥30 days after last treatment), at which time disease relatedness was recorded but cause of death was not. Of the 19 deaths, 10 were disease related, 6 were not disease related, and 3 were attributed to unknown reasons. Overall, 14 of the 25 deaths (24% of enrolled patients) were attributed to disease.
ALK status.
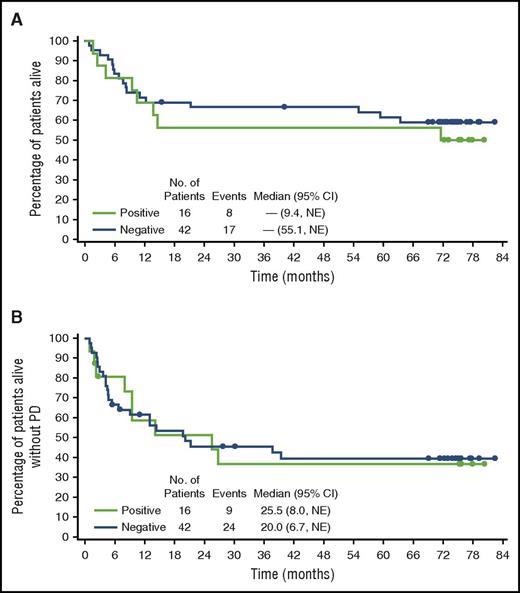
Patients with ALK– disease (n = 42; ORR, 88%; CR, 52%) had similar response rates to patients with ALK+ disease (n = 16; ORR, 81%; CR, 69%) as reported previously per independent review.13 The average age among patients with ALK+ disease was 32.5 years; among patients with ALK– disease, it was 53.4 years. PFS and OS plots according to ALK status are shown in Figure 2. The estimated probability of survival at 5 years for patients with ALK– disease was 61% (95% CI, 47%-76%); for patients with ALK+ disease, it was 56% (95% CI, 32%-81%). The estimated 5-year PFS rates were 39% (95% CI, 24%-55%) for patients with ALK– disease and 37% (95% CI, 11%-62%) for patients with ALK+ disease. Median PFS was 20 months (95% CI, 6.7, NE) and 25.5 months (95% CI, 8.0, NE) for ALK– and ALK+ patients, respectively. Among the 38 patients who achieved a CR, 28 patients had ALK– disease and 10 had ALK+ disease. Ten of the 28 ALK– CR patients (36%) and 5 of the 10 ALK+ CR patients (50%) experienced progressive disease or died. PFS rate at 5 years was 60% (95% CI, 40%-79%) among ALK– CR patients and 50% among ALK+ CR patients (95% CI, 19%-81%).
OS and PFS by ALK status. End-of-study (A) OS and (B) PFS were comparable among patients with ALK+ and ALK– systemic ALCL.
OS and PFS by ALK status. End-of-study (A) OS and (B) PFS were comparable among patients with ALK+ and ALK– systemic ALCL.
SCT.
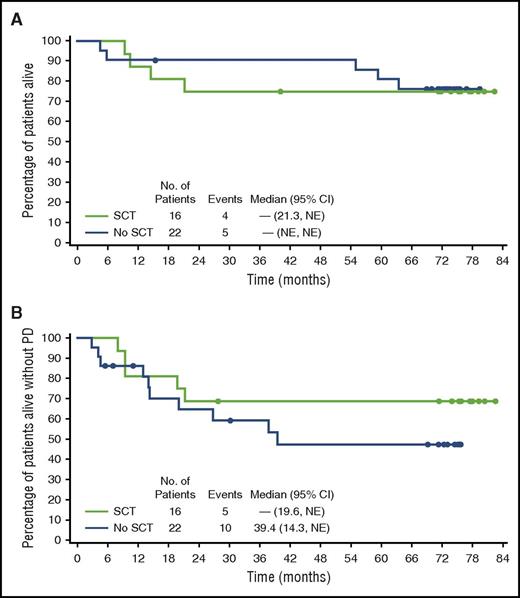
Sixteen of the 38 CR patients received a consolidative SCT as their next therapy subsequent to brentuximab vedotin, of whom 11 (69%) were alive and free of progressive disease at last follow-up. A flowchart for CR patients by transplant status is provided in supplemental Figure 3. Overall, patients who underwent SCT (n = 16) had a 5-year PFS rate of 69% (95% CI, 46%-91%) with median PFS not reached (95% CI, 19.6, NE), and 5-year OS rate of 75% (95% CI, 54%-96%) with median OS not reached (95% CI, 21.3, NE) (Figure 3). Among these 16 patients who received an SCT, 5-year outcomes were similar between patients who underwent autologous SCT (n = 8; PFS, 75%; OS, 88%) compared with patients who received allogeneic SCT (n = 8; PFS, 63%; OS, 63%). Of the 8 patients who received an allogeneic SCT, 6 had undergone an autologous SCT prior to study entry.
OS and PFS by SCT status. End-of-study (A) OS and (B) PFS in patients with a best response of CR subset by receipt of consolidative SCT.
OS and PFS by SCT status. End-of-study (A) OS and (B) PFS in patients with a best response of CR subset by receipt of consolidative SCT.
Median PFS for the 22 patients who did not proceed to SCT was 39.4 months (95% CI, 14.3, NE) with 5-year PFS rate of 48% (95% CI, 25%-70%); 5-year OS rate was 81% (95% CI, 65%-98%) with median not reached (endpoints of 95% CI not estimable). Of the 22 patients who did not receive SCT, 12 (54%) were alive without progressive disease at last follow-up.
A characterization of CR patients who did and did not receive consolidative SCT is shown in Table 2. In general, CR patients who underwent SCT (n = 16) were comparable to those who did not receive SCT (n = 22) with respect to age, disease burden at initial diagnosis, and time from initial diagnosis to first dose of brentuximab vedotin. Characteristics of the patients who underwent SCT were further divided by transplant type (8 autologous, 8 allogeneic). Patients who underwent autologous SCT were largely ALK– (7 of 8, 88%), whereas the majority of patients with allogeneic SCT were ALK+ (6 of 8, 75%). The baseline sum of the products of diameters (SPD; dominant nodes or nodal masses) was lower for patients who received autologous SCT (median, 11.5 cm2) compared with those who received allogeneic SCT (median, 24.6 cm2).
Characterization of CR patients (n = 38) by transplant status and transplant type
| Characteristics . | CR patients who received SCT . | . | |
|---|---|---|---|
| Autologous (n = 8) . | Allogeneic (n = 8) . | No SCT (n = 22) . | |
| Median age in years (range) | 48.0 (17-61) | 46.0 (17-61) | 58.0 (14-76) |
| Female, n (%) | 5 (63) | 4 (50) | 8 (36) |
| ECOG performance status, n (%) | |||
| Grade 0 | 4 (50) | 2 (25) | 9 (41) |
| Grade 1 | 4 (50) | 6 (75) | 13 (59) |
| ALK status, n (%) | |||
| Positive | 1 (13) | 6 (75) | 3 (14) |
| Negative | 7 (88) | 2 (25) | 19 (86) |
| Disease status relative to most recent prior therapy, n (%)* | |||
| Relapsed | 3 (38) | 5 (63) | 14 (64) |
| Refractory | 5 (63) | 3 (38) | 8 (36) |
| Primary refractory disease, n (%)† | 5 (63) | 5 (63) | 13 (59) |
| Stage at initial diagnosis, n (%) | |||
| I/II | 5 (63) | 4 (50) | 8 (36) |
| III/IV | 2 (25) | 4 (50) | 10 (45) |
| Unknown | 1 (13) | 0 | 4 (18) |
| Median time in months from initial diagnosis to first dose (range) | 12.4 (5.2-32.4) | 23.9 (6.2-108.0) | 23.1 (4.4-186.5) |
| Median time in months from most recent relapse to first dose (range)‡ | 1.8 (0.8-2.8) | 1.2 (0.5-2.7) | 1.9 (0.4-2.9) |
| Baseline B symptoms, n (%)§ | 3 (38) | 5 (63) | 3 (14) |
| Fever | 2 (25) | 4 (50) | 0 |
| Night sweats | 2 (25) | 3 (38) | 3 (14) |
| Median baseline SPD of dominant nodes or nodal masses per investigator (cm2, range)|| | 11.5 (4.5-19.4) | 24.6 (11.7-76.8) | 10.7 (2.0-51.3) |
| Baseline bone marrow involvement, n (%) | 0 | 1 (13) | 1 (5) |
| Characteristics . | CR patients who received SCT . | . | |
|---|---|---|---|
| Autologous (n = 8) . | Allogeneic (n = 8) . | No SCT (n = 22) . | |
| Median age in years (range) | 48.0 (17-61) | 46.0 (17-61) | 58.0 (14-76) |
| Female, n (%) | 5 (63) | 4 (50) | 8 (36) |
| ECOG performance status, n (%) | |||
| Grade 0 | 4 (50) | 2 (25) | 9 (41) |
| Grade 1 | 4 (50) | 6 (75) | 13 (59) |
| ALK status, n (%) | |||
| Positive | 1 (13) | 6 (75) | 3 (14) |
| Negative | 7 (88) | 2 (25) | 19 (86) |
| Disease status relative to most recent prior therapy, n (%)* | |||
| Relapsed | 3 (38) | 5 (63) | 14 (64) |
| Refractory | 5 (63) | 3 (38) | 8 (36) |
| Primary refractory disease, n (%)† | 5 (63) | 5 (63) | 13 (59) |
| Stage at initial diagnosis, n (%) | |||
| I/II | 5 (63) | 4 (50) | 8 (36) |
| III/IV | 2 (25) | 4 (50) | 10 (45) |
| Unknown | 1 (13) | 0 | 4 (18) |
| Median time in months from initial diagnosis to first dose (range) | 12.4 (5.2-32.4) | 23.9 (6.2-108.0) | 23.1 (4.4-186.5) |
| Median time in months from most recent relapse to first dose (range)‡ | 1.8 (0.8-2.8) | 1.2 (0.5-2.7) | 1.9 (0.4-2.9) |
| Baseline B symptoms, n (%)§ | 3 (38) | 5 (63) | 3 (14) |
| Fever | 2 (25) | 4 (50) | 0 |
| Night sweats | 2 (25) | 3 (38) | 3 (14) |
| Median baseline SPD of dominant nodes or nodal masses per investigator (cm2, range)|| | 11.5 (4.5-19.4) | 24.6 (11.7-76.8) | 10.7 (2.0-51.3) |
| Baseline bone marrow involvement, n (%) | 0 | 1 (13) | 1 (5) |
SPD, sum of the products of diameters.
Relapse = best response of CR if a patient only had 1 prior therapy, or best response of CR or PR to most recent prior therapy if a patient had more than 1 prior therapy; refractory = best response of PR, stable disease (SD), or progressive disease (PD) if a patient had only 1 prior therapy, or best response of SD or PD to most recent prior therapy if a patient had more than 1 prior therapy.
No CR, or relapse within 3 months of frontline therapy.
For those with relapsed disease status to most recent prior therapy.
B symptoms present at the time of cycle 1, day 1.
SPD of up to 6 of the largest dominant nodes or nodal masses.
Cycle 4 PET.
Assessments of tumor response occurred at cycles 4 and 7. Of the 48 patients who received a cycle 4 PET scan, 20 patients had positive results; among whom 3 had negative results at the time of the cycle 7 scan. Patients who had a negative result at cycle 4 fared better than patients who had a positive result at cycle 4. The former had an estimated 82% probability (95% CI, 67%-96%) of remaining free of progression or death at 5 years compared with 45% (95% CI, 23%-67%) for the latter. The 5-year PFS for patients who had a positive result at cycle 4 was 16% (95% CI, 0%-32%); for patients who had a negative result, it was 64% (95% CI, 45%-83%). Median PFS for patients who had a positive result was 6.7 months (95% CI, 4.2-20.0 months) and was not reached (95% CI, 21.3, NE) in patients who had a negative result (supplemental Figure 2).
Age.
We performed a subgroup analysis comparing enrolled patients 12 to 39 years old (n = 16) with patients 40 years and older (n = 42). Similar outcomes were observed between the 2 age groups (supplemental Figure 2). Patients younger than 40 years had a 5-year survival rate of 63% (95% CI, 39%-86%), and in those 40 years and older, the 5-year survival rate was 59% (95% CI, 44%-74%). Median OS was not reached in either group. Older patients appeared to remain disease-free longer with a median PFS of 20.0 months (95% CI, 6.7, NE) compared with 14.1 months (95% CI, 4.7-37.7 months) in the younger patient population.
Patients on-study and in remission at end of study.
At the time of study closure, which occurred ∼5 years from the last patient’s end of treatment visit, 16 of the 38 patients who achieved a CR per the investigator remained on-study and in remission without the start of new therapy other than consolidative SCT (Figure 4). These 16 patients were treated for a median of 28.5 weeks (range, 6.0-50.9 weeks) with a median of 9 cycles of brentuximab vedotin. Of the 16 patients, 8 achieved CR by cycle 8.
Patients who were in remission and in follow-up at study closure. Includes patients who were in remission according to the investigator and on-study at the time of study closure. Patients are shaded according to ALK status. A consolidative SCT was performed in 8 patients, and 8 patients received no further therapy after completing brentuximab vedotin. Allo-SCT, allogeneic SCT; Auto-SCT, autologous SCT; EOS, end of study.
Patients who were in remission and in follow-up at study closure. Includes patients who were in remission according to the investigator and on-study at the time of study closure. Patients are shaded according to ALK status. A consolidative SCT was performed in 8 patients, and 8 patients received no further therapy after completing brentuximab vedotin. Allo-SCT, allogeneic SCT; Auto-SCT, autologous SCT; EOS, end of study.
These patients, among whom 11 had ALK– disease and 5 had ALK+ disease, were observed for a median of 75.4 months (range, 69-82.4 months) from their first dose of brentuximab vedotin. Of the 16 patients, 8 underwent consolidative SCT (4 autologous, 4 allogeneic), of whom 4 patients (50%) had ALK– disease. The remaining 8 patients (8 of 58, 14%) remained in sustained remission without consolidative SCT or any additional anticancer therapy after receiving brentuximab vedotin. A complete characterization of the patients who achieved sustained CR subset by transplant status compared with CR patients who did not achieve sustained remission is provided in Table 3. No apparent differences were revealed in the demographics or disease characteristics between the 16 patients with sustained CR and the 22 CR patients who experienced disease progression, death, or were lost to follow-up. Of the 16 patients with sustained CR, disease characteristics among patients who underwent consolidative SCT were similar to those in patients without SCT.
Characterization of CR patients in remission at end of study (n = 16) by transplant status
| Characteristics . | CR patients in remission at EOS (n = 16) . | All other CR (n = 22) . | |
|---|---|---|---|
| SCT (n = 8) . | No SCT (n = 8) . | ||
| Median age in years (range) | 48.5 (17-61) | 59.5 (14-76) | 50.0 (17-74) |
| Female, n (%) | 4 (50) | 0 | 13 (59) |
| ECOG performance status, n (%) | |||
| Grade 0 | 2 (25) | 2 (25) | 11 (50) |
| Grade 1 | 6 (75) | 6 (75) | 11 (50) |
| ALK status, n (%) | |||
| Positive | 4 (50) | 1 (13) | 5 (23) |
| Negative | 4 (50) | 7 (88) | 17 (77) |
| Disease status relative to most recent prior therapy, n (%)* | |||
| Relapsed | 5 (63) | 6 (75) | 11 (50) |
| Refractory | 3 (38) | 2 (25) | 11 (50) |
| Primary refractory disease, n (%)† | 4 (50) | 3 (38) | 16 (73) |
| Stage at initial diagnosis, n (%) | |||
| I/II | 3 (38) | 2 (25) | 12 (55) |
| III/IV | 4 (51) | 2 (25) | 10 (46) |
| Unknown | 1 (13) | 4 (50) | 0 |
| Median time in months from initial diagnosis to first dose (range) | 21.7 (6.2-103.5) | 21.83 (7.2-113.2) | 19.55 (186.5) |
| Median time in months from most recent relapse to first dose (range)‡ | 1.2 (0.5-2.5) | 1.6 (0.4-2.9) | 2.1 (0.7-2.8) |
| Baseline B symptoms, n (%)§ | 4 (50) | 1 (13) | 6 (27) |
| Fever | 3 (38) | 0 | 3 (14) |
| Night sweats | 2 (25) | 1 (13) | 5 (23) |
| Median baseline SPD of dominant nodes or nodal masses per investigator (cm2, range)|| | 15.7 (4.5-76.8) | 12.1 (3.2, 47.8) | 11.9 (2.0, 51.3) |
| Baseline bone marrow involvement, n (%) | 0 | 0 | 2 (9) |
| Characteristics . | CR patients in remission at EOS (n = 16) . | All other CR (n = 22) . | |
|---|---|---|---|
| SCT (n = 8) . | No SCT (n = 8) . | ||
| Median age in years (range) | 48.5 (17-61) | 59.5 (14-76) | 50.0 (17-74) |
| Female, n (%) | 4 (50) | 0 | 13 (59) |
| ECOG performance status, n (%) | |||
| Grade 0 | 2 (25) | 2 (25) | 11 (50) |
| Grade 1 | 6 (75) | 6 (75) | 11 (50) |
| ALK status, n (%) | |||
| Positive | 4 (50) | 1 (13) | 5 (23) |
| Negative | 4 (50) | 7 (88) | 17 (77) |
| Disease status relative to most recent prior therapy, n (%)* | |||
| Relapsed | 5 (63) | 6 (75) | 11 (50) |
| Refractory | 3 (38) | 2 (25) | 11 (50) |
| Primary refractory disease, n (%)† | 4 (50) | 3 (38) | 16 (73) |
| Stage at initial diagnosis, n (%) | |||
| I/II | 3 (38) | 2 (25) | 12 (55) |
| III/IV | 4 (51) | 2 (25) | 10 (46) |
| Unknown | 1 (13) | 4 (50) | 0 |
| Median time in months from initial diagnosis to first dose (range) | 21.7 (6.2-103.5) | 21.83 (7.2-113.2) | 19.55 (186.5) |
| Median time in months from most recent relapse to first dose (range)‡ | 1.2 (0.5-2.5) | 1.6 (0.4-2.9) | 2.1 (0.7-2.8) |
| Baseline B symptoms, n (%)§ | 4 (50) | 1 (13) | 6 (27) |
| Fever | 3 (38) | 0 | 3 (14) |
| Night sweats | 2 (25) | 1 (13) | 5 (23) |
| Median baseline SPD of dominant nodes or nodal masses per investigator (cm2, range)|| | 15.7 (4.5-76.8) | 12.1 (3.2, 47.8) | 11.9 (2.0, 51.3) |
| Baseline bone marrow involvement, n (%) | 0 | 0 | 2 (9) |
The categories of CR in remission at end of study include patients with a best response of CR without new treatment and free of known PD per investigator or death due to disease.
Relapse = best response of CR if a patient only had 1 prior therapy, or best response of CR or PR to most recent prior therapy if a patient had more than 1 prior therapy; refractory = best response of PR, SD, or PD if a patient had only 1 prior therapy, or best response of SD or PD to most recent prior therapy if a patient had more than 1 prior therapy.
No CR, or relapse within 3 months of frontline therapy.
For those with relapsed disease status to most recent prior therapy.
B symptoms present at the time of cycle 1, day 1.
SPD of up to 6 of the largest dominant nodes or nodal masses.
Peripheral neuropathy
Rate and resolution.
Peripheral neuropathy is a common adverse event associated with accumulated exposure to brentuximab vedotin. Events of peripheral neuropathy, identified by a standardized Medical Dictionary for Regulatory Activities query, occurred in 33 of the 58 enrolled patients (57%). The incidence of peripheral neuropathy appeared to be similar between the age groups analyzed. Slightly more than half of patients younger than 40 years (n = 9) and those 40 years and older (n = 24) experienced symptoms (56% and 57%, respectively), whereas patients younger than 60 years (n = 22) experienced symptoms at a slightly lower rate than did patients 60 years and older (n = 11; 54% and 65%, respectively).
Patients were monitored for improvement or resolution of symptoms until ∼2 years into LTFU. Resolution was defined as recovered or resolved with sequelae, or a return to baseline or the lower-grade severity for preexisting peripheral neuropathy events. Improvement was defined as a decrease in severity by at least 1 grade from worst grade as of the latest assessment. Among the 33 patients who experienced events, 30 (91%) experienced resolution or improvement, of whom 22 patients (67%) reported complete resolution and 8 patients (24%) reported improvement or some resolution at their last assessment. The remaining 3 patients who were without improvement or resolution had discontinued the study because they had died. Median time to resolution or improvement for all peripheral neuropathy events was 14.1 weeks (range, 0.3-177.7 weeks), and median time to resolution or improvement by grade 2 severity was 14.1 weeks (range, 0.7-161.1 weeks) and 24.3 weeks (range, 2.1-177.7 weeks) for grade 3 severity. There were 11 patients with ongoing neuropathy at last follow-up, of which 8 patients had grade 1 severity and 3 patients had grade 2 severity. Median time to the onset of peripheral neuropathy among the 33 patients who experienced at least 1 event was 15 weeks (range, 0.1-51.9 weeks) with a median time to worst grade of 16.9 weeks (range, 0.1-51.9 weeks). Median time to the onset of grade 2 peripheral neuropathy was 17.7 weeks (range, 2.1-45.1 weeks) and 36.1 weeks (range, 6.0-51.9) for grade 3 severity.
Baseline peripheral neuropathy.
At the time of enrollment, 16 (28%) of the 58 enrolled patients had preexisting neuropathies originating from prior therapies or other causes. The tolerability of brentuximab vedotin treatment was comparable between patients who had preexisting symptoms and those who did not have such symptoms. Treatment durations were similar between patients with baseline symptoms (n = 16; median, 8.0 cycles) and patients without symptoms (n = 42; median, 7.0 cycles) (supplemental Table 1). Median time from first treatment to first dose reduction in patients with and those without symptoms was 17 weeks (range, 9.1-26.1 weeks) and 19 weeks (range, 11.4-21.3 weeks), respectively. Duration of dose reductions was also similar between the 2 groups, with a median duration of 6 weeks for patients with baseline peripheral neuropathy (range, 6-6 weeks), and 6 weeks for patients without (range, 2.7-55.4 weeks). Of the 16 patients with preexisting symptoms of peripheral neuropathy, 14 achieved an objective response to brentuximab vedotin with 11 patients achieving a CR. Median response duration was 18.5 months (95% CI, 5.6, NE) for the subset with an objective response and 20 months (95% CI, 8.3, NE) for patients with a CR.
Patients on-study and in remission at end of study.
Of the 16 CR patients who were on-study and in remission at the time of study closure, 15 experienced at least 1 treatment-emergent event associated with peripheral neuropathy. Among these 15 patients, 12 had complete resolution of symptoms and 3 experienced improvement. Median time to resolution or improvement was 17 weeks. Types of neuropathy among the 15 patients included peripheral sensory neuropathy (n = 10), paresthesia (n = 3), neuralgia (n = 1), peripheral motor neuropathy (n = 2), and demyelinating polyneuropathy (n = 1). Of the 2 patients with peripheral motor neuropathy, 1 patient experienced complete resolution and the second reported some improvement but no resolution at the last assessment. The CR patient with demyelinating polyneuropathy experienced grade 3 symptoms that improved to grade 2 and then to grade 1.
Discussion
In this phase 2 pivotal trial, patients with relapsed or refractory systemic ALCL treated with single-agent brentuximab vedotin experienced a high rate of durable remissions, which has translated into improved survival duration compared with historic control participants. Investigators assessed survival and progression for ∼5 years from the last patient’s end of treatment visit, at which time the median PFS rate was 39% among all enrolled patients and 57% among CR patients. Early response appeared to correlate with improved outcomes, with an estimated 5-year PFS rate of 64% among patients who achieved CR by the cycle 4 response assessment.
Patients who responded to brentuximab vedotin could have received either an allogeneic or an autologous consolidative SCT. The decision to send a CR patient to SCT, as well as the type of SCT was within the purview of the investigator and took into consideration the patient’s eligibility, ALK status, and receipt of prior autologous SCT. PFS was improved in CR patients who received consolidative SCT (69% vs 48% in patients without SCT); however, OS was slightly higher in the patients who did not receive SCT. Among patients who underwent autologous SCT, the estimated 75% PFS rate at 5 years compares favorably with previous estimates of roughly 56% in patients who have received high-dose chemotherapy and autologous SCT.
We analyzed survival and progression results for all enrolled patients who were subcategorized by ALK– disease, ALK+ disease, and age to identify potential factors prognostic for survival and response duration. Long-term outcomes had no clear associations with age and ALK status; however, analyses are limited given the small patient population.
At the time of study closure, 28% of enrolled patients remained on-study and in remission without starting new therapy other than consolidative SCT. These patients had been observed for a median of ∼6 years from the start of treatment. Eight of these patients received a consolidative SCT, of whom 4 had ALK– disease. The remaining 8 patients (14% of all enrolled patients) remained in sustained remission without receiving SCT or any new anticancer therapy. Characteristics of patients in sustained remission who underwent consolidative SCT were similar to patients who did not receive consolidative SCT with respect to baseline SPD, ECOG status, and time from relapse to first dose of brentuximab vedotin. However, patients who did not receive SCT tended to be slightly older and were men.
Patients who entered the trial with preexisting neuropathies endured treatment durations similar to patients without baseline symptoms, and the majority achieved an objective response to brentuximab vedotin. Median response duration among patients with preexisting neuropathies was comparable to responders overall. Together, these results show that heavily pretreated patients with persisting neuropathies were able to tolerate therapy with appropriate dose modifications and derived clinical benefit from treatment with single-agent brentuximab vedotin. Furthermore, peripheral neuropathies that were observed overall were manageable and reversible, with resolution or improvement in symptoms reported by 91% of affected patients at their last follow-up assessment.
Several agents have been approved for the treatment of patients with relapsed or refractory PTCL including systemic ALCL: pralatrexate, a next-generation antifolate; and 2 potent histone deacetylase inhibitors, romidepsin and belinostat. Response rates for patients with relapsed or refractory ALCL from these pivotal studies in mixed PTCL populations ranged from 0% to 35% (pralatrexate, 35% ORR in ALCL; romidepsin, 24% in ALK– ALCL; and belinostat, 15% in ALK– and 0% in ALK+ ALCL).15-17 Although no formal comparison is possible for this single-arm pivotal trial, the response rates observed (86% ORR, 57% CR per central review and 86% ORR, 66% CR per investigator assessment) in this population with relapsed or refractory systemic ALCL are encouraging.
Systemic ALCL is a rare malignancy and, prior to the present study, was often included only as a subset of aggressive lymphomas in large prospective studies of salvage therapy. Although single-agent and multiagent regimens of chemotherapy were commonly used to address relapsed or refractory disease, both pre–autologous SCT and post–autologous SCT, limited data were available to support the benefit of these regimens in systemic ALCL. The ECHELON-2 trial is an ongoing randomized phase 3 trial to evaluate brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone vs standard-of-care cyclophosphamide, doxorubicin, vincristine, and prednisone for frontline treatment of CD30-expressing PTCLs, including systemic ALCL (#NCT01777152). This trial, together with the results presented here, will further define the usefulness of brentuximab vedotin for the treatment of patients with systemic ALCL.
Overall, these end-of-study survival outcomes and freedom-from-progression results, encompassing 5 years of follow-up assessments per the investigator, demonstrate that among a subset of patients with relapsed or refractory systemic ALCL, single-agent brentuximab vedotin may be a potentially curative treatment option.
Presented in part at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 5 December 2016.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the patients who participated in the study and the study staff who helped to take care of them. The authors also acknowledge the medical writing assistance of Katrina Sebolt, employed by Seattle Genetics, Inc.
This work was supported by Seattle Genetics, Inc, through the joint financial support of Seattle Genetics, Inc, and Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceuticals Limited.
Authorship
Contribution: B.P., K.F., D.H., J.M.P., and D.A.K. contributed to the analysis and interpretation of the data and wrote the manuscript; B.P., R.A., P.B., N.L.B., J.D.R., T.I., J.M., R.R., M.F., J.M.C., and A.S. contributed to the acquisition of the data; R.A., P.B., N.L.B., J.D.R., T.I., J.M., R.R., M.F., J.M.C., and A.S. critically reviewed the manuscript; and all authors contributed to the concept and design of the study and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.M.P., K.F., and D.A.K. are employees of and have equity ownership of Seattle Genetics. D.H. is an employee of and has equity ownership of Takeda. P.B. received funding from Seattle Genetics and Takeda. J.M.C. received funding from Seattle Genetics, Takeda, Roche, and Bristol-Myers Squibb. B.P., M.F., T.I., J.M., J.D.R., A.S., and R.R. received funding from Seattle Genetics; R.A. received funding from Kura, Celgene, Genentech, Infinity, Seattle Genetics, Agensys, Bristol-Myers Squibb, Merck, Millennium, Regeneron, Janssen, and Pharmacyclics. N.L.B. received funding from Celgene, Seattle Genetics, Genentech, Pfizer, KITE, Merck, Bristol-Myers Squibb, Immune Design, Forty Seven, Affimed, Janssen, Pharmacyclics, Millennium, AstraZeneca, ImaginAb, and Novartis. B.P. and T.I. received honoraria from Takeda. P.B. received honoraria from Takeda, Roche, Gilead, and Bristol-Myers Squibb. M.F. received honoraria from Seattle Genetics. M.F. and R.R. has received consultant fees from Seattle Genetics. T.I. has received consultant fees from Seattle Genetics and Takeda. J.M. has received consultant fees from Celgene. B.P. has received consultant fees from Seattle Genetics. N.L.B. has received consultant fees from Seattle Genetics, KITE, and Gilead. R.A. has received consultant fees from NanoString Technologies, Pharmacyclics, Spectrum, Bristol-Myers Squibb, Forty Seven, Sutro Biopharma, Juno Therapeutics, and Gilead. J.M. is a member of the speakers bureau for Seattle Genetics and Celgene. M.F., B.P., and R.R. have received travel expenses from Seattle Genetics.
Correspondence: Barbara Pro, Robert H. Lurie Comprehensive Cancer Center, 676 N Saint Clair St, Suite 850, Chicago, IL 60611; e-mail: barbara.pro@nm.org.