In this issue of Blood, Zhao et al perform single-cell RNA sequencing (scRNA-seq) of bone marrow–derived CD34+ cells from patients with monosomy 7. Identifying cells with chromosome 7 aberrations, they uncover reduced transcription of genes upholding genomic integrity and concomitant accumulation of somatic mutations.1
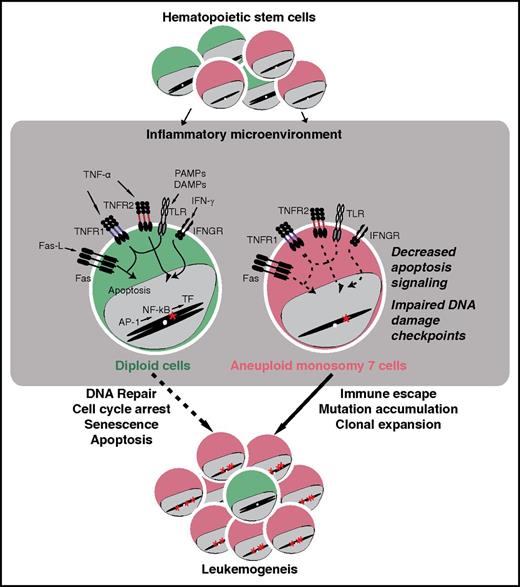
Schematic representation of mechanisms underlying aberrant expansion of aneuploid hematopietic stem cells with −7. During bone marrow failure, hematopietic stem cells are embedded in an inflammatory environment. Although diploid stem cells (green) that accumulate mutations usually are subject to cell-cycle arrest, apoptosis, and effective immune surveillance and eradication, aneuploid stem cells with −7 (red) display downregulation of genes implicated in the regulation of DNA damage checkpoints, cell cycle, and apoptosis, which thereby can facilitate their accumulation of additional mutations and aberrant expansion, ultimately leading to leukemogenesis. AP-1, activator protein 1; DAMP, damage-associated molecular pattern; Fas-L, Fas ligand; IFN-γ, interferon γ; IFNGR, IFN-γ receptor; PAMP, pathogen-associated molecular pattern; TF, transcription factor; TLR, Toll-like receptor; TNF, tumor necrosis factor; TNFR, TNF receptor. The figure has been adapted from Figure 6 in the article by Zhao et al beginning on page 2762.
Schematic representation of mechanisms underlying aberrant expansion of aneuploid hematopietic stem cells with −7. During bone marrow failure, hematopietic stem cells are embedded in an inflammatory environment. Although diploid stem cells (green) that accumulate mutations usually are subject to cell-cycle arrest, apoptosis, and effective immune surveillance and eradication, aneuploid stem cells with −7 (red) display downregulation of genes implicated in the regulation of DNA damage checkpoints, cell cycle, and apoptosis, which thereby can facilitate their accumulation of additional mutations and aberrant expansion, ultimately leading to leukemogenesis. AP-1, activator protein 1; DAMP, damage-associated molecular pattern; Fas-L, Fas ligand; IFN-γ, interferon γ; IFNGR, IFN-γ receptor; PAMP, pathogen-associated molecular pattern; TF, transcription factor; TLR, Toll-like receptor; TNF, tumor necrosis factor; TNFR, TNF receptor. The figure has been adapted from Figure 6 in the article by Zhao et al beginning on page 2762.
In 1960, Nowell and Hungerford2 discovered a small chromosome in the white blood cells of patients with chronic myelogenous leukemia (CML), signifying the first chromosomal abnormality associated with cancer. Cytogenetic analyses have since revealed that chromosomal abnormalities are a common feature of many cancers, with distinct aberrations being associated with specific forms of cancer. Myeloid malignancies encompass a heterogeneous group of clonal hematopoietic stem- and progenitor-cell (HSPC) disorders.3 In addition to CML, they include myeloproliferative neoplasms (MPNs), myelodysplastic syndrome (MDS), and acute myeloid leukemia (AML). Acquired HSPC chromosomal abnormalities are 1 of the main risk factors for developing myeloid malignancies, characterized by impaired hematopoiesis and cytopenias. The incidence of myeloid malignancies increases with age. In the elderly, del(5q) represents the most common chromosomal aberration, followed by the complete or partial loss of chromosome 7, termed monosomy 7 (−7). In children, approximately 20% of leukemias are of myeloid origin, with −7 representing a common chromosomal aberration. Importantly, −7 is associated with refractory cytopenias and a high risk of progression to AML.4 Dissecting how abnormalities affecting large chromosomal regions mechanistically give rise to distinct cancers is challenging. Because chromosomal composition varies among species, animal models are not useful.
Human genetics have provided insights into −7. Inherited bone marrow failure syndromes caused by mutations in genes required for DNA repair, chromosomal stability, and telomere elongation are associated with an increased risk of acquiring somatic mutations that often manifest as myeloid malignancies. Furthermore, several rare autosomal-dominant or de novo monogenic causes of familial MDS/AML have recently been elucidated. Interestingly, heterozygous GATA2 germline mutations are associated bone marrow failure as well as MPNs, MDS, and AML, predominately with −7. Overall, GATA2 haploinsufficiency explains 37% of pediatric cases of −7.5 Providing the first human examples of adaptation by aneuploidy, de novo or inherited gain-of-function mutations in SAMD9 or SAMD9L encoded on chromosome 7q frequently acquire 7q segmental uniparental disomy in HSPCs and their progeny, whereas others develop −7 and subsequent MDS or AML.6,7 In addition, recurrent microdeletions or mutations in chromosome 7 loci encoding SAMD9/SAMD9L, CUX1, LUC7L2, CUL1, and EZH2 have been found in myeloid neoplasms.8 Despite these insights, understanding how −7 in stem cells contributes to the development of myeloid malignancies remains enigmatic. Stem cells are scarce and hence difficult to study. Moreover, in vitro models of human cellular differentiation cannot account for the microenvironment or immune surveillance.
Harnessing new molecular techniques for scRNA-seq, Zhao et al sorted and sequenced a total of 391 HSPCs from 4 healthy donors and 588 HSPCs from 5 patients, 3 with −7, 1 with additional trisomy 1, and 2 with trisomy 8 as determined by clinical cytogenetics. Through statistical analyses of single-cell transcriptome data, they were able to resolve HSPCs into specific subtypes based upon gene expression profiles. Moreover, using 3 independent bioinformatical methods, they detected aneuploid cells with reduced expression of genes encoded by chromosome 7 genes or higher expression of those encoded by chromosome 1 or 8. The patterns of decreased or increased gene expression were consistent with the defined chromosomal abnormalities in each patient and the relative frequencies of such aberrant cells. Analyses uncovered abnormal differentiation profiles of −7 HSPCs. Comparing gene expression in HSPCs bioinformatically assigned as −7 with diploid cells, Zhao et al uncovered prominently downregulated genes and gene pathways in addition to a limited number of upregulated genes. Marked −7 cell heterogeneity was noted among patients. Nonetheless, comparing CD38+ HSPCs in all 3 donors with −7 revealed some common transcriptional features generally categorized into functional groups implicated in DNA damage checkpoint, cell cycle, apoptosis, immune response, and hematopoietic differentiation, respectively (see figure). On the basis of these findings, they hypothesized that the downregulation of genes required for maintenance of DNA stability might result in genomic instability. Indeed, analyzing a single patient in whom a sufficient number of −7 cells were sequenced, they detected a higher rate of single-nucleotide polymorphisms in more differentiated CD34+CD38+ HSPCs relative to diploid counterparts and more quiescent CD34+CD38− HSPCs with −7.
Stemming from aberrant HSPCs, myeloid malignancies are increasingly viewed as a spectrum of diseases, ranging from clonal hematopoiesis to MDS and AML. The findings by Zhao et al elegantly offer insight into HSPC biology in the context of −7. Their work, as well as recent work by Giustacchini et al9 investigating stem cells in BCR-ABL CML, provides the first example of how scRNA-seq enables in vivo studies of rare aberrant hematopoietic stem cells, illuminating dysregulated cellular pathways that may facilitate transformation to myeloid malignancies. Their data sets represent resources for investigating the early steps of human HSPC differentiation and gaining insights into early leukemogenesis induction at the single-cell level. Notably, data obtained so far are correlative. Future efforts need to focus on validating findings in greater numbers of patients, in addition to identifying more definitive causal relationships between genes and function. Combined with insights from systematic efforts to delineate how distinct chromosomal abnormalities as well as gene mutations interact to determine transformation to leukemia,10 evaluation of HSPC genetics at the single-cell level may in the future prove useful for monitoring treatment and prognosis of patients with myeloid neoplasms. Furthermore, knowing the functional consequences of chromosome gain or loss will help in exploring gene targets for new directed therapies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal