Abstract
Anticoagulant-associated heavy menstrual bleeding (HMB) is an underrecognized but not uncommon problem in clinical practice. Premenopausal women should be advised of the potential effect of anticoagulant therapy on menstrual bleeding at the time of treatment initiation. Consequences of HMB should be assessed and treated on an ongoing basis. In the acute setting, the decision to withhold anticoagulants is based on an individual patient’s risk of thrombosis and the severity of the bleeding. For women who require long-term anticoagulation, a levonorgestrel intrauterine system, tranexamic acid (during menstrual flow), high-dose progestin-only therapy, or combined hormonal contraceptives are effective for controlling HMB. The risk of thrombosis during anticoagulant therapy with these treatments is not well studied but is likely to be low. Selection of type of hormonal therapy is based on patient preference, other indications for and contraindications to therapy, adverse effect profile, and ongoing thrombotic risk factors. Women who do not respond to medical treatment or who do not wish to retain their fertility should be considered for surgical management.
Case 1 vignette
A 22-year-old woman with a history of recurrent unprovoked venous thromboembolism (VTE) presented to the emergency department with heavy menstrual bleeding (HMB) of 8 days duration. She had been started on rivaroxaban 20 mg orally once per day some months earlier because of poor international normalized ratio (INR) control on warfarin. After rivaroxaban was initiated, she experienced heavy and prolonged monthly menstrual bleeding that resulted in iron deficiency anemia and a requirement for iron infusions. To reduce HMB, rivaroxaban was stopped for a few days during menses; during one of these interruptions, she developed recurrent pulmonary embolism. Insertion of a levonorgestrel-eluting intrauterine system (LNG-IUS) did not improve her HMB. An ultrasound revealed normal anatomy, and there was no personal or family history of bleeding. The patient reported that her HMB had a significant negative effect on her quality of life.
Case 2 vignette
A 44-year-old woman had a history of provoked deep vein thrombosis that had been treated with warfarin for 3 months. Shortly after warfarin was discontinued, she developed recurrent unprovoked pulmonary embolism and was started on therapeutic doses of rivaroxaban. Coincident with initiating rivaroxaban, she reported HMB. She described passing blood clots associated with abdominal cramps. An earlier pelvic ultrasound had revealed no structural abnormality. On 1 occasion, she required hospital admission and packed red blood cell transfusion because of symptomatic anemia.
Introduction
Physicians and patients share concerns about bleeding complications associated with anticoagulants; overall, patients treated with direct oral anticoagulants (DOACs) have a lower rate of life-threating bleeding when compared with patients treated with low-molecular-weight heparin/vitamin K antagonists (LMWH/VKAs).1 Despite this, there is an increasing focus on the role that DOACs may play in causing abnormal uterine bleeding (AUB) including HMB.2,3 Because HMB is not usually life-threatening, is chronic in nature, and is not usually a separate prespecified end point in clinical trials of anticoagulant therapy, it is underrecognized by physicians. There are limited data on HMB in women who are receiving anticoagulants and, to the best of our knowledge, there are no published or proposed practice guidelines for the management of anticoagulant-associated HMB,4 despite the major impact HMB has on quality of life, especially in women who require long-term anticoagulation. HMB is likely to be associated with a need for direct treatment of its consequences and compromise of anticoagulant compliance, paradoxically increasing the risk of recurrent thromboembolism.5
Definition and brief discussion of the etiology of HMB
A normal menstrual cycle is defined in terms of frequency of 24 to 38 days (the regularity of which can vary between ± 2 and 20 days) with a duration of flow between 4.5 and 8 days and a volume of monthly blood loss between 5 and 80 mL per cycle.6 The term “menorrhagia” has been discarded and replaced by “heavy menstrual bleeding” by the International Federation of Gynecology and Obstetrics classification because of a lack of both specificity and standardization.7 HMB is defined as menstrual blood loss of more than 80 mL per cycle or clinically excessive menstrual blood loss that interferes with a woman’s physical, social, emotional, and/or material quality of life.8,9 AUB is a broader term that includes abnormal bleeding during a menstrual cycle (menstrual bleeding) or abnormal bleeding outside a regular menstrual cycle (intermenstrual bleeding).
The International Federation of Gynecology and Obstetrics has classified the causes of AUB into 9 categories. Abnormalities of the uterus that cause AUB include polyps, adenomyosis, leiomyoma, and malignancy or hyperplasia. Other causes include coagulopathy, ovulatory dysfunction, endometrial dysfunction, iatrogenic etiologies, and those not yet classified. Anticoagulant therapy–associated AUB falls into the nonstructural coagulopathy category, which also includes other hemostatic disorders, such as von Willebrand disease, platelet function disorders, coagulation factor deficiencies, or defects of fibrinogen.7
Does anticoagulant use cause HMB?
VKAs
HMB (with varying definitions) has been reported with the use of VKAs. In observational studies, the incidence of HMB has ranged from 22% to 65% in women treated with VKAs.10-13 A retrospective study reported an increase in HMB from 44% to 71% of patients before and after treatment with VKAs. VKAs significantly increased the duration of menstruation,11,14 flooding, passage of clots, and intermenstrual bleeding.11
DOACs
Cohort studies report that the incidence of HMB in women treated with rivaroxaban ranges from 20% to 27%.15-17 Again, the definition used for HMB varies between studies. A post hoc analysis of a cohort from the EINSTEIN DVT-PE study found that AUB occurred more frequently in the rivaroxaban group compared with the LMWH/VKA group (hazard ratio, 2.13; 95% confidence interval [CI], 1.57-2.89).18 In non-hormonally–treated women, the incidence density of AUB was 30.7% per year in those receiving rivaroxaban compared with 13.4% per year in women treated with warfarin. Prospective data from the EINSTEIN CHOICE study demonstrated a decrease in menstrual flow length and intensity with 10 mg of rivaroxaban compared with 20 mg of rivaroxaban.19 An observational study reported a high incidence of mucosal bleeding, especially uterine and/or ovarian bleeding, in women with VTEs who were treated with rivaroxaban.20
A cohort study reported that HMB occurred in 9.3% of women treated with apixaban.17 In a cohort from the AMPLIFY study, clinically relevant non-major vaginal bleeding occurred in 28 (2.5%) of 1122 women treated with apixaban and in 24 (2.1%) of 1106 women treated with warfarin (odds ratio [OR], 1.2; 95% CI, 0.67-2.0). Although the absolute number of vaginal bleeding events was comparable between recipients of apixaban and enoxaparin or warfarin, the relative occurrence of vaginal bleeds among all clinically relevant non-major bleeds was higher in women treated with apixaban (OR, 3.4; 95% CI, 1.8-6.7).21 In the Hokusai study, vaginal bleeding occurred in 158 patients (9%) treated with edoxaban compared with 126 patients (7.1%) treated with enoxaparin or warfarin.22 A post hoc analysis of a cohort of 1280 women from the pooled RE-COVER I, RE-COVER II, and RE-MEDY trials reported a significantly lower rate of AUB in women treated with LMWH and dabigatran compared with those treated with LMWH and warfarin (5.9% vs 9.6%; OR, 0.59; 95% CI, 0.39-0.90).23
Although DOACs have not been directly compared in clinical trials, data derived from a German DOAC registry demonstrated that the frequency of vaginal bleeding (both cycle-related and non-cycle–related bleeding) was comparable for all factor Xa inhibitors (50 [32%] of 156 with rivaroxaban, 5 [28%] of 18 with apixaban, and 1 [25%] of 4 with edoxaban).24
Unfractionated heparin/LMWH
In theory, unfractionated heparin (UFH)/LMWH should have an effect on menstrual bleeding similar to that of other anticoagulants. However, no study has reported or specifically investigated the effect of UFH/LMWH on menstrual bleeding. The effect of UFH/LMWH on bleeding will be difficult to evaluate because most UFH/LMWH is used for a short duration and/or at a prophylactic dose. In addition, many patients who receive extended therapeutic doses of UFH/LMWH (such as those with cancer-associated thrombosis or those who are pregnant) are not representative of women with regular menstruation.
In summary, HMB associated with anticoagulant use is common. Current evidence suggests the direct factor Xa inhibitors may be associated with a higher risk of this complication than warfarin therapy, but the risk may be lower with dabigatran. More data are needed to confirm this hypothesis.
How to assess HMB in clinical and research settings
In the clinical setting, women treated with oral anticoagulants usually do not spontaneously report their change in menstrual bleeding pattern since they initiated or modified their anticoagulant treatment. Hence, HMB is likely underrecognized by physicians. Discussing the potential effect of anticoagulant treatment on menstrual bleeding at the time of anticoagulant initiation will raise patient awareness of the potential for HMB.
Menstrual bleeding history in reproductive age women who are treated with anticoagulants should be specifically sought. A requirement to change sanitary pads or tampons more often than hourly, clots at least 1 inch in diameter, or a low ferritin level are clinical predictors for HMB.25 An inherited bleeding disorder should be suspected when patients have long-standing HMB or HMB since their menarche. Suspicion of a bleeding disorder on the basis of clinical or family history should trigger further evaluations.26 Women who report increased bleeding should be assessed for bleeding severity, its consequences, and the impact it has on their quality of life. A complete blood count, serum ferritin, INR in women receiving VKAs, serum creatinine in those receiving DOACs, and pregnancy testing should be performed. Other concomitant drugs that could potentiate bleeding should be reviewed. Referral to a gynecologist should be considered to exclude other causes of AUB.
In the research setting, the severity of menstrual bleeding can be assessed by using quantitative or qualitative methods. The volume of menstrual blood loss can be measured by using the alkaline hematin method but its complexity has precluded widespread use.27 The pictorial blood assessment chart is a semi-objective measurement tool that has been validated in the diagnosis of HMB and in the assessment of response to HMB-related treatments.28 However, its sensitivity and specificity vary widely.29 For a qualitative assessment, several menstrual bleeding–specific questionnaires that incorporate quality-of-life assessment have been used.30,31 However, a standardized and validated questionnaire is needed to attenuate variability in outcomes and to increase generalizability.
Management of anticoagulant-associated HMB
Outpatient management
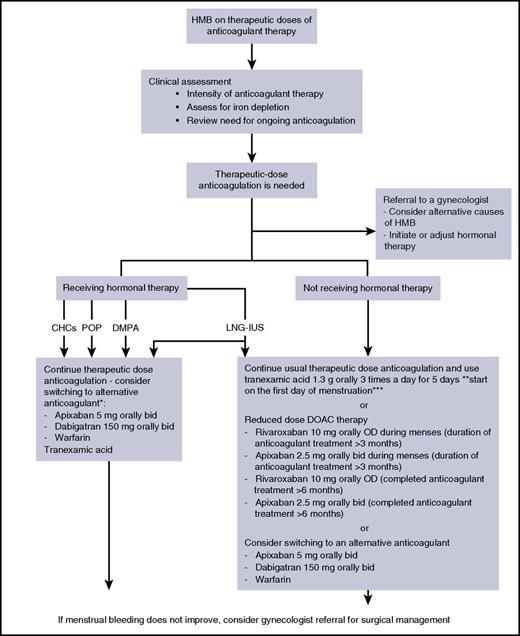
As outlined in Figure 1, patients with iron depletion should receive iron therapy, along with specific HMB-related treatments. Although gynecologists usually initiate specific HMB-related treatments, hematologists are frequently asked to provide an opinion regarding thrombotic risk and anticoagulant management. Likewise, hematologists may be asked to advise on the management of iron deficiency anemia if that has occurred as a complication of the HMB. HMB-related therapies in anticoagulated patients include hormonal therapy, tranexamic acid, anticoagulant management, and surgical interventions.
Suggested strategies for outpatient management of HMB associated with anticoagulants. (*) Ensure good anticoagulant control if on warfarin therapy and compliance if receiving DOAC therapy. If started on combined hormonal contraceptive (CHC), high-dose oral progestogen, or depot medroxyprogesterone acetate (DMPA), hormonal therapy must be discontinued before cessation of anticoagulants. **The recommended dose in the United States; in Europe, the recommended dose is 1 g orally 3 times per day for up to 4 days (dose may be increased to maximum of 4 g/d). ***Thrombotic risk unknown when combined with reduced-dose DOACs. bid, twice per day; OD, once per day; POP, progestin-only pill; tid, 3 times per day.
Suggested strategies for outpatient management of HMB associated with anticoagulants. (*) Ensure good anticoagulant control if on warfarin therapy and compliance if receiving DOAC therapy. If started on combined hormonal contraceptive (CHC), high-dose oral progestogen, or depot medroxyprogesterone acetate (DMPA), hormonal therapy must be discontinued before cessation of anticoagulants. **The recommended dose in the United States; in Europe, the recommended dose is 1 g orally 3 times per day for up to 4 days (dose may be increased to maximum of 4 g/d). ***Thrombotic risk unknown when combined with reduced-dose DOACs. bid, twice per day; OD, once per day; POP, progestin-only pill; tid, 3 times per day.
Hormonal therapy.
CHCs
Although not studied in women who receive anticoagulants, the use of cyclical combined oral contraceptives (COCs) significantly decreases menstrual blood loss and increases hemoglobin levels in women with HMB.32-34 Combined contraceptive patches or vaginal rings have not been specifically studied in women with HMB.
Major adverse effects of COCs include breakthrough bleeding, nausea, headache, and abdominal bloating. The most commonly reported adverse effect with extended or continuous COCs is breakthrough vaginal bleeding.
Progestin-only contraceptives.
The progestin-only pill containing low-dose progestin is an effective contraceptive but has not been studied for the management of HMB. A meta-analysis concluded that the use of short-course cyclic high-dose progestin (norethisterone [norethindrone] 5 mg given 2 or 3 times per day for 7-11 days) for HMB treatment was less effective than tranexamic acid, danazol, and the progesterone-releasing intrauterine system.35 An extended regimen of high-dose progestin (norethisterone 5 mg given 3 times per day for 21 days) seems to be effective in reducing menstrual blood loss in women with AUB,36 although it has not been evaluated in women receiving anticoagulants.
DMPA, an injectable progestin, provides effective contraception and has been used for the management of HMB. A study reported that amenorrhea occurred in 50% of normal menstruating women during the first year of administration.37 Short-term treatment with DMPA also reduces menstrual blood loss in perimenopausal women with HMB.38
Major adverse effects of progestin-only contraceptives are irregular spotting and breakthrough bleeding. Other common adverse effects of DMPA are weight gain, greasy skin and hair, acne, and abdominal bloating.
LNG-IUS.
The efficacy of LNG-IUS in reducing HMB has been evaluated in patients treated with VKAs and in patients with bleeding disorders. Observational studies have shown a reduction in the amount and duration of menstrual bleeding with LNG-IUS.39-42 To date, however, only 1 randomized trial has been published. That study enrolled 40 women with HMB receiving VKAs after cardiac valve replacement. Patients were randomly assigned to LNG-IUS or to a no-treatment group. In the LNG-IUS group, there was a significant decrease in the pictorial blood assessment chart bleeding score and the number of bleeding days per cycle at 3 and 6 months compared with baseline preinsertion, whereas no change was noted in the control group. The mean of hemoglobin and ferritin levels were significantly increased at 3 months but not at 6 months in those randomly assigned to LNG-IUS.43
LNG-IUS may increase pelvic pain and breast tenderness.44 Breakthrough bleeding often occurs in the first 3 to 6 months. Other serious but rare complications of LNG-IUS include pelvic infection, device expulsion, and uterine perforation.
Risk of VTE in users of hormonal therapy during anticoagulant treatment.
Anticoagulants reduce the risk of recurrent thromboembolic events, and recent evidence suggests that the effect of therapeutic-dose anticoagulants is sufficient to overcome the incremental prothrombotic risk associated with hormonal contraceptives. In post hoc analyses of the EINSTEIN DVT and EINSTEIN PE studies, women receiving hormonal therapy (CHCs and progestin-only contraceptives) during the anticoagulant period had a risk of recurrent VTEs comparable to that for women not using hormonal therapy (adjusted hazard ratio, 0.56; 95% CI, 0.23-1.39).18
The results of that study have challenged the World Health Organization Guideline for CHCs, which states that established VTEs during anticoagulant therapy is a condition that represents an unacceptable health risk for CHCs when used for contraceptive purposes.45 However, the results support the guideline of the International Society on Thrombosis and Hemostasis, which suggests that hormonal therapy can be continued in selected patients after initiation of anticoagulants.46
Taken together, these observations suggest that informed patients may choose to continue CHCs or progestin-only therapy while receiving therapeutic dose anticoagulants or while in a process of transitioning to other hormonal therapies. This treatment will provide effective contraception and will reduce the risk of new or recurrent HMB. In addition, by suppressing ovulation, CHCs, DMPA, and possibly progestin-only pills can reduce the risk of ovarian hemorrhage.47 It is important to note that adequate anticoagulant therapy is required to safely use CHCs and progestin-only therapies associated with an increased risk of VTEs. These agents should be discontinued before anticoagulants are stopped.
Risk of VTE in users of hormonal therapy without anticoagulant treatment.
Because data are limited for women treated with anticoagulants, data from the general population not receiving anticoagulants can help inform management decisions by providing information on potential risks of VTEs with hormonal therapy, as well as the safety of reduced-intensity anticoagulation or withdrawal of anticoagulant therapy while continuing hormonal therapy in women with serious bleeding. When compared with a nonuser group, patients in the general population receiving COCs have about a fourfold increase in their risk of VTE.48 The combined contraceptive patch and vaginal ring also increase the risk of VTE.49 Estrogen dose and subtype of progesterone play a major role in thrombotic risk with COCs.50,51 The use of low-dose progestin-only pills for contraceptive purposes is not associated with an increased risk of VTE.52 However, the risk of VTE is increased by about fivefold with progestin use for therapeutic indications,53,54 and by about twofold with DMPA.52,55 LNG-IUS is effective for HMB treatment in women who receive anticoagulants and does not seem to increase the risk of thrombosis when used in women with no prior thrombosis.49,56
Tranexamic acid.
Tranexamic acid, an antifibrinolytic agent, is effective in the treatment of HMB in women who are not receiving anticoagulants.57 However, a post-marketing report suggests that it increases the risk of thromboembolic events. The product monograph indicates that tranexamic acid is contraindicated in patients with active VTEs or with a history of VTEs.
Despite a label warning from the US Food and Drug Administration, the association of tranexamic acid and thrombotic risk in patients with VTEs has not been definitively confirmed. The use of tranexamic acid was not associated with an increased risk for VTEs in 1 case-control study (OR, 0.55; 95% CI, 0.31-0.97).58 A nested case-control study in women with HMB did not demonstrate a statistically significant association between tranexamic acid use and the risk of VTEs (adjusted OR, 3.2; 95% CI, 0.65-15.78).59 It is worth noting that tranexamic acid use for bleeding associated with severe trauma and general or obstetrical surgery does not seem to significantly increase the risk of thrombosis.60-63 Thus, current evidence does not support the hypothesis that tranexamic acid is associated with an unacceptable risk of thrombosis in women who received anticoagulants. How tranexamic acid interacts with hormonal therapies used to treat HMB is unknown.
Management of anticoagulants.
Modification of anticoagulant treatment in the setting of HMB has become more common with the widespread use of DOACs. With warfarin, dose reduction or temporary interruption is not practical. However, DOACs can be transiently withheld because of their short half-life and rapid return to therapeutic levels once they are resumed. Dose reduction and temporary interruption of rivaroxaban have been reported in observational studies.15,20,22 As noted above, recent evidence suggests that a 10-mg dose of rivaroxaban decreased menstrual bleeding without an increased risk of thrombosis when compared with a 20-mg dose of rivaroxaban.19 The effectiveness of a lower-dose DOAC for prevention of recurrent thrombosis in patients who have received at least 6 months of therapy has been demonstrated with apixaban and rivaroxaban.64,65 One observational study reported successful HMB management by switching from rivaroxaban to apixaban in 5 (70%) of 7 women.17 In our practice, we do not temporarily discontinue or reduce the dose of rivaroxaban during the active treatment period (usually within 3 months of acute VTE). In women with HMB who are beyond 3 months from their acute VTE, we advise some patients taking rivaroxaban to reduce their dose to 10 mg during their menstrual period. Use of dabigatran for long-term secondary prevention of VTEs is also reasonable.23 Use of usual doses of apixaban might be an alternative option on the basis of limited evidence.17 Low-dose apixaban (2.5 mg given twice per day) probably attenuates HMB similarly to low-dose rivaroxaban, but evidence supporting this recommendation is lacking. It is important to note that in patients who require extended anticoagulant treatment, there is no evidence to support the use of low-dose rivaroxaban (10 mg orally once per day) or low-dose apixaban (2.5 mg orally twice per day) in combination with estrogen-containing hormonal therapy, DMPA, or tranexamic acid for the treatment of HMB. Whether the reduced-dose DOACs provide sufficient anticoagulant effect to overcome the prothrombotic risk of these therapies remains unknown. At this time, avoiding this combination of treatments seems reasonable.
Surgical treatment.
Endometrial ablation has been widely used in healthy women with HMB who are refractory to hormonal therapy and do not wish to preserve their fertility. Small studies have demonstrated that hysteroscopic endometrial ablation to avoid hysterectomy in women who receive anticoagulants resulted in a reduction in menstrual bleeding (or amenorrhea) in 80% of them.66,67
Uterine artery embolization is another option for HMB associated with a fibroid or myoma. Although fertility may be preserved, amenorrhea has been reported in 1% to 7% of patients after this intervention.68
Emergent management
In acute bleeding settings, should a patient discontinue anticoagulant therapy or should a reversal agent be used? Would a reversal agent increase the thrombotic risk? The answers to those questions are based on an individual patient’s risk profile. In patients with hemodynamic instability from bleeding, the anticoagulant should be withheld and a reversal agent should be considered. Other surgical interventions that might be considered in consultation with a gynecologist include intrauterine balloon tamponade, endometrial ablation, uterine artery embolization, and hysterectomy.69-71 Patients with a recent history of thrombosis (<1 month) may be at a higher risk of recurrent thrombosis, and thus, attempts should be made to maintain some form of anticoagulation. In the latter situation, LMWH at intermediate or prophylactic doses might be considered, although this strategy has not been shown to be safe or effective in this situation. In patients judged to be at moderate to high long-term risk of recurrent thrombosis, reinstitution of anticoagulant therapy should be strongly considered as soon as bleeding is controlled.
Another common question from gynecologists is, Can a specific-HMB-related hormonal therapy be used to control the bleeding, and if so, what kinds of HMB-related hormonal therapy can be used with minimal impact on the risk of thrombosis? In most patients with acute HMB, specific hormonal therapy, including high-dose intravenous or oral estrogen, high-dose progestin, or COCs, is usually needed along with anticoagulant management to control bleeding. To the best of our knowledge, no study has reported the risk of thrombosis with HMB-related hormonal therapy used to suppress menstrual bleeding in the acute setting. However, high-dose estrogen therapy is likely to be associated with an increased risk of thrombosis.
Although it has not specifically been studied in the acute HMB setting, we would consider using tranexamic acid in patients with a remote VTE event (>3 months) because their risk of recurrence is lower than those with a recent history of VTE. Tranexamic acid is given in a single dose, and bleeding is reassessed. With persistent bleeding, it can be repeated as required at 6- to 8-hour intervals. The recommended intravenous dose is 10 mg/kg per dose. The dosage should be adjusted according to the patient’s creatinine clearance.
Other medical therapies that might be considered in the acute setting include epsilon-aminocaproic acid72 and the selective progesterone receptor modulator ulipristal acetate. The latter has been shown to be effective in reducing HMB associated with uterine fibroids.73,74 Trials investigating the use of this drug in women with HMB without uterine fibroids are ongoing.75 How this drug changes the risk of VTE compared with other hormonal contraceptive pills is unknown.
Back to case 1 vignette
At the emergency department, the patient’s blood pressure was 108/73 mmHg, and her heart rate was 134 bpm. Her hemoglobin level dropped to 4.5 g/dL. Because her last thrombotic episode had occurred more than 3 months ago, the potential morbidity from bleeding was felt to outweigh thrombotic risk. Therefore, rivaroxaban was held. A single dose of tranexamic acid was given. During admission, the patient was transfused with 4 units of red blood cells, and a selective progesterone antagonist receptor was initiated. Endometrial ablation and hysterectomy were discussed if HMB should recur. The patient was switched from rivaroxaban to warfarin. Subsequent visits revealed no further HMB. At present, the patient does not require further HMB-related treatment or surgery; she remained on warfarin at follow-up several years after her bleeding event.
Back to case 2 vignette
The patient developed HMB after being treated with rivaroxaban. We discussed switching to warfarin or an alternate DOAC, but she preferred therapy once per day and did not wish to undergo INR monitoring. Because her second VTE event was more than 6 months ago, we advised the patient to decrease the dose of rivaroxaban to 10 mg once per day after informing her that this was an off-label dose. We also referred her to a gynecologist for further investigation. With a reduced dose of rivaroxaban, her HMB has resolved.
Acknowledgment
The authors thank Mark Crowther for providing case vignettes and sharing his expertise in the management of these patients; he holds a Career Investigator award from the Heart and Stroke Foundation of Canada and the Leo Pharma Chair in Thromboembolism Research.
Authorship
Contribution: K.B., S.H.O., and S.M.B. conceived this article; K.B. performed the literature review and wrote the first draft; and S.H.O. and S.M.B reviewed and edited the final paper.
Conflict-of-interest disclosure: S.H.O received funding from Children’s Oncology Group/Bristol-Myers Squibb as principal investigator of an apixaban study and served as a consultant to Pfizer for another study of apixaban. S.M.B. received funding support from the Eli Lilly Canada/May Cohen Chair in Women’s Health at McMaster University. K.B. declares no competing financial interests.
Correspondence: Kochawan Boonyawat, Department of Medicine, Ramathibodi Hospital, 270 Rama VI Rd, Rachathewi, Bangkok 10400, Thailand; e-mail:kochawan.boonyawat@medportal.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal