In this issue of Blood, Münzer et al used a specific platelet/megakaryocyte (MK) deletion of casein kinase (CK) 2β mouse model to elegantly demonstrate that CK2 regulates both platelet formation and activation by altering microtubule structure, which results in impaired intracellular Ca2+ release.1
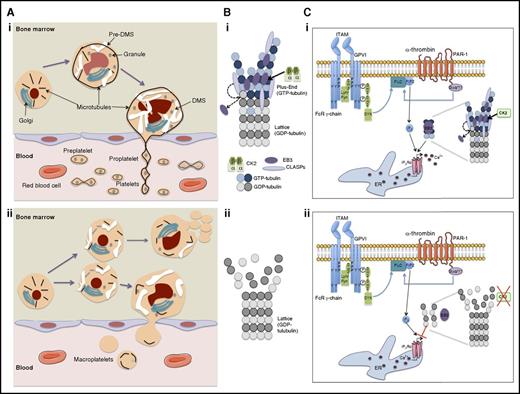
Schematic showing how ck2β deletion induces a defect in proplatelet formation with a premature MK fragmentation and how it impairs α-thrombin/PAR-1 and collagen/glycoprotein VI (GPVI) signaling. (A) Megakaryocyte maturation in bone marrow and platelet release into blood circulation in (i) presence or (ii) absence of CK2β. (B) End-binding protein 3 (EB3)-dependent stabilization of tubulin polymerization in (i) presence or (ii) absence of CK2β. (C) Regulation of intracellular Ca2+ release during Ca2+-dependent platelet activation in (i) presence or (ii) absence of CK2β. CLASPs, cytoplasmic linker associated proteins; DMS, demarcation membrane system; ER, endoplasmic reticulum; FcR, Fc receptor; Gαq/11, Gq protein; GDP, guanosine diphosphate; GTP, guanosine triphosphate; ITAM, immunoreceptor tyrosine-based activation motif; p, phosphorylated; PIP2, phosphatidyl inositol diphosphate; PLC, phospholipase; SH3, sarc homology 3 domain; SYK, spleen tyrosine kinase; Y, tyrosine.
Schematic showing how ck2β deletion induces a defect in proplatelet formation with a premature MK fragmentation and how it impairs α-thrombin/PAR-1 and collagen/glycoprotein VI (GPVI) signaling. (A) Megakaryocyte maturation in bone marrow and platelet release into blood circulation in (i) presence or (ii) absence of CK2β. (B) End-binding protein 3 (EB3)-dependent stabilization of tubulin polymerization in (i) presence or (ii) absence of CK2β. (C) Regulation of intracellular Ca2+ release during Ca2+-dependent platelet activation in (i) presence or (ii) absence of CK2β. CLASPs, cytoplasmic linker associated proteins; DMS, demarcation membrane system; ER, endoplasmic reticulum; FcR, Fc receptor; Gαq/11, Gq protein; GDP, guanosine diphosphate; GTP, guanosine triphosphate; ITAM, immunoreceptor tyrosine-based activation motif; p, phosphorylated; PIP2, phosphatidyl inositol diphosphate; PLC, phospholipase; SH3, sarc homology 3 domain; SYK, spleen tyrosine kinase; Y, tyrosine.
CK2 is a ubiquitous, constitutively active serine threonine kinase. It is a tetrameric protein composed of 2 regulatory β chains that dimerize to bind to 2 homologous catalytic chains (α and α′). In contrast with other kinases, CK2 has hundreds of potential substrates and is involved in many cellular processes, including survival, proliferation, transcription, and signaling.2 The importance of these functions is demonstrated by the embryonic lethality of ck2β knockout mice.
CK2 is expressed in blood cells, including platelets, yet few studies have investigated the role of CK2 in the function of hematopoietic cells. CK2 is involved in the growth and proliferation of numerous cancers, including acute lymphocytic leukemia, acute myeloid leukemia, and their chronic forms (chronic lymphocytic leukemia and chronic myeloid leukemia), mainly by inducing an overactivation of certain signaling pathways such as the phosphatidylinositol 3-kinase (PI3K)/AKT, JAK/STAT, and NF-κB pathways.3 This has led to the development of several CK2 inhibitors. Keeping in mind that most of these CK2 inhibitors may exert off-target effects, they have been useful in showing that CK2 is involved in platelet activation (aggregation and secretion) through the regulation of PI3K signaling.4
To precisely study the effects of CK2 in both thrombopoiesis and platelet functions, Münzer et al performed a megakaryocyte/platelet-specific genetic deletion of ck2β (ck2β−/−) in mice.1 This led to 2 important discoveries (see figure): (1) CK2 is involved in platelet production and survival. The ck2β knockout induced a macrothrombocytopenia resulting from an MK fragmentation inside the bone marrow, which led to ineffective platelet production and a slight decrease in platelet half-life. The decrease in platelet count stimulates megakaryopoiesis in bone marrow as well as in the spleen but without noticeable development of fibrosis. (2) CK2 regulates Ca2+-triggered platelet activation and exerts an important role in thrombus formation under high shear.
Thrombocytopenic phenotypes related to premature MK fragmentation in the bone marrow have rarely been described. The first demonstration was in Wasp-deficient mice in which it was reported that MK fragmentation might be linked to an MK trafficking defect.5 Subsequently, it was shown in patients that a germ line mutation in cytochrome C led to thrombocytopenia by platelet-like release in the marrow as a result of an increased MK apoptosis.6 Finally, it was recently shown that the double cdc42/rac1 knockout leads to macrothrombocytopenia with MK fragmentation in the marrow. This fragmentation was related to a defect in microtubule organization, but surprisingly, this double knockout barely affected the actin cytoskeleton.7
In the case of this conditional ck2β knockout, it was expected that thrombocytopenia would be related to an increase in apoptosis. This is because in hematopoietic cells, CK2 regulates the PI3K/AKT pathway and thus BAD, a key molecule in platelet survival and production. However, in the study by Münzer et al, the authors demonstrated that in their ck2β knockout mice, thrombocytopenia is the consequence of an alteration in microtubule dynamics. Indeed, it has recently been emphasized that CK2 plays an important role in the regulation of microtubule cytoskeleton and in morphogenesis.8 In particular, CK2 is associated with microtubules and regulates their stability. CK2 function, as a microtubule-associated protein, is illustrated during the neurite outgrowth, a process that strongly resembles proplatelet production. In platelets, the absence of CK2β decreases microtubule stability, probably by impairing tubulin polymerization.1 Thus, the mechanism of thrombocytopenia seems quite similar to that in the cdc42/rac1 null mice.7 However, because the defect in MK maturation is not restricted to proplatelet formation, additional mechanisms may be involved. Indeed, CK2 also regulates the actin cytoskeleton through CK2-interacting protein 1 (CKIP-1) and by activating WASP through phosphorylation.8 N-WASP is involved in MK terminal differentiation and proplatelet formation.
The microtubule defect induces a decrease in inositol 1,4,5-triphosphate receptor type 1 (IP3R1) binding to polymerized microtubules and an impaired activation of platelets to different stimuli such as thrombin and collagen, which leads to a reduced intracellular release of cytosolic Ca2+; (see figure). Overall, this defect has functional consequences such as decreased platelet adherence to collagen at high shear rates and altered formation of stable thrombi.1 Thus, ck2β null mice were protected from arterial thrombosis with only a minor bleeding tendency. Two CK2 inhibitors decreased Ca2+ influx and secretion in human platelets, suggesting that all these results can be extended to humans.1
Overall, this work sheds light on CK2 as an important target for future antithrombotic therapy. CK2 inhibitors have recently been developed for cancer treatment despite their possible toxic effects. However, the pleiotropic effects of CK2 might not limit the use of inhibitors, because several other kinases are redundant on most CK2 substrates.9 Recent clinical trials underscore that CK2 inhibitors are, on the whole, well tolerated. Nevertheless long-term administration may be limited by the role of CK2 in γδ T cells and the possible induction of an immune deficiency. Conversely, it seems unlikely that CK2 mutations might be involved in inherited macrothrombocytopenia because the kinase is indispensable for embryonic development. In conclusion, this study unravels the role of the CK2 pathway in platelet production and function.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal