Key Points
IRd was associated with a consistent PFS benefit vs placebo-Rd in RRMM patients with high-risk and standard-risk cytogenetics.
The addition of ixazomib to Rd overcomes the poor PFS associated with high-risk cytogenetics in patients with RRMM.
Abstract
Certain cytogenetic abnormalities are known to adversely impact outcomes in patients with multiple myeloma (MM). The phase 3 TOURMALINE-MM1 study demonstrated a significant improvement in progression-free survival (PFS) with ixazomib-lenalidomide-dexamethasone (IRd) compared with placebo-lenalidomide-dexamethasone (placebo-Rd). This preplanned analysis assessed the efficacy and safety of IRd vs placebo-Rd according to cytogenetic risk, as assessed using fluorescence in situ hybridization. High-risk cytogenetic abnormalities were defined as del(17p), t(4;14), and/or t(14;16); additionally, patients were assessed for 1q21 amplification. Of 722 randomized patients, 552 had cytogenetic results; 137 (25%) had high-risk cytogenetic abnormalities and 172 (32%) had 1q21 amplification alone. PFS was improved with IRd vs placebo-Rd in both high-risk and standard-risk cytogenetics subgroups: in high-risk patients, the hazard ratio (HR) was 0.543 (95% confidence interval [CI], 0.321-0.918; P = .021), with median PFS of 21.4 vs 9.7 months; in standard-risk patients, HR was 0.640 (95% CI, 0.462-0.888; P = .007), with median PFS of 20.6 vs 15.6 months. This PFS benefit was consistent across subgroups with individual high-risk cytogenetic abnormalities, including patients with del(17p) (HR, 0.596; 95% CI, 0.286-1.243). PFS was also longer with IRd vs placebo-Rd in patients with 1q21 amplification (HR, 0.781; 95% CI, 0.492-1.240), and in the “expanded high-risk” group, defined as those with high-risk cytogenetic abnormalities and/or 1q21 amplification (HR, 0.664; 95% CI, 0.474-0.928). IRd demonstrated substantial benefit compared with placebo-Rd in relapsed and/or refractory MM (RRMM) patients with high-risk and standard-risk cytogenetics, and improves the poor PFS associated with high-risk cytogenetic abnormalities. This trial was registered at www.clinicaltrials.gov as #NCT01564537.
Introduction
Treatment of patients with multiple myeloma (MM) has advanced considerably over the past 15 years with the introduction and widespread use of proteasome inhibitors and immunomodulatory drugs, which have become the backbones of therapy in the first-line and relapsed settings.1-5 However, despite these recent advances, outcomes for patients with high-risk cytogenetic abnormalities remain poor.6-10 “High-risk” abnormalities on fluorescence in situ hybridization (FISH) have typically been considered to comprise the translocations t(4;14) and t(14;16) and chromosome 17p deletion [del(17p)],7,9,11-15 each of which has been shown to be an independent poor prognostic marker in MM.16-19 Additionally, gain of 1q21 on FISH has been shown to confer poor prognosis17,20,21 ; in some reports, 1q21 amplification is considered a high-risk abnormality,21,22 including in a 2016 consensus paper from the International Myeloma Working Group,10 whereas other reports classify it as conferring intermediate or standard risk.23,24
To date, although data on the use of novel agent–based treatments have demonstrated that these regimens improve outcomes in patients with MM vs previous or existing standards of care, progression-free survival (PFS) and overall survival (OS) nonetheless remain poorer for patients with high-risk cytogenetic abnormalities compared with those with standard-risk cytogenetics.11-13,15 The International Myeloma Working Group recently advised that newly diagnosed MM patients with high-risk cytogenetics should receive a combination of a proteasome inhibitor with lenalidomide or pomalidomide and dexamethasone,10 noting the positive impact of these regimens on outcomes in patients with specific poor-prognosis abnormalities. Nevertheless, there remains a need for additional active therapeutic options for patients with high-risk cytogenetics, including regimens that allow for prolonged treatment and thus may offer extended disease control.
The phase 3, randomized, double-blind, placebo-controlled TOURMALINE-MM1 (NCT01564537) study evaluated the efficacy and safety of the oral proteasome inhibitor ixazomib in combination with lenalidomide-dexamethasone (Rd; ixazomib-lenalidomide-dexamethasone [IRd]) in 722 patients with relapsed and/or refractory MM (RRMM). The findings demonstrated a 35% improvement in PFS with IRd compared with placebo-lenalidomide-dexamethasone (placebo-Rd) (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.587-0.939; P = .01), with limited additional toxicity with this all-oral triplet regimen.25 These data led to the approval (first in the United States and subsequently in the European Union and many other countries) of ixazomib, in combination with Rd, for the treatment of patients with MM who have received at least 1 prior therapy. A key secondary end point of the TOURMALINE-MM1 study was OS in patients with del(17p), making it 1 of the first trials to be powered to prospectively assess survival in this patient subgroup; an additional secondary end point was outcomes in patients with high-risk cytogenetic abnormalities. Here, we report prespecified and post hoc analyses of the efficacy and safety of IRd vs placebo-Rd according to cytogenetic risk status, including analyses in patients with specific high-risk cytogenetic abnormalities. We also evaluated the impact of the size of the “high-risk” clone on the clinical outcome in MM patients with high-risk cytogenetic abnormalities by using different cutoffs for defining positivity.
Methods
Study design and participants
The global, randomized, double-blind, placebo-controlled, phase 3 TOURMALINE-MM1 study enrolled patients from 147 sites in 26 countries between 28 August 2012 and 27 May 2014. Adult patients with RRMM who had received 1 to 3 prior lines of therapy were eligible, including primary refractory patients; patients who were refractory to prior lenalidomide or proteasome inhibitor–based therapy were not eligible. Full eligibility criteria have been previously reported.25 Patients were randomized double-blind in a 1:1 ratio to receive ixazomib 4 mg orally or matching placebo on days 1, 8, and 15 of 28-day cycles, together with lenalidomide 25 mg orally on days 1 to 21 and dexamethasone 40 mg orally on days 1, 8, 15, and 22. Treatment was continued until disease progression or unacceptable toxicity. Response and disease progression were evaluated by an independent review committee blinded to both patient assignment and investigator assessment.
The study was performed in accordance with the International Conference on Harmonization, the Guidelines for Good Clinical Practice, appropriate regulatory requirements, and with approval of institutional review boards at individual enrolling institutions. All patients provided written informed consent.
The primary end point was PFS; key secondary end points were OS in the intent-to-treat population and OS in patients with del(17p). Other prespecified secondary end points included overall response rate, rate of complete response (CR) plus very-good-partial response (VGPR), duration of response, time to progression (TTP), PFS in patients with high-risk cytogenetics, and safety. A list of all study end points has been reported previously.25
Assessments
Cytogenetic abnormalities were rigorously assessed on CD138+ sorted cells from bone marrow samples collected at study entry using FISH at a Clinical Laboratory Improvement Amendments (CLIA)-certified central laboratory. If central laboratory results were not available, local laboratory cytogenetic results were used. High-risk cytogenetic abnormalities were defined as del(17p), t(4;14), and/or t(14;16). Standard-risk cytogenetics were defined as the absence of high-risk abnormalities in evaluable samples. Cutoff values for defining the presence of del(17p), t(4;14), and t(14;16) were, per protocol, based on the false-positive rates (technical cutoffs) of the Kreatech FISH probes used. These were 5%, 3%, and 3% positive cells, respectively. Additionally, patients were assessed for the presence of 1q21 amplification by FISH (3% cutoff). Post hoc analyses were performed using different cutoff values for defining the presence of del(17p), t(4;14), and 1q21 amplification abnormalities. Efficacy data were analyzed in: patient subgroups defined by the presence of each individual cytogenetic abnormality [ie, del(17p) alone or in combination with 1 of the translocations; t(4;14) alone; t(14;16) alone; or 1q21 amplification alone]; high-risk patients [defined by any of del(17p), t(4;14), or t(14;16)] vs standard-risk patients; and an expanded high-risk subgroup [defined as patients with any of del(17p), t(4;14), t(14;16), or 1q21 amplification]. Safety data were evaluated according to the presence or absence of high-risk features.
Statistical analysis
Group sequential design was used in the study and total sample size was calculated to provide 80% power (2-sided α, 0.05) to test for a 30% improvement in OS (assumed HR of 0.77), which is also sufficiently powered to demonstrate PFS superiority (HR, 0.74). Kaplan-Meier methodology was used to estimate time-to-event distributions, with stratified log-rank tests and Cox models (α = 0.05, 2-sided) used for interarm comparisons of time-to-event end points. All statistical analyses were performed using SAS 9.2 and above.
Data were collected, analyzed, and interpreted by the authors and the sponsor. All authors had full access to the data and agreed to be accountable for the accuracy and integrity of the data and analyses.
Results
Patients and cytogenetic assessment
A total of 722 patients were enrolled in the TOURMALINE-MM1 study (IRd, n = 360; placebo-Rd, n = 362). Of these, 552 patients (76%) had cytogenetic results (274 and 278 in the IRd and placebo-Rd groups, respectively), including 137 with the high-risk cytogenetic abnormalities del(17p), t(4;14), or t(14;16) (high-risk group; IRd, n = 75; placebo-Rd, n = 62), 172 with 1q21 amplification alone (IRd, n = 80; placebo-Rd, n = 92), and 309 with any of the assessed cytogenetic abnormalities del(17p), t(4;14), t(14;16), or 1q21 amplification (expanded high-risk group; IRd, n = 155; placebo-Rd, n = 154). In 133 (97%) of the high-risk patients, cytogenetic results were confirmed by the central laboratory, and local laboratory data were used for the remaining 4 patients (3%). In the IRd and placebo-Rd groups, 36 and 33 patients (10% overall), respectively, had del(17p) [alone or in combination with t(4;14) or t(14;16)], 36 and 25 patients (8% overall) had t(4;14) alone, and 3 and 4 patients (1% overall), respectively, had t(14;16) alone; efficacy and safety have not been analyzed in this latter subgroup due to the small numbers of patients included. Baseline demographic and disease characteristics in the high-risk, standard-risk, and expanded high-risk groups are shown in Table 1.
Baseline characteristics of the high-risk, standard-risk, and expanded high-risk cytogenetics populations
| . | IRd . | Placebo-Rd . | ||||
|---|---|---|---|---|---|---|
| High risk, n = 75 . | Standard risk, n = 199 . | Expanded high risk, n = 155 . | High risk, n = 62 . | Standard risk, n = 216 . | Expanded high risk, n = 154 . | |
| Median age (range), y | 67.0 (45-86) | 67.0 (38-91) | 67.0 (38-91) | 66.0 (45-89) | 66.0 (30-88) | 66.0 (43-89) |
| Male sex | 41 (55) | 120 (60) | 85 (55) | 25 (40) | 127 (59) | 76 (49) |
| White race | 63 (84) | 174 (87) | 136 (88) | 51 (82) | 181 (84) | 125 (81) |
| ISS stage at screening | ||||||
| I or II | 68 (91) | 170 (85) | 136 (88) | 51 (82) | 189 (88) | 131 (85) |
| III | 7 (9) | 29 (15) | 19 (12) | 11 (18) | 27 (13) | 23 (15) |
| Creatinine clearance, mL/min | ||||||
| <30 | 1 (1) | 2 (1) | 2 (1) | 0 | 5 (2) | 1 (<1) |
| 30-50 | 6 (8) | 16 (8) | 8 (5) | 11 (18) | 31 (14) | 21 (14) |
| >50 | 68 (91) | 181 (91) | 145 (95) | 51 (82) | 179 (83) | 132 (86) |
| Lines of prior therapy | ||||||
| 1 | 45 (60) | 111 (56) | 92 (59) | 34 (55) | 124 (57) | 92 (60) |
| 2 or 3 | 30 (40) | 88 (44) | 63 (41) | 28 (45) | 92 (43) | 62 (40) |
| Disease status | ||||||
| Relapsed | 53 (71) | 157 (79) | 116 (75) | 47 (76) | 164 (76) | 110 (71) |
| Refractory | 9 (12) | 21 (11) | 18 (12) | 10 (16) | 24 (11) | 28 (18) |
| Relapsed and refractory | 13 (17) | 20 (10) | 21 (14) | 5 (8) | 28 (13) | 16 (10) |
| Prior therapies | ||||||
| PI-exposed | 57 (76) | 133 (67) | 106 (68) | 49 (79) | 145 (67) | 105 (68) |
| PI-naive | 18 (24) | 66 (33) | 49 (32) | 13 (21) | 71 (33) | 49 (32) |
| Prior lenalidomide | 9 (12) | 28 (14) | 20 (13) | 8 (13) | 20 (9) | 16 (10) |
| Prior thalidomide | 33 (44) | 89 (45) | 76 (49) | 27 (44) | 106 (49) | 72 (47) |
| Thalidomide-refractory | 11 (15) | 17 (9) | 23 (15) | 10 (16) | 30 (14) | 25 (16) |
| Prior SCT | 47 (63) | 115 (58) | 95 (61) | 28 (45) | 120 (56) | 72 (47) |
| . | IRd . | Placebo-Rd . | ||||
|---|---|---|---|---|---|---|
| High risk, n = 75 . | Standard risk, n = 199 . | Expanded high risk, n = 155 . | High risk, n = 62 . | Standard risk, n = 216 . | Expanded high risk, n = 154 . | |
| Median age (range), y | 67.0 (45-86) | 67.0 (38-91) | 67.0 (38-91) | 66.0 (45-89) | 66.0 (30-88) | 66.0 (43-89) |
| Male sex | 41 (55) | 120 (60) | 85 (55) | 25 (40) | 127 (59) | 76 (49) |
| White race | 63 (84) | 174 (87) | 136 (88) | 51 (82) | 181 (84) | 125 (81) |
| ISS stage at screening | ||||||
| I or II | 68 (91) | 170 (85) | 136 (88) | 51 (82) | 189 (88) | 131 (85) |
| III | 7 (9) | 29 (15) | 19 (12) | 11 (18) | 27 (13) | 23 (15) |
| Creatinine clearance, mL/min | ||||||
| <30 | 1 (1) | 2 (1) | 2 (1) | 0 | 5 (2) | 1 (<1) |
| 30-50 | 6 (8) | 16 (8) | 8 (5) | 11 (18) | 31 (14) | 21 (14) |
| >50 | 68 (91) | 181 (91) | 145 (95) | 51 (82) | 179 (83) | 132 (86) |
| Lines of prior therapy | ||||||
| 1 | 45 (60) | 111 (56) | 92 (59) | 34 (55) | 124 (57) | 92 (60) |
| 2 or 3 | 30 (40) | 88 (44) | 63 (41) | 28 (45) | 92 (43) | 62 (40) |
| Disease status | ||||||
| Relapsed | 53 (71) | 157 (79) | 116 (75) | 47 (76) | 164 (76) | 110 (71) |
| Refractory | 9 (12) | 21 (11) | 18 (12) | 10 (16) | 24 (11) | 28 (18) |
| Relapsed and refractory | 13 (17) | 20 (10) | 21 (14) | 5 (8) | 28 (13) | 16 (10) |
| Prior therapies | ||||||
| PI-exposed | 57 (76) | 133 (67) | 106 (68) | 49 (79) | 145 (67) | 105 (68) |
| PI-naive | 18 (24) | 66 (33) | 49 (32) | 13 (21) | 71 (33) | 49 (32) |
| Prior lenalidomide | 9 (12) | 28 (14) | 20 (13) | 8 (13) | 20 (9) | 16 (10) |
| Prior thalidomide | 33 (44) | 89 (45) | 76 (49) | 27 (44) | 106 (49) | 72 (47) |
| Thalidomide-refractory | 11 (15) | 17 (9) | 23 (15) | 10 (16) | 30 (14) | 25 (16) |
| Prior SCT | 47 (63) | 115 (58) | 95 (61) | 28 (45) | 120 (56) | 72 (47) |
Data are n (%) unless otherwise stated.
ISS, International Staging System; PI, proteasome inhibitor; SCT, stem cell transplant.
Efficacy by cytogenetic risk status
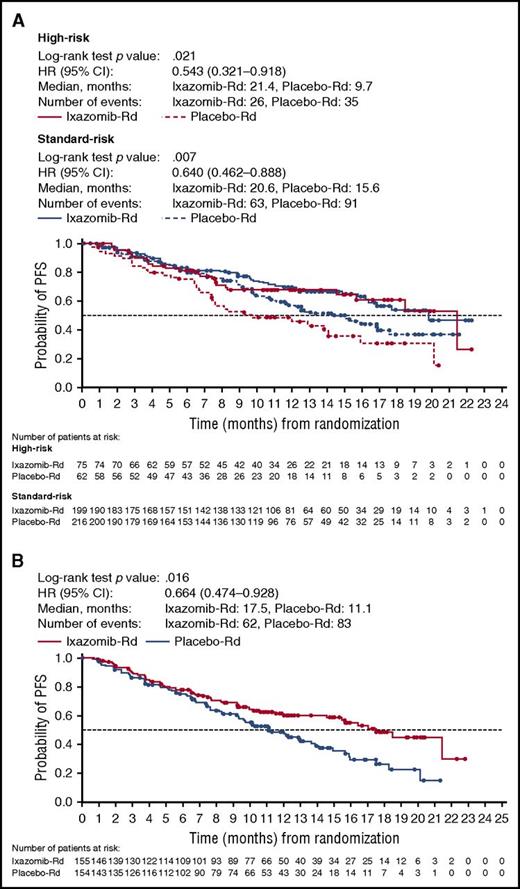
The addition of ixazomib to Rd decreased the risk of disease progression in high-risk patients (Figure 1A; HR, 0.543 [95% CI, 0.321-0.918], log-rank test P value, .021) and median PFS was 21.4 and 9.7 months with IRd and placebo-Rd, respectively. PFS was also longer with IRd vs placebo-Rd among subgroups of patients defined by the presence or absence of individual high-risk cytogenetic abnormalities (Figure 2A-B). In the del(17p) and t(4;14) subgroups, the respective HRs were 0.596 (95% CI, 0.286-1.243) and 0.645 (95% CI, 0.250-1.663), with median PFS of 21.4 vs 9.7 months, and 18.5 vs 12.0 months, respectively, in the IRd vs placebo-Rd groups. PFS was also improved with IRd vs placebo-Rd in standard-risk patients (Figure 1A; HR, 0.640 [95% CI, 0.462-0.888], P = .007) and median PFS was 20.6 and 15.6 months, respectively.
Kaplan-Meier estimates of PFS by cytogenetic risk status. (A) PFS in high-risk and standard-risk patients. (B) PFS in expanded high-risk patients.
Kaplan-Meier estimates of PFS by cytogenetic risk status. (A) PFS in high-risk and standard-risk patients. (B) PFS in expanded high-risk patients.
Kaplan-Meier estimates of PFS according to presence of individual cytogenetic abnormalities. PFS in patients with (A) del(17p), alone or in combination with t(4;14) and/or t(14;16); (B) t(4;14) alone; and (C) amp 1q21 alone.
Kaplan-Meier estimates of PFS according to presence of individual cytogenetic abnormalities. PFS in patients with (A) del(17p), alone or in combination with t(4;14) and/or t(14;16); (B) t(4;14) alone; and (C) amp 1q21 alone.
Among patients with 1q21 amplification alone (IRd, n = 80; placebo-Rd, n = 92), the HR for PFS was 0.781 (95% CI, 0.492-1.240) in favor of IRd and the medians were 15.4 vs 11.3 months in the IRd vs placebo-Rd groups, respectively (Figure 2C). Combining patients with 1q21 amplification alone with the high-risk group, to form the expanded high-risk group, PFS was improved with IRd vs placebo-Rd (Figure 1B). In patients in the expanded high-risk group, the HR was 0.664 (95% CI, 0.474-0.928; P = .016), and with 62 and 83 events, respectively, in the IRd and placebo-Rd groups, the median PFS was 17.5 vs 11.1 months.
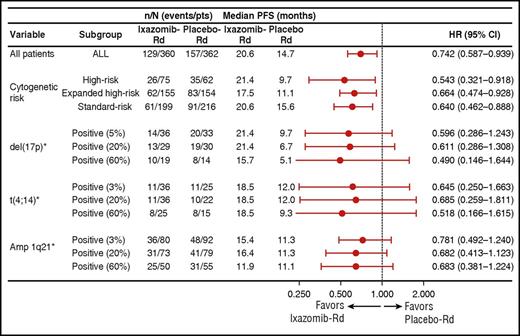
The PFS benefit with IRd vs placebo-Rd was consistent and independent of the size of the clone, as shown by different cutoff values for defining positivity for del(17p), t(4;14), and 1q21 amplification (Figure 3). For del(17p), using the protocol-specified cutoff of 5%, the HR was 0.596 (95% CI, 0.286-1.243), whereas using cutoff values of 20% and 60% positive cells, the HRs were 0.611 (95% CI, 0.286-1.308) and 0.490 (95% CI, 0.146-1.644), respectively, with subgroup sizes becoming smaller with increasing cutoff value (Figure 3). For t(4;14), using the protocol-specified cutoff of 3%, the HR was 0.645 (95% CI, 0.250-1.663), whereas using cutoff values of 20% and 60%, the HRs were 0.685 (95% CI, 0.259-1.811) and 0.518 (95% CI, 0.166-1.615), respectively (Figure 3). For 1q21 amplification alone, the HR for PFS was 0.781 (95% CI, 0.492-1.240) using a 3% cutoff, and 0.682 (95% CI, 0.413-1.123) and 0.683 (95% CI, 0.381-1.224) when using cutoff values of 20% and 60%, respectively (Figure 3).
Forest plot of PFS among patient subgroups defined by cytogenetic abnormalities, including post hoc analyses of different cutoff values for individual abnormalities. *del(17p) subgroup includes patients with del(17p) alone or in combination with t(4;14) or t(14;16); t(4;14) subgroup includes patients with t(4;14) alone; amp 1q21 subgroup includes patients with amp 1q21 alone.
Forest plot of PFS among patient subgroups defined by cytogenetic abnormalities, including post hoc analyses of different cutoff values for individual abnormalities. *del(17p) subgroup includes patients with del(17p) alone or in combination with t(4;14) or t(14;16); t(4;14) subgroup includes patients with t(4;14) alone; amp 1q21 subgroup includes patients with amp 1q21 alone.
Overall response rate and rates of VGPR or better and of CR or better were also higher with IRd vs placebo-Rd in both high-risk and standard-risk cytogenetics patient subgroups (Table 2). Median time to response in the IRd vs placebo-Rd groups was 1.1 vs 2.1 months in high-risk patients and 1.1 vs 1.9 months in standard-risk patients; respective values for median duration of response were 20.5 vs 11.3 months in high-risk patients and not reached vs 15.0 months in standard-risk patients (Table 3). Data on TTP in the patient subgroups reflected PFS (Table 3).
Response rates in the IRd and placebo-Rd groups according to cytogenetic subgroup
| . | Overall response rate, n (%) . | VGPR or better, n (%) . | CR or better, n (%) . | |||
|---|---|---|---|---|---|---|
| Patients (N, ixazomib vs placebo group) . | IRd . | Placebo-Rd . | IRd . | Placebo-Rd . | IRd . | Placebo-Rd . |
| All (360 vs 362) | 282 (78) | 259 (72) | 173 (48) | 141 (39) | 42 (12) | 24 (7) |
| Standard risk (199 vs 216) | 160 (80) | 158 (73) | 191 (51) | 94 (44) | 24 (12) | 16 (7) |
| High risk* (75 vs 62) | 59 (79) | 37 (60) | 34 (45) | 13 (21) | 9 (12) | 1 (2) |
| del(17p)† (36 vs 33) | 26 (72) | 16 (48) | 14 (39) | 5 (15) | 4 (11) | 0 |
| t(4;14) alone (36 vs 25) | 32 (89) | 19 (76) | 19 (53) | 7 (28) | 5 (14) | 1 (4) |
| Amp 1q21 alone (80 vs 92) | 57 (71) | 63 (62) | 35 (44) | 37 (40) | 7 (9) | 8 (9) |
| Expanded high risk‡ (155 vs 154) | 116 (75) | 100 (65) | 69 (45) | 50 (33) | 16 (10) | 9 (6) |
| . | Overall response rate, n (%) . | VGPR or better, n (%) . | CR or better, n (%) . | |||
|---|---|---|---|---|---|---|
| Patients (N, ixazomib vs placebo group) . | IRd . | Placebo-Rd . | IRd . | Placebo-Rd . | IRd . | Placebo-Rd . |
| All (360 vs 362) | 282 (78) | 259 (72) | 173 (48) | 141 (39) | 42 (12) | 24 (7) |
| Standard risk (199 vs 216) | 160 (80) | 158 (73) | 191 (51) | 94 (44) | 24 (12) | 16 (7) |
| High risk* (75 vs 62) | 59 (79) | 37 (60) | 34 (45) | 13 (21) | 9 (12) | 1 (2) |
| del(17p)† (36 vs 33) | 26 (72) | 16 (48) | 14 (39) | 5 (15) | 4 (11) | 0 |
| t(4;14) alone (36 vs 25) | 32 (89) | 19 (76) | 19 (53) | 7 (28) | 5 (14) | 1 (4) |
| Amp 1q21 alone (80 vs 92) | 57 (71) | 63 (62) | 35 (44) | 37 (40) | 7 (9) | 8 (9) |
| Expanded high risk‡ (155 vs 154) | 116 (75) | 100 (65) | 69 (45) | 50 (33) | 16 (10) | 9 (6) |
t(4;14) and/or t(14;16) and/or del(17p).
Alone or in combination with t(4;14) or t(14;16).
t(4;14) and/or t(14;16) and/or del(17p) and/or amp 1q21.
Duration of response, PFS, and time to progression in the IRd and placebo-Rd groups according to cytogenetic subgroup
| Patients (N, ixazomib vs placebo group) . | Median duration of response, mo . | Median PFS, mo . | Median time to progression, mo . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IRd . | Placebo-Rd . | IRd . | Placebo-Rd . | HR . | 95% CI . | IRd . | Placebo-Rd . | HR . | |
| All (360 vs 362) | 20.5 (n = 282) | 15.0 (n = 259) | 20.6 | 14.7 | 0.742 | 0.587-0.939 | 21.4 | 15.7 | 0.712 |
| Standard risk (199 vs 216) | NR (n = 160) | 15.0 (n = 158) | 20.6 | 15.6 | 0.640 | 0.462-0.888 | 20.6 | 15.9 | 0.626 |
| High risk* (75 vs 62) | 20.5 (n = 59) | 11.3 (n = 37) | 21.4 | 9.7 | 0.543 | 0.321-0.918 | 21.4 | 12.0 | 0.534 |
| del(17p)† (36 vs 33) | 20.5 (n = 26) | 12.0 (n = 16) | 21.4 | 9.7 | 0.596 | 0.286-1.243 | 21.4 | 12.9 | 0.590 |
| t(4;14) alone (36 vs 25) | 17.5 (n = 32) | 7.2 (n = 19) | 18.5 | 12.0 | 0.645 | 0.250-1.663 | 18.5 | 12.0 | 0.645 |
| Amp 1q21 alone (80 vs 92) | 16.6 (n = 56) | 11.3 (n = 63) | 15.4 | 11.3 | 0.781 | 0.492-1.240 | 16.4 | 12.3 | 0.787 |
| Expanded high risk‡ (155 vs 154) | 20.5 (n = 115) | 11.3 (n = 100) | 17.5 | 11.1 | 0.664 | 0.474-0.928 | 18.5 | 12.1 | 0.672 |
| Patients (N, ixazomib vs placebo group) . | Median duration of response, mo . | Median PFS, mo . | Median time to progression, mo . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IRd . | Placebo-Rd . | IRd . | Placebo-Rd . | HR . | 95% CI . | IRd . | Placebo-Rd . | HR . | |
| All (360 vs 362) | 20.5 (n = 282) | 15.0 (n = 259) | 20.6 | 14.7 | 0.742 | 0.587-0.939 | 21.4 | 15.7 | 0.712 |
| Standard risk (199 vs 216) | NR (n = 160) | 15.0 (n = 158) | 20.6 | 15.6 | 0.640 | 0.462-0.888 | 20.6 | 15.9 | 0.626 |
| High risk* (75 vs 62) | 20.5 (n = 59) | 11.3 (n = 37) | 21.4 | 9.7 | 0.543 | 0.321-0.918 | 21.4 | 12.0 | 0.534 |
| del(17p)† (36 vs 33) | 20.5 (n = 26) | 12.0 (n = 16) | 21.4 | 9.7 | 0.596 | 0.286-1.243 | 21.4 | 12.9 | 0.590 |
| t(4;14) alone (36 vs 25) | 17.5 (n = 32) | 7.2 (n = 19) | 18.5 | 12.0 | 0.645 | 0.250-1.663 | 18.5 | 12.0 | 0.645 |
| Amp 1q21 alone (80 vs 92) | 16.6 (n = 56) | 11.3 (n = 63) | 15.4 | 11.3 | 0.781 | 0.492-1.240 | 16.4 | 12.3 | 0.787 |
| Expanded high risk‡ (155 vs 154) | 20.5 (n = 115) | 11.3 (n = 100) | 17.5 | 11.1 | 0.664 | 0.474-0.928 | 18.5 | 12.1 | 0.672 |
NR, not reached.
t(4;14) and/or t(14;16) and/or del(17p).
Alone or in combination with t(4;14) or t(14;16).
t(4;14) and/or t(14;16) and/or del(17p) and/or amp 1q21.
At a preplanned analysis for OS, after a median follow-up of ∼23 months in the overall study population, OS data were not mature. The TOURMALINE-MM1 study used a sequential testing procedure for the primary and key secondary end points.25 Thus, in the absence of statistical significance for OS in the intent-to-treat population, formal statistical analyses of OS in patient subgroups defined by cytogenetics should not be conducted and are not reported here. At data cutoff for the preplanned analysis for OS, 81 of 360 patients in the IRd group had died, including 15 of 75 patients in the high-risk group and 37 of 199 patients in the standard-risk group; in the placebo-Rd group, 90 of 362 patients had died, including 24 of 62 patients in the high-risk group and 47 of 216 patients in the standard-risk group. When analyzed by individual cytogenetic abnormality, in the IRd and placebo-Rd groups, respectively, 9 of 36 patients (25%) and 15 of 33 patients (45%) with del(17p) [alone or in combination with t(4;14) or t(14;16)] had died, and 4 of 36 patients (11%) and 7 of 25 patients (28%) with t(4;14) alone had died.
Safety
The overall safety profiles in the high-risk and standard-risk patients in each group are consistent with data reported for the overall population (Table 4).25 As seen in the overall population, in both high-risk and standard-risk patients, common adverse events were primarily of grade 1 or 2 severity, and included diarrhea, constipation, neutropenia, and anemia (supplemental Table 1, available on the Blood Web site). Rates of adverse events of clinical importance were also consistent with previous reports (supplemental Table 2).25
Overall safety profile with IRd and placebo-Rd among high-risk and standard-risk patients
| . | High risk . | Standard risk . | ||
|---|---|---|---|---|
| IRd, n = 74 . | Placebo-Rd, n = 62 . | IRd, n = 200 . | Placebo-Rd, n = 214 . | |
| Median treatment duration, mo | 16.3 | 9.9 | 16.1 | 14.7 |
| Any adverse event | 73 (99) | 61 (98) | 197 (99) | 214 (100) |
| Any grade ≥3 adverse event | 49 (66) | 45 (73) | 149 (75) | 140 (65) |
| Any serious adverse event | 31 (42) | 32 (52) | 90 (45) | 101 (47) |
| Adverse event resulting in dose reduction of any drug | 30 (41) | 26 (42) | 119 (60) | 110 (51) |
| Adverse event resulting in discontinuation of any drug | 13 (18) | 16 (26) | 55 (28) | 42 (20) |
| Adverse event resulting in discontinuation of regimen | 6 (8) | 8 (13) | 42 (21) | 31 (14) |
| On-study death | 0 | 6 (10) | 9 (5) | 13 (6) |
| . | High risk . | Standard risk . | ||
|---|---|---|---|---|
| IRd, n = 74 . | Placebo-Rd, n = 62 . | IRd, n = 200 . | Placebo-Rd, n = 214 . | |
| Median treatment duration, mo | 16.3 | 9.9 | 16.1 | 14.7 |
| Any adverse event | 73 (99) | 61 (98) | 197 (99) | 214 (100) |
| Any grade ≥3 adverse event | 49 (66) | 45 (73) | 149 (75) | 140 (65) |
| Any serious adverse event | 31 (42) | 32 (52) | 90 (45) | 101 (47) |
| Adverse event resulting in dose reduction of any drug | 30 (41) | 26 (42) | 119 (60) | 110 (51) |
| Adverse event resulting in discontinuation of any drug | 13 (18) | 16 (26) | 55 (28) | 42 (20) |
| Adverse event resulting in discontinuation of regimen | 6 (8) | 8 (13) | 42 (21) | 31 (14) |
| On-study death | 0 | 6 (10) | 9 (5) | 13 (6) |
Per the primary report from the study, exposure and safety data are reported from a prespecified analysis at a median follow up of ∼23 months. One patient with high-risk cytogenetics who was randomized to the ixazomib arm did not receive ixazomib and was not included in the ixazomib group safety population. Among patients with standard-risk cytogenetics, 1 patient randomized to the ixazomib arm did not receive ixazomib, and 2 patients randomized to the placebo arm accidentally received ixazomib and were conservatively included in the ixazomib group for analyses of exposure and safety.
Discussion
This subgroup analysis of the TOURMALINE-MM1 phase 3 study according to FISH cytogenetics showed that the addition of ixazomib to Rd overcomes the poor PFS associated with high-risk cytogenetic abnormalities in patients with RRMM. Reflecting the findings reported for the intent-to-treat population,25 the primary end point of PFS was prolonged with IRd vs placebo-Rd in patients with high-risk abnormalities and those with standard-risk cytogenetics, together with patient subgroups defined by the presence of individual high-risk cytogenetic abnormalities. The consistent benefit with IRd was also demonstrated in the cytogenetic subgroups in terms of higher response rates and prolonged TTP. These findings suggest that the triplet regimen of IRd represents an active and tolerable treatment option, producing rapid responses, for patients with RRMM who have high-risk or standard-risk cytogenetics on FISH.
In this protocol-specified subgroup analysis high-risk cytogenetics were defined as the presence of t(4;14), t(14;16), and/or del(17p).25 This is consistent with the FISH abnormalities used to define “high risk” in other phase 3 studies and analyses [with or without the rare t(14;20) translocation] in patients with MM.7,9,11-15 The presence of these abnormalities has previously been shown to be associated with poor outcomes, including poor PFS and OS, relative to outcomes seen in patients without these abnormalities.7,9,14 Consequently, there remains an ongoing unmet need in patients with MM not only to improve absolute outcomes in high-risk patients but also to provide long-term disease control and overcome the poor prognosis associated with these cytogenetic abnormalities. The findings from our analyses showed that, in patients with high-risk cytogenetics, there was an ∼12-month improvement in median PFS with IRd vs placebo-Rd, and the HR for progression or death was 0.543 (95% CI, 0.321-0.918), corresponding to an 84% improvement in PFS with IRd. Of note, after a median follow-up of ∼15 months (intent-to-treat population), the median PFS with IRd was 21.4 months in high-risk patients, which was similar to the 20.6 months seen in standard-risk patients, whereas the respective medians in the placebo-Rd group were 9.7 and 15.6 months.
The PFS benefit with IRd vs placebo-Rd was also seen in analyses according to the presence or absence of each cytogenetic abnormality. The individual abnormalities t(4;14),16,17 t(14;16),17,18 and del(17p)17,19 have been shown to be independent poor prognostic markers in MM. The findings from our analyses showed a consistent PFS benefit with IRd vs placebo-Rd across the different markers, with a HR of 0.596 (95% CI, 0.286-1.243) in patients with del(17p) and 0.645 (95% CI, 0.250-1.663) in patients with t(4;14).
Our results and data from other studies12,26 in RRMM suggest that Rd is a less-than-optimal treatment of patients with high-risk cytogenetics, particularly del(17p), further supporting the recommendation in the 2016 International Myeloma Working Group consensus paper that a triplet regimen containing an immunomodulatory drug and a proteasome inhibitor should be used for the treatment of MM patients with high-risk cytogenetic abnormalities.10 Indeed, the combination of carfilzomib plus Rd has shown benefit in patients with high-risk cytogenetic abnormalities, although outcomes remained poorer than those for patients with standard-risk cytogenetics.12 Similarly, previous work has indicated that prolonged administration of bortezomib therapy may improve the poor prognosis associated with del(17p).27 Although other studies have reported limited benefit with bortezomib in patients with high-risk cytogenetics, these studies often involved a short course of bortezomib therapy, further highlighting the importance of prolonged proteasome inhibitor therapy in improving outcomes for patients with high-risk cytogenetics.28,29 In TOURMALINE-MM1, patients received continuous IRd therapy until disease progression or unacceptable toxicity. It is therefore possible that the feasibility of continuous ixazomib treatment may contribute to the similar outcomes seen with IRd in patients with high-risk and those with standard-risk cytogenetics, indicating that IRd may be able to overcome the negative impact of high-risk cytogenetic abnormalities, including del(17p). Although the mechanism by which proteasome inhibitors have particular activity in patients with high-risk cytogenetic abnormalities is not well understood, and is likely to differ by particular cytogenetic abnormality, 1 hypothesis for increased activity in patients with del(17p), in which 1 p53 allele is lost,30 involves increased levels of p53 as a result of proteasome activity inhibition, which then triggers apoptosis.31,32 Thus, long-term inhibition of the proteasome may be of importance for maintaining levels of this important tumor suppressor.
Although some evidence suggests that the effect of clone size may vary depending on the cytogenetic abnormality present,33 the link between the size of the del(17p) clone in tumors and its negative impact on the clinical outcome of patients is still the subject of debate. Most of the recent publications reporting the effect of novel therapies on high-risk MM patients have used different “single” cutoffs (ranging from the presence of a single cell in the ELOQUENT-2 trial,26 1.5%-7.5% in the SWOG S0777 trial,34 to a cutoff threshold of 60% of cells in the ASPIRE trial12 ), further limiting cross-trial comparisons and making it difficult to extrapolate the relationship between clone size and clinical outcome in those studies. In 2012, the European Myeloma Network provided guidelines regarding the cutoffs for FISH testing in MM studies involving cytogenetic assessments across multiple laboratories.35 In contrast, in TOURMALINE-MM1, all cytogenetic testing was done using the same assay at a central laboratory, which allowed us to use the protocol-specified technical cutoff (false-positive rate) of the FISH probe used [5% positivity for del(17p)]. Using this cutoff for positivity, 10% of the patients in TOURMALINE-MM1 had tumors carrying the del(17p) abnormality, similar to what has been observed in other studies.36 Furthermore, in the placebo-Rd group, poorer outcomes in high-risk vs standard-risk patients showed that the presence of 5% del(17p)+ cells within a tumor is sufficient to impart a poor clinical outcome, supporting the use of the false-positive rate, or technical cutoffs for each probe, to identify high-risk patients with del(17p).
We also performed post hoc analyses using cutoffs of 20% and 60% of cells to assess the impact of the size of the clone carrying high-risk features on clinical outcomes. Median PFS data suggest that the size of the del(17p) clone impacts long-term outcomes (median PFS with IRd vs placebo-Rd was 21.4 vs 9.7 months using a 5% cutoff, 21.4 vs 6.7 months using a 20% cutoff, and 15.7 vs 5.1 months using a 60% cutoff) (Figure 3). HRs ranging from 0.490 (95% CI, 0.146-1.644; 60% cutoff) to 0.611 (95% CI, 0.286-1.308; 20% cutoff) demonstrate a consistent PFS benefit with IRd vs placebo-Rd, regardless of the cutoff value for del(17p). However, due to the small number of patients in the group with ≥60% positive cells, findings should be interpreted with caution. In patients with t(4;14), a consistent benefit with ixazomib was seen using the protocol-specified 3% cutoff and in the post hoc analyses using 20% and 60% cutoffs (HRs of 0.518-0.685).
In addition to the previously defined high-risk cytogenetic abnormalities, the 2016 International Myeloma Working Group consensus paper includes gain of 1q on FISH as a high-risk feature10 ; this abnormality has also been shown to confer poor prognosis in other studies and analyses in MM17,20,21 and has been suggested as 1 of the most important markers of poor prognosis with current treatments.21 We therefore undertook a post hoc analysis of outcomes according to the presence of this abnormality, and incorporated patients with 1q21 amplification within the expanded high-risk group. As with the other high-risk subgroups and abnormalities, our findings showed a consistent PFS benefit with IRd vs placebo-Rd in patients with amp 1q21 alone (3% cutoff; HR, 0.781; 95% CI, 0.492-1.240) and in the expanded high-risk cytogenetics subgroup (HR, 0.664; 95% CI, 0.474-0.928). Interestingly, in patients with an isolated 1q21 amplification, a greater magnitude of PFS benefit with IRd was noted with the 20% and 60% cutoffs (HR, 0.682 and 0.683, respectively) than with a 3% cutoff (HR, 0.781; 95% CI, 0.492-1.240), suggesting that, in contrast to del(17p), the negative prognostic impact of 1q21 and benefit of the addition of ixazomib to Rd are more dependent on the clone size. The improvement in median PFS with IRd vs placebo-Rd appeared somewhat shorter in patients with amp 1q21 alone as compared with the other high-risk abnormalities, suggesting that IRd can improve, but not overcome, the adverse outcome associated with this abnormality. These data are of particular interest because few studies have analyzed the ability of novel therapies to impact the prognostic value of 1q21 amplification.37 It should, however, be noted that 1q21 amplification frequently cosegregates with other cytogenetic abnormalities such as del(1p), which may also contribute to the poorer outcomes seen in these patients.38,39
As might be expected, our analyses of treatment exposure and toxicities indicated that the safety profile of IRd was generally consistent in high-risk and standard-risk patients, and with the findings in the intent-to-treat population.25 Importantly, the median treatment duration with IRd appeared consistent between high-risk and standard-risk patients, whereas in the placebo-Rd group, the median duration appeared ∼5 months shorter in high-risk vs standard-risk patients. The manageable toxicity and tolerability of the IRd regimen thus appear to enable prolonged proteasome inhibitor–based treatment, thought to be important for improved outcomes in patients with high-risk cytogenetic abnormalities,27 as noted earlier.
In conclusion, IRd demonstrated substantial benefit vs placebo-Rd, with limited additional toxicity, in patients with RRMM and high-risk or standard-risk cytogenetics, and appeared to improve the poor PFS associated with high-risk cytogenetic abnormalities. A consistent PFS benefit was seen across individual adverse cytogenetic abnormalities and across a range of cutoff values used to define the presence of the abnormalities. The tolerable all-oral IRd regimen may therefore represent an important treatment option for patients with RRMM, regardless of cytogenetics, but notably for patients with high-risk abnormalities requiring prolonged active therapy to control their aggressive disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge writing support from Steve Hill and Jane Saunders of FireKite, an Ashfield company, part of UDG Healthcare plc, during the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc, and complied with Good Publication Practice 3 ethical guidelines.40
This work was supported by grants from Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Authorship
Contribution: P.M., P.G.R., D.B., J.L., H.v.d.V., D.-L.E., and A.d.B. contributed to the study concept and design; H.A.-L., D.B., J.L., H.v.d.V., D.-L.E., and A.d.B. analyzed and interpreted the data and drafted the manuscript; and all authors collected and assembled the data, provided the study materials or patients, contributed to critically revising the manuscript, and reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: A. Palumbo has received research funding from Amgen, Novartis, Bristol-Myers Squibb (BMS), Genmab A/S, Celgene, Janssen-Cilag, Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, Sanofi Aventis, Merck, and Binding Site, and received personal fees from Amgen, Novartis, BMS, Genmab A/S, Celgene, Janssen-Cilag, Millennium Pharmaceuticals, Inc, Sanofi Aventis, and Merck. S.K. has received institutional consultancy funding from Merck, Millennium Pharmaceuticals, Inc, Celgene, Sanofi, Amgen, Janssen, Glycomimetics, and R01, and has received personal fees from Skyline Diagnostics, Noxxon, and Kesios. C.L. has received grant funding from Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and received personal fees from Celgene, Janssen, and BMS outside of the submitted work. P.M. reports third-party funding from Millennium Pharmaceuticals, Inc and Celgene, and received personal fees from BMS, Janssen, and Novartis. J.S.-M. has received funding for advisory boards from Novartis, Celgene, Janssen, Millennium Pharmaceuticals, Inc, Onyx, BMS, Merck Sharp & Dohme Corp (MSD), and Amgen. P.G.R. has received funding for advisory boards from Celgene, Novartis, and Millennium Pharmaceuticals, Inc. T.M. has received funding for advisory boards from Janssen-Cilag, Novartis, Millennium Pharmaceuticals, Inc and BMS. M.C. has received personal fees from Millennium Pharmaceuticals, Inc, Celgene, Amgen, and Janssen outside of the submitted work. N.J.B. has received personal fees from Millennium Pharmaceuticals, Inc outside of the submitted work. P.G. has received personal fees from Celgene, Roche, and Novartis outside of the submitted work. H.v.d.V. is an employee of Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and stockholder in Johnson & Johnson. D.-L.E. was an employee of Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, at the time of the study, and stockholder in Johnson & Johnson. D.B. is an employee of Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. The remaining authors declare no competing financial interests.
Correspondence: Hervé Avet-Loiseau, University Cancer Center of Toulouse Institut National de la Santé, Toulouse, France; e-mail: avet-loiseau.h@chu-toulouse.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal