Key Points
Intravenous fluids are used when treating VOE, but guidelines are lacking, and how IVF tonicity affects sickle red cell biomechanics is unknown.
Modifying extracellular fluid tonicity alters deformability, adhesivity, and occlusion risk for sRBCs in microfluidic vascular models.
Abstract
Abnormal sickle red blood cell (sRBC) biomechanics, including pathological deformability and adhesion, correlate with clinical severity in sickle cell disease (SCD). Clinical intravenous fluids (IVFs) of various tonicities are often used during treatment of vaso-occlusive pain episodes (VOE), the major cause of morbidity in SCD. However, evidence-based guidelines are lacking, and there is no consensus regarding which IVFs to use during VOE. Further, it is unknown how altering extracellular fluid tonicity with IVFs affects sRBC biomechanics in the microcirculation, where vaso-occlusion takes place. Here, we report how altering extracellular fluid tonicity with admixtures of clinical IVFs affects sRBC biomechanical properties by leveraging novel in vitro microfluidic models of the microcirculation, including 1 capable of deoxygenating the sRBC environment to monitor changes in microchannel occlusion risk and an “endothelialized” microvascular model that measures alterations in sRBC/endothelium adhesion under postcapillary venular conditions. Admixtures with higher tonicities (sodium = 141 mEq/L) affected sRBC biomechanics by decreasing sRBC deformability, increasing sRBC occlusion under normoxic and hypoxic conditions, and increasing sRBC adhesion in our microfluidic human microvasculature models. Admixtures with excessive hypotonicity (sodium = 103 mEq/L), in contrast, decreased sRBC adhesion, but overswelling prolonged sRBC transit times in capillary-sized microchannels. Admixtures with intermediate tonicities (sodium = 111-122 mEq/L) resulted in optimal changes in sRBC biomechanics, thereby reducing the risk for vaso-occlusion in our models. These results have significant translational implications for patients with SCD and warrant a large-scale prospective clinical study addressing optimal IVF management during VOE in SCD.
Introduction
Sickle cell disease (SCD) is an inherently biophysical disorder, in which hypoxia-driven intracellular hemoglobin S (Hb S) polymerization confers sickle red blood cells (sRBCs) with increased adhesion and decreased deformability through changes at the membrane and cytoplasmic levels, which predisposes to occlusion in the microcirculation.1-3 Although therapies for SCD often focus on manipulating these pathologic biomechanical properties,4-6 evidence-based guidelines for the optimal treatment of some SCD complications remain elusive.7,8
Specifically, vaso-occlusive pain episodes (VOE), the most common SCD complication,9 remain difficult to treat despite improvements in our understanding of their pathophysiology.7,8,10 In particular, intravenous fluids (IVFs) are a common part of the backbone of therapy during VOE, as patients are often dehydrated.4,11-14 However, the specific type of IVF to administer during VOE remains controversial, and this issue was not adequately addressed by the most recent National Heart, Lung, and Blood Institute guidelines on the treatment of SCD.12,15 Many clinicians use normal saline (NS), a hyperosmolar IVF, during the treatment of VOE.11,16 Others discourage the use of NS because of concerns that patients with SCD-related kidney dysfunction may not effectively excrete its high-sodium, hyperosmolar load.13,14,17 Further, exposure to NS could theoretically worsen vaso-occlusion, as increased plasma osmolality can lead to sRBC dehydration, increased intracellular Hb S concentration (ie, mean corpuscular hemoglobin concentration [MCHC]), and increased rate of polymerization under deoxygenated conditions, which can affect cytoplasmic and membrane properties of sRBCs.1,18-20 Other providers, therefore, advocate administering hypotonic IVFs during VOE.13,14 Reducing plasma osmolality with these IVFs may improve the rheological behavior of sRBCs by improving their hydration and diluting the MCHC, which could improve deformability and allow sRBCs to escape the capillary bed before deoxygenation can cause Hb S polymerization and vaso-occlusion.1,19,21 A limited study by Rosa et al reported that in 3 patients with SCD, sustaining hyponatremia (ie, sodium = 120-125 mEq/L) reduced the frequency of VOE compared with the same patients when they were treated conventionally.22 Another small study of 7 patients by Guy et al reported positive results using the rapid administration of the hypotonic IVF 1/2NS (sodium = 77 mEq/L) to treat VOE, as most patients experienced pain improvement and decreased circulating sickle cells after treatment.23 However, poor patient tolerance of prolonged hyponatremia, including seizures, has precluded the widespread use regimens like these to treat VOE.24
Robust preclinical, mechanistic studies elucidating how such hyponatremia and reduced extracellular tonicity may improve sRBC biomechanics in the microvasculature are lacking. As the pathophysiology of vaso-occlusion is complex, involving many factors external to the sRBCs, including white blood cells, platelets, and the endothelium,4,6,9,10,25-29 studies in human or animal models are too biologically complex to control for all external biophysical parameters that influence vaso-occlusion pathophysiology. Other confounders of in vivo systems include complex genetic variations imparted by individual circulatory systems (ie, microvascular vessel size, geometry, shear stress).30-33 Other models, such as in vitro bulk rheological measurements in blood suspensions and static adhesion assays, may not reflect the biophysical behavior of blood at the microcirculatory level under physiological flow conditions. Microfluidic devices, however, serve as ideal preclinical, reductionist models to study hematologic processes under physiologic conditions at the micron scale and show considerable promise for biomedical research related to SCD and other blood disorders.34 We and others have demonstrated that experimental findings from microfluidic approaches may correlate with patient clinical course.35-41
In this work, we investigate how admixtures of IVFs with various sodium and tonicity levels alter sRBC predisposition to vaso-occlusion using microfluidic devices that model biophysical components of the human microvasculature. We demonstrate that altering extracellular tonicity can alter sRBC deformability, transit time, and occlusion in capillary-like networks under normoxic and hypoxic conditions, as well as sRBC adhesion to both human endothelium and the subendothelial matrix protein laminin under postcapillary venule shear conditions. These results may have clinical implications for the treatment of VOE.
Materials and methods
All blood samples were drawn according to institutional review board–approved protocols per the Declaration of Helsinki. Additional methods are included in supplemental Materials, available on the Blood Web site.
Generation of fluids and measuring changes in MCHC and MCV
Premade, clinical grade IVFs (Baxter; NS [sodium = 154 mEq/L], 5% dextrose in water [D5W; sodium = 0 mEq/L], D5+1/2NS [5% dextrose in water with 77 mEq/L sodium], D5+1/4NS [5% dextrose in water with 34 mEq/L sodium]) were mixed with phosphate-buffered saline (PBS; Sigma, sodium = 137 mEq/L) to prepare admixtures of various osmolalities and sodium concentrations for experiments.42 MCHC values were determined as the quotient of measured hemoglobin/hematocrit, as previously described.43 Relative mean corpuscular volume (MCV) values were calculated on the basis of changes in MCHC values from baseline (supplemental Materials).
Deformability and occlusion experiments
Microfluidic devices with geometries of the human capillary system were fabricated as previously described.36 To isolate sRBCs, whole blood was drawn into citrate tubes and washed 3 times in PBS to remove other blood components. Packed sRBCs were then diluted to 0.5% hematocrit (HCT) in the various admixtures, mixed for 10 minutes, and perfused into microfluidic devices via a syringe pump (Harvard Apparatus) for 2 minutes at 0.3 µL/min, resulting in velocities similar to that seen in the human capillary system.44-46 Transit times, velocities, and propensity for occlusion of sRBCs under normoxic conditions were tracked using video microscopy (supplemental Movie 1).
Adhesion experiments
Human placental laminin.
Microfluidic devices consisting of 4 lanes with separate inlets and outlets connected by microchannels were bonded to glass slides and stored overnight at 4°C after coating lanes with 10 µg/mL human placental laminin (Sigma), as previously described.47 Devices were slowly brought to room temperature and each lane washed with PBS twice before each experiment. sRBCs were isolated from citrated whole blood after removing plasma and were washed 3 times in PBS. sRBCs were then suspended in PBS to 0.2% HCT and perfused into each lane.47 Adherent cells were counted along the straight portion of each lane. sRBCs in each lane were exposed to different admixtures at 1 to 5 dyne/cm2 for 3 minutes, and a postflow cell count was performed.
Human umbilical vein endothelial cells.
The 4-chamber microfluidic device was “endothelialized” with human placental laminin (human umbilical vein endothelial cells), as previously described.37,38 Fluorescently stained sRBCs (supplemental Materials) were first washed and isolated from whole blood drawn into citrate tubes, as described earlier, and diluted to 25% HCT in the various admixtures, mixed for 10 minutes, and perfused for 5 minutes across the endothelium at 1 to 5 dyne/cm2. Video fluorescence microscopy was used to quantify sRBCs adherent on a single plane of endothelium between the straightest portions of the device, as curved geometries can affect adhesion characteristics in vitro.41
Deoxygenation experiments
For transit time measurements under deoxygenated conditions, we used a system that diffusively couples an oxygen gas reservoir to a microfluidic.39 Whole blood was drawn into EDTA tubes (BD Biosciences) and washed 3 times in PBS to remove other blood components. Packed sRBCs were then resuspended in the 3:1 admixtures to 0.5% HCT, as described earlier, and perfused through the capillary device under deoxygenation conditions. Transit times were measured in the device while going from oxygenated to deoxygenated conditions (supplemental Movie 2).
For blood rheology deceleration rate measurements, sRBCs were washed 3 times in the admixtures after removing plasma and then diluted to native HCT in the admixtures. sRBCs were then perfused through our rheological measurement platform at 37°C as oxygen gas tension was cycled from oxygenated to deoxygenated conditions, as previously described.39 Rheological differences were determined for each sample by comparing the exponential deceleration rate of the decaying blood velocity on deoxygenation. Deceleration rates were determined by fitting the deoxygenated blood velocity region to the exponential decay function
where kdecel is the exponential deceleration rate.
Statistical analysis
OriginPro 2016 was used for statistical analyses. Nonparametric Kruskal-Wallis analysis of variance and Mann-Whitney analysis was used for transit times and rheological time constant measurements, respectively. One-way analysis of variance was used to compare MCHC, MCV, and sRBC adhesion to endothelium among the various admixture conditions. The 2-sample proportion test was used to compare pre-/postadhesion to laminin. Nonlinear regression analysis was used to compute R2 values. P < .05 was considered significant.
Results
Effect of fluid tonicity on sRBC MCHC and MCV
To determine the effects of extracellular fluid tonicity and sodium concentrations on sRBC MCHC and MCV, we first diluted PBS with the various IVFs (NS, D5+1/2NS, D5+1/4NS, and D5W with sodium 154, 77, 34, and 0 mEq/L, respectively) to obtain a panel of admixtures with physiologically comparable osmolalities (normal range, ∼275-295 mOsm/kg).42 A 3:1 dilution (ie, 75% volume PBS and 25% volume IVF) resulted in admixtures with osmolalities of 275 to 313 mOsm/kg and sodium concentrations of 103 to 141 mEq/L, so we implemented a 3:1 dilution for all experimental protocols. Vapor pressure osmometry was used to confirm such physiologic osmolalities can be similarly observed using 3:1 admixtures of patient plasma and IVFs (supplemental Methods; supplemental Table 1).
Next, to determine how exposure to high volumes of these clinical IVFs of various tonicities may affect the MCHC and MCV of sRBCs, changes in these parameters from baseline were measured in quadruplicate, using samples from 3 patients with SCD (Figure 1A-B). Expectedly, sRBC MCHC decreased when blood was mixed with hypotonic fluids. However, MCHC slightly increased when sRBCs were washed in NS admixtures (Figure 1A). A similar tonicity-driven divergence in MCV was observed, with all hypotonic fluids increasing MCV compared with the NS admixtures, although D5W admixtures caused the greatest increase in MCV (Figure 1B). Interestingly, similar changes were observed with blood from normal donors, although with greater variability (supplemental Figure 1A-B).
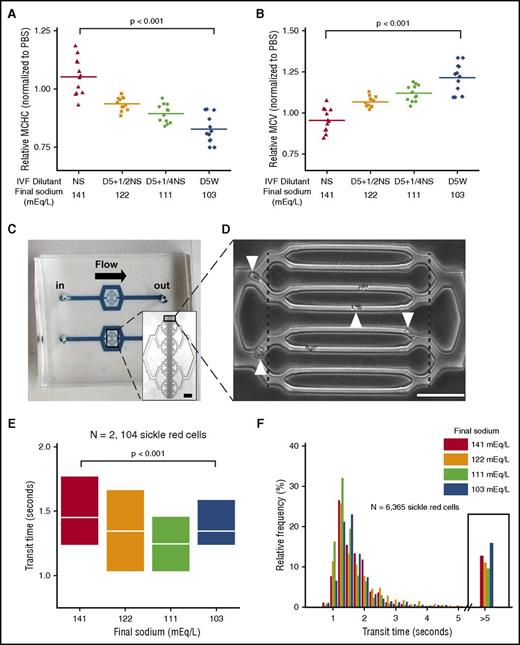
Fluid admixtures with various tonicities alter sRBC biomechanics and transit times in a microfluidic model of the capillary bed. (A-B) Using commonly used IVFs, increased extracellular fluid tonicity increases cytoplasmic viscosity (MCHC) and hypotonic fluids increase size (MCV) of sRBCs, as demonstrated in results from N = 3 patient blood samples. (C) The capillary-sized microdevice measures 1.5 cm from inlet to outlet. (Inset) 4× view; scale bar, 100µm. (D) The smallest channels (8 are shown in the figure) are ∼6 µm wide × ∼13 µm tall. Transit times of sRBCs from 4 patient blood samples were measured as the time required for each cell to traverse the distance between the dashed lines. Occlusion and transit time data were analyzed from 28 microchannels for each experiment (20× view; scale bar, 50µm; white arrowheads are transiting sRBCs). (E) Transit times of sRBCs that traversed unobstructed microchannels were measured after exposure to the different IVF admixtures. White lines and boxes represent median values and 25th and 75th percentiles, respectively. (F) Relative frequency plots show transit times of all sRBCs from the 4 patient blood samples in the different conditions, including stuck sRBCs that have very prolonged transit times, defined as more than 5 seconds. Admixtures with the highest and lowest tonicities were associated with an increased frequency of stuck sRBCs and other poorly deformable cells with very prolonged transit times (ie, >5 s; black boxed data) that caused transient obstruction before finally traversing microchannels.
Fluid admixtures with various tonicities alter sRBC biomechanics and transit times in a microfluidic model of the capillary bed. (A-B) Using commonly used IVFs, increased extracellular fluid tonicity increases cytoplasmic viscosity (MCHC) and hypotonic fluids increase size (MCV) of sRBCs, as demonstrated in results from N = 3 patient blood samples. (C) The capillary-sized microdevice measures 1.5 cm from inlet to outlet. (Inset) 4× view; scale bar, 100µm. (D) The smallest channels (8 are shown in the figure) are ∼6 µm wide × ∼13 µm tall. Transit times of sRBCs from 4 patient blood samples were measured as the time required for each cell to traverse the distance between the dashed lines. Occlusion and transit time data were analyzed from 28 microchannels for each experiment (20× view; scale bar, 50µm; white arrowheads are transiting sRBCs). (E) Transit times of sRBCs that traversed unobstructed microchannels were measured after exposure to the different IVF admixtures. White lines and boxes represent median values and 25th and 75th percentiles, respectively. (F) Relative frequency plots show transit times of all sRBCs from the 4 patient blood samples in the different conditions, including stuck sRBCs that have very prolonged transit times, defined as more than 5 seconds. Admixtures with the highest and lowest tonicities were associated with an increased frequency of stuck sRBCs and other poorly deformable cells with very prolonged transit times (ie, >5 s; black boxed data) that caused transient obstruction before finally traversing microchannels.
Effect of fluid tonicity on sRBC deformability
Our group recently used atomic force microscopy to show that prolonged exposure to NS stiffens sRBCs and prolongs transit times in capillary-sized microchannels compared with isotonic and hypotonic fluids.48 Intrigued by these results, we investigated the effect of brief (10 minutes) exposure to IVF admixtures of various tonicities on sRBC deformability and occlusion in the microcirculation by measuring sRBC transit times in a model of the human capillary system (supplemental Movie 1).
In the capillary model (Figure 1C),36 sRBCs must readily deform to safely transit through the microchannel system without getting stuck (Figure 1D; supplemental Figure 2). sRBCs (N = 2104) from 4 separate patients with SCD were analyzed. Interestingly, admixtures with the highest and lowest tonicities (sodium concentrations of 103 and 141 mEq/L, respectively) were associated with prolonged transit times and decreased velocities through the microchannels compared with those with intermediate tonicities (Figure 1E; supplemental Figure 3). On one end, sRBCs exposed to the higher tonicity fluids exhibited longer transit times, likely in part because of sRBC dehydration and an increase in MCHC, which stiffens sRBCs and decreases their deformability.20,48 On the other end, sRBCs exposed to the excessively hypotonic fluid admixtures exhibited increased transit times likely in part as a result of decreased deformability from cellular overhydration, pathologically altering the ideal surface area-to-volume relationship needed for microchannel transit. sRBCs exposed to intermediate tonicities exhibited transit times between these 2 extremes. Thus, there appear to be competing effects of MCV and MCHC on the microchannel transit time of sRBCs. However, when RBCs isolated from healthy donors were placed in similar tonicity conditions, such changes in transit times were not observed (supplemental Figure 4), which has been previously described.49
Next, as increased transit times in the capillary bed can increase the rate of sickling and obstruction,1,26 we quantified subpopulations of sRBCs within each experiment that exhibited significantly prolonged transit times in the microfluidic system. We analyzed 6365 sRBCs. Interestingly, the number of sRBCs with very prolonged transit times (ie, >5 s), which included stuck cells (ie, sRBCs with cessation of flow within microchannels), was elevated in the highest and lowest sodium conditions (Figure 1F). These data suggest that sRBCs exposed to these tonicities may give rise to sRBC populations that cause transient or permanent microvascular occlusion through specific changes in MCHC and/or MCV, further indicating that stiffness and swelling mediate sRBC transit time.
Admixture tonicity alters microchannel occlusion under normoxic conditions
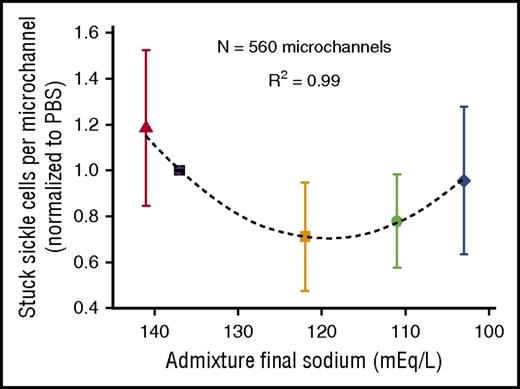
We then analyzed each fluid admixture’s contribution to sRBC microchannel occlusion. Overall, we analyzed 8857 sRBCs that traversed or occluded 560 microchannels (N = 20 experiments; N = 28 microchannels per experiment). Although all conditions resulted in some degree of occlusion, the relationship between fluid sodium concentration and percentage of microchannels occluded exhibited a strong parabolic relationship (R2 = 0.99), with admixtures with the highest and lowest sodium concentrations showing increased propensity for microchannel occlusion (Figure 2). These results reiterate that optimal deformability of sRBCs in the microchannels is associated with exposure to extracellular fluid tonicities between these 2 admixtures, with higher tonicities increasing sRBC stiffness and decreasing deformability, and very low tonicities overhydrating sRBCs and decreasing deformability.20,48 Exposure to the admixtures with intermediate tonicities appears to optimize sRBC deformability by minimizing stiffness and overhydration, thereby decreasing occlusion risk.
Microchannel occlusion depends on fluid admixture final sodium and tonicity. The average number of sRBCs occluding microchannels was dependent on final sodium concentrations and tonicities of the fluid admixtures. Data represent results from a total of 8857 sRBCs analyzed from 4 patient samples (N = 5 conditions per sample; N = 20 total experiments; N = 300-500 red cells/experiment; N = 28 microchannels/experiment; N = 560 total microchannels). Boxes and bars represent mean ± standard error of the mean.
Microchannel occlusion depends on fluid admixture final sodium and tonicity. The average number of sRBCs occluding microchannels was dependent on final sodium concentrations and tonicities of the fluid admixtures. Data represent results from a total of 8857 sRBCs analyzed from 4 patient samples (N = 5 conditions per sample; N = 20 total experiments; N = 300-500 red cells/experiment; N = 28 microchannels/experiment; N = 560 total microchannels). Boxes and bars represent mean ± standard error of the mean.
Effect of fluid tonicity on sRBC occlusion under hypoxic conditions
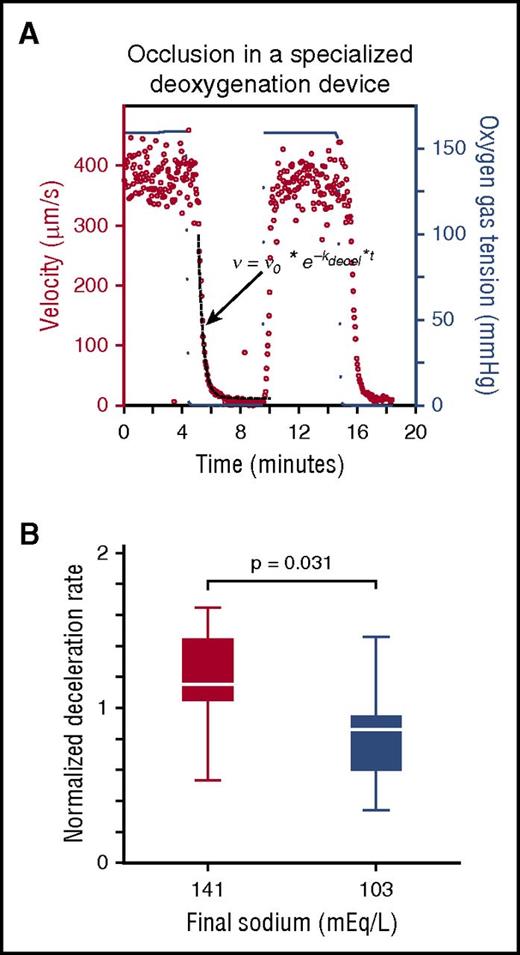
Exposure to the highest and lowest fluid tonicities were both consistently associated with reduced sRBC deformability in our models, suggesting adverse effects of increases in MCHC and MCV, respectively. We thus explored which of these factors may dominate risk for vaso-occlusion under hypoxic conditions, which is paramount in studying SCD pathophysiology. Specifically, we assessed the effect that the highest- and lowest-sodium admixtures have on sRBCs when perfused through 2 microfluidic platforms under deoxygenated conditions. First, patient sRBCs were resuspended in the above admixtures and perfused through the same capillary microfluidic device, but in a deoxygenation chamber (supplemental Figure 5A; supplemental Movie 2).39 Exposure to the high-sodium admixture increased transit times compared with that of the low-sodium admixture under slow deoxygenation (supplemental Figure 5B). Exposure to 0% oxygen rapidly induced occlusion in both conditions in this model, rendering further deformability differences between the admixtures difficult to measure. Therefore, we took an alternative approach to monitoring sickle cell blood rheology. Whole sickle blood samples (N = 10) were washed and carefully resuspended at native HCT in these 2 admixtures and perfused through a separate microfluidic system that recapitulates the venous circulation and enables rapid switching from oxygenated to deoxygenated conditions, as previously described.39 Our previous findings show that the deceleration rate of blood in this model clinically correlates with hospitalizations and disease severity in patients with SCD. Using this platform, we quantified the rate of velocity change of blood in the different fluid tonicities after deoxygenation (ie, changes in oxygen gas tension) by fitting the blood velocity to an exponential decay function (dashed line in Figure 3A) and calculating the exponential deceleration rate, kdecel (Equation 1).
Under deoxygenated conditions, sickle blood rheology is differentially affected by fluid admixtures with opposing tonicities. (A) Representative rheological plot of whole sickle blood resuspended in a fluid (fluid with final sodium = 141 mEq/L shown). Blood velocity (red dots, left side y-axis) responds to changes in oxygen tension (blue dots, right side y-axis). Blood velocity exponential deceleration rates were calculated by fitting the deoxygenated blood velocity region to an exponential decay function (dashed black line, equation). (B) Deceleration rates for blood samples were calculated from N = 10 patients with SCD. Samples exposed to admixtures with sodium concentrations of 141 mEq/L were significantly higher than blood samples exposed to sodium concentrations of 103 mEq/L.
Under deoxygenated conditions, sickle blood rheology is differentially affected by fluid admixtures with opposing tonicities. (A) Representative rheological plot of whole sickle blood resuspended in a fluid (fluid with final sodium = 141 mEq/L shown). Blood velocity (red dots, left side y-axis) responds to changes in oxygen tension (blue dots, right side y-axis). Blood velocity exponential deceleration rates were calculated by fitting the deoxygenated blood velocity region to an exponential decay function (dashed black line, equation). (B) Deceleration rates for blood samples were calculated from N = 10 patients with SCD. Samples exposed to admixtures with sodium concentrations of 141 mEq/L were significantly higher than blood samples exposed to sodium concentrations of 103 mEq/L.
The exponential deceleration rates for blood samples in high-sodium fluids were significantly higher than those in the low-sodium samples under deoxygenated conditions (P = .031), indicating a more rapid decrease in microchannel velocity of sRBCs after deoxygenation in the microfluidic device at this higher tonicity (Figure 3B). Under deoxygenated conditions, the rise in MCHC associated with low sRBC deformability after exposure to high-sodium concentrations appears to be a more dominant factor than the effect of sRBC overswelling characteristic of excessively hypotonic fluids. These data may suggest a correlation between sRBC exposure to higher-sodium fluids and adverse SCD clinical outcomes under certain conditions.39
Effect of fluid tonicity on sRBC adhesion to endothelium and laminin
The contribution of extracellular fluid tonicity on sRBC adhesion to endothelium and the subendothelial matrix protein laminin, a protein component of the microvascular wall that adheres to the Lu/BCAM receptor on sRBCs and is believed to play a role in VOE,47,50 has not been elucidated. Thus, we aimed to determine how manipulating the tonicity of the extracellular milieu might affect sRBC adhesion to these vascular components. To that end, parallel microchannels of a 4-chambered microfluidic device (Figure 4A) were endothelialized with human umbilical vein endothelial cells (Figure 4B), as previously described,37,38 to determine the effects these various fluid admixtures have on the adhesive interactions between sRBCs and human endothelium under postcapillary venular shear stress. We also investigated how varying extracellular tonicity alters specific sRBC–laminin interactions after immobilizing human placental laminin to nonendothelialized channels and perfusing sRBCs at similar shear stresses.
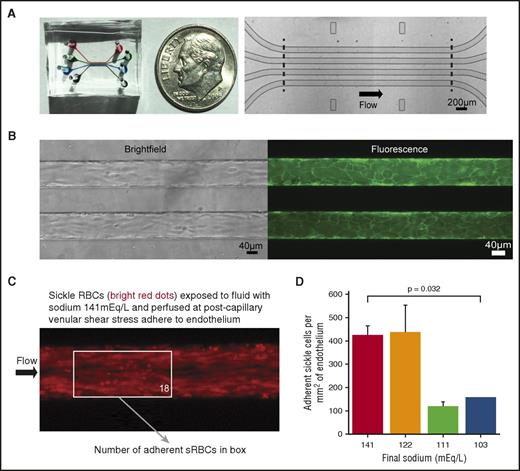
Sickle red blood cells exposed to lower sodium fluids are less adherent to human endothelium under postcapillary venular shear stress. (A) Macroscopic (left) and microscopic (right) images of the 4-chamber microfluidic device, whereby 4 lanes with separate inlets and outlets are each 46 µm tall, 100 µm wide, and 4 mm in length along the uncurving portion of the device. (B) Brightfield (left) and fluorescence (right, membrane dye) microscopy images of the microfluidic device endothelialized to confluence. (C) Fluorescence images of stained sRBCs adhered to human umbilical vein endothelial cells. sRBCs exposed to fluids with higher sodium levels adhere to endothelium under a postcapillary venular shear stress of 1 dyne/cm2. (Boxed number corresponds to number of sRBCs adherent to endothelium in that region of interest.) (D) In total, sRBCs were isolated from 6 patients. sRBCs were less adherent when exposed to the most hypotonic admixtures. Bars and lines represent mean ± standard deviation from 3 patients for each experimental condition, except the most hypotonic (N = 2).
Sickle red blood cells exposed to lower sodium fluids are less adherent to human endothelium under postcapillary venular shear stress. (A) Macroscopic (left) and microscopic (right) images of the 4-chamber microfluidic device, whereby 4 lanes with separate inlets and outlets are each 46 µm tall, 100 µm wide, and 4 mm in length along the uncurving portion of the device. (B) Brightfield (left) and fluorescence (right, membrane dye) microscopy images of the microfluidic device endothelialized to confluence. (C) Fluorescence images of stained sRBCs adhered to human umbilical vein endothelial cells. sRBCs exposed to fluids with higher sodium levels adhere to endothelium under a postcapillary venular shear stress of 1 dyne/cm2. (Boxed number corresponds to number of sRBCs adherent to endothelium in that region of interest.) (D) In total, sRBCs were isolated from 6 patients. sRBCs were less adherent when exposed to the most hypotonic admixtures. Bars and lines represent mean ± standard deviation from 3 patients for each experimental condition, except the most hypotonic (N = 2).
For endothelialized experiments, fluorescently stained sRBCs were diluted to 25% HCT in our 3:1 admixtures and perfused across nonactivated endothelium (Figure 4C). Among the admixtures, sRBCs exposed to sodium concentrations of 141 and 122 mEq/L were more adherent than cells exposed to the most hypotonic fluid admixtures (ie, sodium concentrations 111 and 103 mEq/L) when perfused at 1 dyne/cm2 (Figure 4D; supplemental Figure 6; P = .032). Similar experiments using RBCs from healthy donors exhibited a marked decrease in adhesion to endothelium compared with sRBCs in all conditions, while showing no difference in adhesion in the various tonicity fluids among themselves (supplemental Figure 7).
Next, we investigated the effect of different admixtures on the sRBC/laminin interaction. sRBCs were first perfused into lanes of laminin-coated devices, and adherent, immobilized cells were quantified (Figure 5A). The various admixtures were then perfused across adherent cells at postcapillary venular shear stress, and the cells remaining after flow were enumerated. A total of N = 1750 sRBCs were analyzed, ∼100 cells per experiment. Compared with the high-sodium admixture, all hypotonic fluid admixtures led to a significant decrease in sRBC–laminin adhesion at 4.5 dyne/cm2 (Figure 5B; supplemental Figure 8), suggesting only brief exposure to mildly hypotonic conditions is needed to overcome the laminin/sRBC interaction under this postcapillary shear stress.
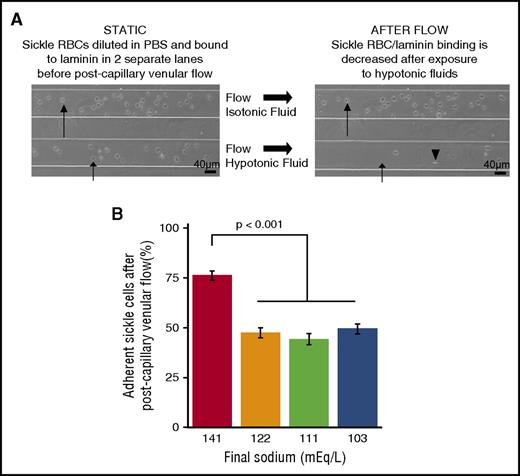
Admixture tonicity affects adhesion of sickle red cells to human laminin under post-capillary venular shear stress. (A) Brightfield microscopy images showing that sRBCs adhered to laminin become less adherent when exposed to hypotonic fluids at postcapillary shear stress. (Long arrow) sRBC exposed to isotonic fluid remaining adhered after flow. (Short arrow) sRBC exposed to hypotonic fluid becoming nonadherent and moving (arrowhead)). (B) sRBCs adhered to laminin-coated slides became less adherent after exposure to hypotonic fluids at postcapillary venule shear stress compared with admixtures containing higher sodium levels. (Bars and lines represent mean percentages ± standard error of the mean from N = 3 patients.)
Admixture tonicity affects adhesion of sickle red cells to human laminin under post-capillary venular shear stress. (A) Brightfield microscopy images showing that sRBCs adhered to laminin become less adherent when exposed to hypotonic fluids at postcapillary shear stress. (Long arrow) sRBC exposed to isotonic fluid remaining adhered after flow. (Short arrow) sRBC exposed to hypotonic fluid becoming nonadherent and moving (arrowhead)). (B) sRBCs adhered to laminin-coated slides became less adherent after exposure to hypotonic fluids at postcapillary venule shear stress compared with admixtures containing higher sodium levels. (Bars and lines represent mean percentages ± standard error of the mean from N = 3 patients.)
Discussion
sRBC biomechanical properties are dependent on intrinsic (ie, membrane and cytosolic contents) and extrinsic (ie, environmental osmolality, surface area-to-volume ratio, oxygen tension) factors to the red cell.4 Reduced sRBC deformability and increased adhesion, which are directly linked to VOE, can result from cellular dehydration and can subsequently disrupt microvascular blood flow.2,4,51 Clinically, IVFs are used to affect blood rheology under various circumstances; namely, to maintain blood volume and prevent dehydration.52 However, despite no supporting evidence, hyperosmolar IVFs such as NS are often used to treat VOE in SCD and are often infused as boluses, sometimes at ∼25% of total intravascular volume.11,16 We recently showed that such bolus volumes of NS negatively affect the pain of some patients with SCD during VOE.16
By leveraging novel in vitro microfluidic models, we have investigated how exposure to fluids with various sodium concentrations and tonicities alter sRBC biomechanics and propensity for vaso-occlusion. Our results may have clinical implications related to SCD, as IVF management during VOE remains an important and unresolved issue in clinical care for patients with this disease. Further, these data suggest that by directly altering the hydration status of sRBCs via exposure to fluids of varying tonicities, sRBC deformability, adhesion, and propensity for microvascular occlusion can be altered (Figure 6A-D). Specifically, hypotonic admixtures with sodium concentrations 111 to 122 mEq/L had an optimal effect on sRBC biomechanics in our models. Interestingly, this is a similar range noted in historical clinical results that investigated the effect of hyponatremia on MCHC and VOE.22
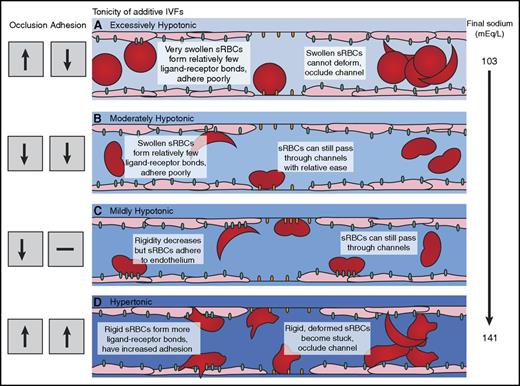
Effect of extracellular fluid tonicity on sickle red blood cell deformability and adhesion. (A) Exposure to excessively hypotonic admixtures increases occlusion risk for sRBCs resulting from excessive cellular hydration and swelling, although decreased microvascular adhesion is observed. (B) sRBCs exposed to moderately hypotonic admixtures exhibit increased deformability and decreased adhesion in our microfluidic models. (C) Exposure to mildly hypotonic admixtures increases sRBC adhesion to endothelium but decreases adhesion to laminin. Optimal deformability through microchannels, with decreased occlusion, is maintained. (D) Admixtures with increased sodium levels increase adhesion and occlusion risk in our microvascular models. Increased occlusion from exposure to these higher sodium levels was observed under both normoxic and hypoxic conditions.
Effect of extracellular fluid tonicity on sickle red blood cell deformability and adhesion. (A) Exposure to excessively hypotonic admixtures increases occlusion risk for sRBCs resulting from excessive cellular hydration and swelling, although decreased microvascular adhesion is observed. (B) sRBCs exposed to moderately hypotonic admixtures exhibit increased deformability and decreased adhesion in our microfluidic models. (C) Exposure to mildly hypotonic admixtures increases sRBC adhesion to endothelium but decreases adhesion to laminin. Optimal deformability through microchannels, with decreased occlusion, is maintained. (D) Admixtures with increased sodium levels increase adhesion and occlusion risk in our microvascular models. Increased occlusion from exposure to these higher sodium levels was observed under both normoxic and hypoxic conditions.
sRBCs exposed to admixtures with sodium concentrations of 103 mEq/L displayed significantly reduced deformability and increased risk for occlusion in our normoxic models (Figure 6A), suggesting that excessive hydration of sRBCs may be detrimental in certain in vivo settings. Admixtures with sodium concentrations of 111 mEq/L appeared to optimize changes in sRBC deformability (Figure 6B), likely in part because of ideal changes in MCHC, the most sensitive predictor of Hb S polymerization and microvascular occlusion,1,4 and also an optimization of the surface area-to-volume ratio necessary for sRBCs to traverse capillary microchannels. Admixtures with sodium concentrations of 122 mEq/L maintained this improved deformability in our models (Figure 6C). sRBCs exposed to the highest sodium levels of 141 mEq/L had reduced deformability and increased propensity for occlusion in our models (Figure 6D), which was likely in part a result of induced changes in intrinsic properties of sRBCs (ie, increased viscosity at the cytoplasmic level and increased rigidity at the membrane level).3,20,48,53 These results may have clinical implications related to VOE, as the number of occluding cells in the microcirculation, along with rate of occlusion and reopening of capillary microvessels, can affect transit times and increase the risk of exacerbating the dynamic vaso-occlusion process in SCD. sRBCs are also the major contributor to the initiation of vaso-occlusion that leads to pathologic microvascular log jamming,4 and the percentage of capillaries obstructed may affect the degree of ischemia associated with VOE.1 Furthermore, it is well documented that the small increases in intracellular Hb S that result from sRBC dehydration in hyperosmolar conditions can lead to a significant reduction in the delay time for polymerization.1,18 This can lead to exponential Hb S polymer growth in sRBCs: delay time for polymerization is inversely proportional to Hb S concentration to the 30th power (ie, delay time for polymerization ∼ 1/[HbS]30 ),54,55 thereby increasing the rigidity of the sRBC, intracytoplasmic viscosity, and therefore propensity for microvascular obstruction under deoxygenated conditions.1,20,26 Deoxygenation experiments reported here further support this.
Abnormal sRBC adhesion to intact microvascular endothelium and subendothelial matrix components also play an important role in the initiation and propagation of vaso-occlusion, and the pathologic adhesiveness of sRBCs has been associated with vaso-occlusion risk in patients.6,28,29 Also, unlike RBCs from healthy people without SCD, sRBCs pathologically adhere to human vascular endothelium and vascular wall components under postcapillary venular conditions where shear stress is 5 dyne/cm2 or less.3,6,29,56,57 We found that sRBCs exposed to admixtures with sodium concentrations of 141 mEq/L were dehydrated with increased MCHC and decreased MCV and also demonstrated increased adhesion to human endothelium and laminin (Figure 6D). When sRBCs are dehydrated, cytoplasmic viscosity and density increases and intracellular hemoglobin congregates near the membrane, rather than in the cytosol, which can alter the landscape of the sRBC cytoskeleton and membrane, and thus the elastic and adhesive properties of the cell.20,28,53,58,59 In contrast, increased sRBC hydration from hypotonic fluid exposure decreases sRBC membrane rigidity and cytoplasmic viscosity, which may alter cell membrane contact with endothelium and subendothelial matrix proteins under postcapillary venular conditions. As previously described,60 these interactions appear to be tightly dependent on tonicity, shear conditions, and the degree of affinity and concentration of the ligand-receptor involved. This is evident in our models, where sRBCs exposed to hypotonic admixtures with sodium levels of 122 mEq/L showed decreased adhesion to laminin but an increase in adherence to the endothelium under postcapillary flow conditions (Figure 6C). Hebbel et al previously described nonspecific sRBC adhesion to endothelium related to cellular hydration status and shape change under conditions similar to ours, but using bovine endothelium and static assays.56 Their results suggested the possibility that changes in the surface charge topography on sRBC membranes caused by low osmolar saline solutions may also contribute to increased sRBC adhesion to the microvasculature, which could also be relevant to our findings.29,56 Also, as volume increases but surface area is held near constant, the resultant RBC shape tends toward a spherocyte (Figure 6).49,61 Such a shape change could lead to less intimate sRBC membrane contact with adhesive antigens in the microcirculation, as higher-density sRBCs are known to have larger areas of adhesive contact to endothelium compared with lower-density sRBCs.3,28 Further, increased adhesion of the dehydrated sRBC may include the pathologic reorganization, fragmentation, or vesiculation of components of the membrane that accompanies such exposures.3 As the degree of sRBC adhesion to human endothelium has been correlated to clinical severity of vaso-occlusive events,6 our findings may have implications where IVFs are used during the treatment of VOE in SCD.
This research poses a translational question that has not been adequately addressed in the clinical realm and needs to be answered, as there is no consensus with respect to which clinical IVFs to use for patients with SCD in a state of acute vaso-occlusive pain.12,15 As with any other research, our investigation does have limitations. For instance, it is unclear from our experiments which classes of sRBCs (eg, reticulocytes, irreversibly sickled cells, etc.) may be more or less affected by tonicity changes. Further, in our reductionist approach, we purposefully ignored the contributions of other cellular and plasma components, which are well-known contributors to the process of vaso-occlusion.28,31-33 Despite these limitations, it is clear extracellular tonicities of clinical IVFs differentially alter sRBC biomechanical properties at the microvascular level, and our findings suggest that appropriate volumes and infusion rates of hypotonic fluids may improve rheological properties of sRBCs while decreasing their risk of occluding the microcirculation. Overall, our findings strongly suggest that a well-controlled, prospectively randomized clinical trial is needed to determine which clinical IVF, if any, is the optimal choice for treating SCD-related VOE, which could ultimately improve clinical outcomes in patients with SCD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the patients who donated blood for the advancement of science and the improvement of treatments for sickle cell disease. The authors also thank all members of the W.A.L. laboratory who contributed to thoughtful discussion during the experimental process and during preparation of the manuscript. The authors also thank the clinical research coordinators at the Aflac Cancer and Blood Disorders Center at Children’s Healthcare of Atlanta for assistance in obtaining and transporting samples. The authors also thank Yvonne Data at the University of Minnesota Medical Center for assistance with blood sample collection, identification, and transport. In addition, the authors thank the Minnesota Nanofabrication Center for support with device fabrication and assembly, John M. Higgins at the Center for Systems Biology at Harvard Medical School, and the Department of Pathology at Massachusetts General Hospital for assistance with tracking blood velocities in deoxygenation experiments. Last, this work was performed in part at the Georgia Tech Institute for Electronics and Nanotechnology, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (Grant ECCS-1542174).
This work was supported by the National Science Foundation CAREER Award 1150235 (W.A.L.); National Institutes of Health, National Heart, Lung, and Blood Institute grants 5U01-HL117721 (W.A.L.), R01HL121264 (W.A.L.), U54HL112309 (W.A.L.), R21HL130818 (D.K.W.), and R56HL132906 (D.K.W.); and American Heart Association grant 13SDG6450000 (D.K.W.) and predoctoral fellowship grant 16PRE31020025 (X.L.).
Authorship
Contribution: M.A.C., M.E.F., and X.L. designed the study, performed experiments, collected and analyzed data, designed figures, and wrote the manuscript; R.G.M., Y.S., J.C.C., C.E.H., and S.C. assisted in experiments, collected and analyzed data, designed figures, and critically reviewed and revised the manuscript; C.H.J. contributed to study design, provided supervisory support, and critically reviewed and revised the manuscript; D.K.W. and W.A.L. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wilbur A. Lam, Emory University, Departments of Pediatrics and Biomedical Engineering, 2015 Uppergrate Dr, Rm 448, Atlanta, GA 30322; e-mail: wilbur.lam@emory.edu.
References
Author notes
M.E.F. and X.L. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal