Abstract
Multiple myeloma (MM) is a nearly always incurable malignancy of plasma cells, so new approaches to treatment are needed. T-cell therapies are a promising approach for treating MM, with a mechanism of action different than those of standard MM treatments. Chimeric antigen receptors (CARs) are fusion proteins incorporating antigen-recognition domains and T-cell signaling domains. T cells genetically engineered to express CARs can specifically recognize antigens. Success of CAR-T cells (CAR-Ts) against leukemia and lymphoma has encouraged development of CAR-T therapies for MM. Target antigens for CARs must be expressed on malignant cells, but expression on normal cells must be absent or limited. B-cell maturation antigen is expressed by normal and malignant plasma cells. CAR-Ts targeting B-cell maturation antigen have demonstrated significant antimyeloma activity in early clinical trials. Toxicities in these trials, including cytokine release syndrome, have been similar to toxicities observed in CAR-T trials for leukemia. Targeting postulated CD19+ myeloma stem cells with anti-CD19 CAR-Ts is a novel approach to MM therapy. MM antigens including CD138, CD38, signaling lymphocyte–activating molecule 7, and κ light chain are under investigation as CAR targets. MM is genetically and phenotypically heterogeneous, so targeting of >1 antigen might often be required for effective treatment of MM with CAR-Ts. Integration of CAR-Ts with other myeloma therapies is an important area of future research. CAR-T therapies for MM are at an early stage of development but have great promise to improve MM treatment.
Introduction
Despite recent improvements in treatment, multiple myeloma (MM) remains an almost always incurable disease associated with a high morbidity and mortality; ∼30 000 new cases are expected to be diagnosed and ∼12 000 deaths are expected to occur within the United States in 2017.1 Thus, improved treatments for MM are needed.1,2 MM therapies currently in use include cytotoxic chemotherapy, proteasome inhibitors such as bortezomib, agents such as lenalidomide, monoclonal antibodies, and corticosteroids.3,4 T-cell therapies for MM are completely different than traditional MM therapies. Chimeric antigen receptors (CARs) are artificial fusion proteins that incorporate an antigen-recognition domain and T-cell signaling domains.5-8 Clinical trials using CAR-T cells (CAR-Ts) to treat MM are ongoing and have generated some promising early results.9,10
Allogeneic hematopoietic stem cell transplantation and non-CAR autologous T-cell therapies in MM
Allogeneic hematopoietic stem cell transplantation has been used to treat MM.11,12 In studies assessing the efficacy of allogeneic transplant, chronic graft-versus-host disease had a significant protective effect against relapse of MM.11 A graft-versus-tumor effect from donor lymphocyte infusions yields an overall survival benefit for a subset of relapsed patients.12,13 Unfortunately, allogenic transplant and donor lymphocyte infunsions carry high rates of morbidity and mortality, mainly due to graft-versus-host disease, which has prompted investigators to develop autologous T-cell therapies for MM.11-14 Noonan et al have used activated marrow-infiltrating lymphocytes (MILs) to target MM.15,16 MILs are a polyclonal population of T cells from the MM bone marrow microenvironment.15,16 Twenty-seven percent of patients receiving autologous hematopoietic stem cell transplantation (ASCT) followed by MILs achieved a complete response (CR), 27% achieved a partial response (PR), 23% achieved stable disease (SD), and 14% had progressive disease (PD).15,16 These responses were from the combination of ASCT plus MILs, so the amount of the antimyeloma activity attributable to the MILs is uncertain.
Rapoport et al genetically engineered T cells to express a T-cell receptor (TCR) that recognized the cancer testis antigens NY-ESO-1 and LAGE-1 in HLA-A201+ patients.17 Twenty patients with advanced MM were enrolled in a phase 1/2 trial of ASCT followed 2 days later by TCR-modified T-cell infusions.17 With a median follow-up of 21.1 months, the progression-free survival (PFS) was 50%; this result was from the combination of ASCT plus TCR-modified T cells, so the amount of the antimyeloma activity attributable to the TCR-modified T cells is uncertain.17
Chimeric antigen receptors
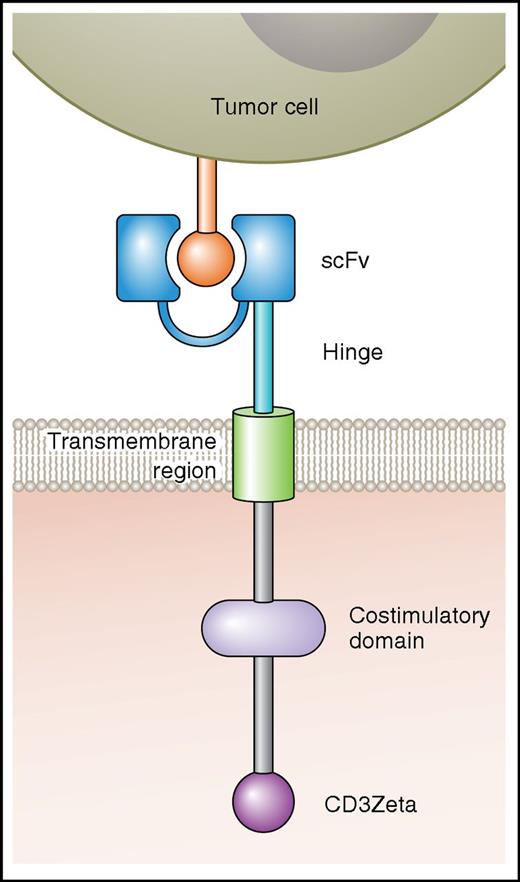
CARs are artificial fusion proteins that incorporate an antigen-recognition domain and T-cell signaling domains (Figure 1).5,6,18 T cells expressing a CAR can specifically recognize a targeted antigen, which is an advantage of CAR T cells over nonspecific cellular therapies such as allogeneic hematopoietic stem cell transplantation.5,6,18-20 CARs are not HLA-restricted, so patients of any HLA type can be treated with CAR-T; this is an advantage of CAR-Ts over T cells engineered to express HLA-restricted TCRs.5,21,22 In addition to an antigen-recognition domain, CARs include hinge and transmembrane regions that connect the extracellular antigen-recognition domain to cytoplasmic signaling domains.5,6,19,23 Signaling domains are of 2 types: costimulatory domains and T-cell activation domains.5,6,8,19,23,24 Examples of costimulatory domains include CD28, 4-1BB, OX40, and immune T-cell costimulator.6-8,25 The T-cell activation domain used in most CARs comes from the CD3ζ molecule.5,6,19,23
A diagram of a CAR is shown. The antigen-binding domain of a CAR is attached to intracellular T-cell signaling moieties by an extracellular hinge domain and a transmembrane region. The CAR antigen-binding domain is usually a scFv derived from a monoclonal antibody. Examples of costimulatory domains are CD28 and 4-1BB. The T-cell activation domain is usually from the CD3Zeta molecule. Professional illustration by Patrick Lane, ScEYEnce Studios.
A diagram of a CAR is shown. The antigen-binding domain of a CAR is attached to intracellular T-cell signaling moieties by an extracellular hinge domain and a transmembrane region. The CAR antigen-binding domain is usually a scFv derived from a monoclonal antibody. Examples of costimulatory domains are CD28 and 4-1BB. The T-cell activation domain is usually from the CD3Zeta molecule. Professional illustration by Patrick Lane, ScEYEnce Studios.
Inclusion of costimulatory domains in CARs has been a critical step in the development of CAR-T therapies.6,7,26-28 First-generation CARs contained the primary activating signaling molecule CD3ζ, which was sufficient to activate a T-cell response,6,19,22 yet, the signal elicited was weak, leading to the creation of second-generation CARs, which added a second costimulatory signal.6-8 The addition of CD28, 4-1BB, OX40, and immune T-cell costimulator led to more robust cytokine production and enhanced cytolytic capacity of CAR-Ts.6-8,27-29 CD28 and 4-1BBB remain the most commonly used costimulatory molecules in clinical trials.6,7 Comparison of first-generation and second-generation CARs has yielded results that confirm the supremacy of second-generation CARs with regard to cytokine production and antitumor activity in mice.6
Implementation of CAR-T therapies
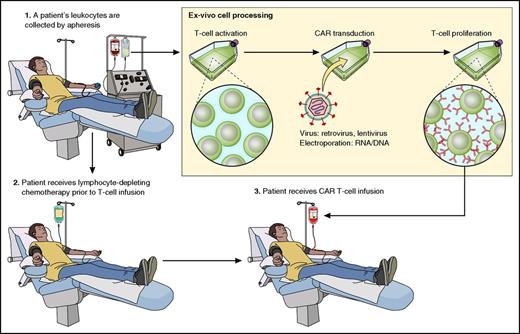
The process of producing CAR-Ts starts by culturing the cells to induce T-cell proliferation (Figure 2). T cells are genetically modified to express a CAR by using γ-retroviruses, lentiviruses, or transposon systems.5,19,23,40 CAR vectors include genetic material that encodes the CAR protein sequence.5,6 CAR design and T-cell culture methods continue to undergo iterations to optimize the many factors that determine patient outcomes after infusions of CAR-Ts.18,24,26,41
A representation of the CAR-T therapy process is shown. Cells are harvested from patients by apheresis. T cells are activated and genetically modified with a CAR gene that will lead to expression of a CAR protein on the T cells. Genetic modification is carried out with a gene-therapy vector such as a γ-retrovirus or a lentivirus. CAR-expressing T cells proliferate ex vivo. Patients often receive a chemotherapy conditioning regimen to deplete endogenous leukocytes with a goal of enhancing CAR-T activity. After completion of the conditioning chemotherapy regimen, CAR-Ts are infused. Professional illustration by Patrick Lane, ScEYEnce Studios.
A representation of the CAR-T therapy process is shown. Cells are harvested from patients by apheresis. T cells are activated and genetically modified with a CAR gene that will lead to expression of a CAR protein on the T cells. Genetic modification is carried out with a gene-therapy vector such as a γ-retrovirus or a lentivirus. CAR-expressing T cells proliferate ex vivo. Patients often receive a chemotherapy conditioning regimen to deplete endogenous leukocytes with a goal of enhancing CAR-T activity. After completion of the conditioning chemotherapy regimen, CAR-Ts are infused. Professional illustration by Patrick Lane, ScEYEnce Studios.
CAR-T toxicity
Toxicities of CAR-T include cytokine release syndrome (CRS), neurologic toxicities, and anaphylaxis.42-45 In particular, CRS has emerged as the most important CAR-T toxicity.43,44,46 CRS features hypotension, fever, and tachycardia among many other abnormalities.43,44,46 CRS has been described to be more severe in patients with higher disease burdens and is associated with increases in serum cytokines including interleukin 6 (IL-6), interferon γ (IFN-γ), and other cytokines.43,44,46 Effective management of CAR-T toxicities includes supportive care and the use of tocilizumab, an IL-6-receptor antagonist, or corticosteroids.42-44 One of the most important goals of the CAR field is to improve the efficacy-to-toxicity ratio of CAR-T therapies.
Rationale for developing CAR-T therapies for MM
One of the main rationales for current efforts to develop CAR-T therapies for MM is the striking activity of anti-CD19 CAR-T against leukemia and lymphoma.5,24,31,33,34,47-51
T cells expressing anti-CD19 CARs can cause complete remissions of B-cell malignancies that are often durable.5,24,25,34,47,52 Impressive results of anti-CD19 CAR-T therapy in acute lymphoid leukemia patients provide a rationale for developing CAR-T against MM because of the extensive bone marrow involvement that is shared by MM and acute lymphoid leukemia.47,48,52,53
Antigen selection
A critical factor in determining the success or failure of a CAR-T therapy is the choice of target antigen. It is important that a selected antigen is uniformly expressed on the malignancy targeted by CAR-T.5,6,20,23 MM is a clonal malignancy, but over time, multiple subclones of MM evolve.54-58 This leads to genetic and phenotypic heterogeneity of the MM cells within the same patient.56,57,59 This phenotypic heterogeneity includes differences in cell-surface antigen expression.58 As antigen expression can differ among MM cells of the same patient, it might be necessary to target >1 antigen to optimize antimyeloma activity of CAR T cells.
To avoid toxicity, the targeted antigen should be absent on essential normal tissues because CAR-Ts might destroy cells expressing the target antigen whether the antigen-expressing cells are normal or malignant. This has been observed in clinical trials involving anti-CD19 CAR-Ts where hypogammaglobinemia results from destruction of all B cells expressing CD19.5,6,31,33 In another example, Lamers et al described epithelial destruction with grade 2-4 liver toxicity when using CAR-Ts engineered to recognize carboxy anhydrase IX (CAIX).60 Liver biopsies obtained from the patients after infusion of CAR-Ts showed discrete cholangitis with T-cell infiltration around the bile ducts as well as CAIX expression on bile duct epithelial cells.60 Similarly, a patient infused with CAR-T targeting ERBB2 was reported to have almost immediate respiratory distress and demonstration of dramatic pulmonary infiltrates on chest radiograph leading to death 5 days after infusion of CAR-Ts.61 Investigators speculated that CAR-Ts localized in the lung due to low expression of ERBB2 on epithelial cells.61 There is no known plasma cell antigen that has strong, uniform expression on all malignant plasma cells and complete absence of expression on normal cells, but several antigens have been studied as targets for antimyeloma CAR-Ts (Table 1).
Target antigens that have been considered for CAR-T therapies of MM
| Antigen . | Expression in nonmalignant cells . | Expression in MM . | Clinical trial of CAR-Ts in myeloma . | References . |
|---|---|---|---|---|
| CD44v6 | Activated T cells, activated monocytes, keratinocytes | Reported to be expressed by 43% of advanced MM cases | No | 62, 63 |
| CD70 | Activated lymphoid cells | Only a portion of the myeloma cells express CD70 in most cases | No | 64, 66 |
| CD56 | NK cells, T cells, neuronal cells | Strongly expressed in 70% of patients with myeloma | No | 67, 68, 70 |
| CD38 | Precursor B cells, plasma cells, T cells, NK cells, myeloid precursors, prostate cells, nervous system, osteoclasts, muscle cells | Strong, uniform expression on myeloma cells | No | 72, 78, 79 |
| CD138 | Plasma cells, salivary glands, liver, skin | Expressed on MM cells | Yes | 36, 81, 82 |
| CD19 | B cells | Minimal expression of CD19 on myeloma plasma cell surface. CD19+ cells may represent cancer stem cell in MM | Yes | 10, 35, 59, 84 |
| Immunoglobulin κ light chain | Mature B cells | Potential target on B cells that represent MM stem cells and that express surface immunoglobulins | Yes | 21, 85 |
| SLAMF7 | Plasma cells, NK cells, CD8+ cells, activated monocytes and B cells, dendritic cells | Strongly expressed by MM cells | No | 88, 89 |
| BCMA | Plasma cells, small subset of B cells | Expressed on MM cells | Yes | 9, 20, 37, 95, 96, 102, 103 |
| Antigen . | Expression in nonmalignant cells . | Expression in MM . | Clinical trial of CAR-Ts in myeloma . | References . |
|---|---|---|---|---|
| CD44v6 | Activated T cells, activated monocytes, keratinocytes | Reported to be expressed by 43% of advanced MM cases | No | 62, 63 |
| CD70 | Activated lymphoid cells | Only a portion of the myeloma cells express CD70 in most cases | No | 64, 66 |
| CD56 | NK cells, T cells, neuronal cells | Strongly expressed in 70% of patients with myeloma | No | 67, 68, 70 |
| CD38 | Precursor B cells, plasma cells, T cells, NK cells, myeloid precursors, prostate cells, nervous system, osteoclasts, muscle cells | Strong, uniform expression on myeloma cells | No | 72, 78, 79 |
| CD138 | Plasma cells, salivary glands, liver, skin | Expressed on MM cells | Yes | 36, 81, 82 |
| CD19 | B cells | Minimal expression of CD19 on myeloma plasma cell surface. CD19+ cells may represent cancer stem cell in MM | Yes | 10, 35, 59, 84 |
| Immunoglobulin κ light chain | Mature B cells | Potential target on B cells that represent MM stem cells and that express surface immunoglobulins | Yes | 21, 85 |
| SLAMF7 | Plasma cells, NK cells, CD8+ cells, activated monocytes and B cells, dendritic cells | Strongly expressed by MM cells | No | 88, 89 |
| BCMA | Plasma cells, small subset of B cells | Expressed on MM cells | Yes | 9, 20, 37, 95, 96, 102, 103 |
BCMA, B-cell maturation antigen; NK, natural killer; SLAMF7, signaling lymphocyte–activating molecule F7.
CD44 variant 6
CD44 is a widely expressed glycoprotein, but CD44 isoform variant 6 (CD44v6) has a more restricted expression pattern.62 CD44v6 has been reported to be expressed by 43% of cases of advanced MM.63 CARs targeting CD44v6 have been constructed and tested.62 A drawback to CD44v6 is that it is expressed on some normal cells including activated T cells, monocytes, and keratinocytes.62
CD70
CD70, which is also known as CD27 ligand, has an expression pattern that is reportedly limited to lymphoid tissues.64 CD70 is under investigation as a CAR target.65,66 Although CD70 might be a good target for some malignancies, the suitability of targeting CD70 in MM is unclear as it has been reported to not be uniformly expressed by the malignant plasma cells in most cases of MM.64
CD56
CD56, a surface glycoprotein mediating cell-cell and cell-matrix interactions, was considered as a potential malignant plasma cell target antigen.67-69 CAR-Ts incorporating anti-CD56 scFv specifically reacted against MM cells in preclinical work.67,70 However, CD56 expression has been demonstrated on natural killer (NK) cells, some T cells, and most importantly, neuronal cells.69 Thus, anticipation of neurologic toxicity because of the expression on neuronal cells has limited development of CAR-Ts targeting CD56.70
CD38
CD38 is a transmembrane glycoprotein involved in cell adhesion, signal transduction, and calcium regulation.71,72 It is normally expressed on precursor B cells, plasma cells, T cells, NK cells, and myeloid precursors.72-74 Among nonhematologic organs, CD38 is expressed on prostate cells, in the nervous system, gut, muscle cells, and on osteoclasts.72,73 Two monoclonal antibodies against CD38 have been tested clinically, daratumumab and isatuximab.75,76 Used as a single agent, daratumumab had a 36% response rate in patients with refractory myeloma.77 Drent et al have demonstrated in preclinical work that anti-CD38 CAR-Ts proliferate, produce cytokines, and lyse CD38+ MM cells; however, these CAR-Ts lysed not only CD38+ MM cells, but also CD38+ normal hematopoietic cells.78 Drent et al have used a process they term affinity optimization in which new anti-CD38 antibodies are generated via light-chain exchange. The CARs are then categorized based on antibody affinity and selected based on ability to lyse CD38+ MM cells but not CD38+ healthy hematopoietic cells.79 The basis for this approach is that MM cells express higher levels of CD38 than most normal hematopoietic cells.78,79 In addition to optimizing scFv affinity, Drent et al and Straathof et al have constructed anti-CD38 CAR-Ts with caspase-9–based suicide genes to eliminate CAR-Ts on demand.78,80
CD138
CD138 also known as syndecan-1 is a heparan-sulfate proteoglycan involved in cell adhesion.81 CD138 is expressed on most plasma cells and MM cells.81,82 MM cells from patients who had relapsed or PD demonstrate higher CD138 levels than MM cells from patients with newly diagnosed disease.82 CD138 expression is found on epithelial cells of organs such as salivary glands, liver, and skin; an antibody-drug conjugate targeting CD138 was associated with dose-limiting toxicities including elevated liver enzymes and palmar-plantar erythrodysesthesia in a small number of patients.20,26,81-83 Five patients with relapsed and refractory MM or plasma cell leukemia were treated on a phase 1 clinical trial of anti-CD138 CAR-Ts.36 All patients had CD138+ MM present in their bone marrow.36 Peripheral blood mononuclear cells (PBMCs) were isolated and cultured with IFN-γ and IL-2 followed by transduction with a lentivirus encoding an anti-CD138 CAR to produce cytokine-induced killer (CIK) cells.36 After conditioning chemotherapy followed by infusion of anti-CD138 CIK cells, the CAR gene was persistently detectable for at least 4 weeks in the blood of all patients.36 Toxicities were limited and consistent with CRS; interestingly, epithelial toxicity was not noted.36,43 The best responses on this trial were SD in 4 patients and PD in 1 patient.36 The average number of CIK cells infused was 0.756 × 107/kg.36 A mean of 31% of the infused cells expressed the anti-CD138 CAR.36
CD19
Several investigators have reported a population of cells that are distinct from the bulk population of malignant MM plasma cells; these cells phenotypically resemble B cells and their role might be that of a cancer stem cell.55,59,84-86 A cancer stem cell is understood to be a cell that has great proliferative potential, the property of self-renewal, and, unlike normal stem cells, a capacity to generate abnormal tissue.55,59,84,85,87 Taking this definition, tumors are thought to be a mix of both cancer stem cells and more differentiated, malignant cells that have limited proliferative capabilities.87 Cancer stem cells are believed to be important in the pathogenesis of acute myeloid leukemia and other malignancies.55,87 Malignant plasma cells have been shown to have a low proliferative index and low cloning efficiency, raising the possibility that a myeloma stem cell could be responsible for tumor propagation in MM.55,59,84,85,87 Circulating B cells that are clonally related to malignant plasma cells have been characterized in patients with MM; in vitro studies suggest that CD138− cells that express B-cell surface antigens including CD45, CD20, and CD19 can give rise to myeloma colonies.55,59,84,85,87 Several studies suggest that the postulated myeloma stem cells have intrinsic drug-resistant properties that may promote relapse.55,85,87 A phase 2 trial showed limited efficacy with administration of the anti-CD20 monoclonal antibody rituximab to heavily pretreated patients with MM; 6 of 19 patients had a PR or SD 3 months after treatment.86
Garfall et al hypothesized that CAR-Ts targeting CD19 would deplete myeloma cells with stem cell properties after myeloablative chemotherapy and standard ASCT.10,35 Ten myeloma patients who had a history of progressive MM after a prior ASCT received a second ASCT followed by an infusion of 5 × 107 anti-CD19 CAR-Ts 12 to 14 days after infusion of stem cells.10,35 The ASCT chemotherapy conditioning regimen was 140 to 200 mg/m2 of melphalan.35 CD19+ cells were detected at low levels among the bone marrow MM plasma cells of 7 of the 9 patients evaluated by flow cytometry pre-ASCT; in these 7 patients, 0.5% to 1.5% of MM cells were CD19+.35 This finding emphasizes that anti-CD19 CAR-Ts are intended to target MM stem cells, not the bulk population of MM cells. All 10 treated patients have progressed; the median PFS was 185 days (range, 42-479 days).35
Immunoglobulin κ light chain
Plasma cells do not generally express cell-surface immunoglobulin, but Ramos et al have used a strategy to target a postulated MM stem cell population that expresses surface immunoglobulins by generating a CAR targeting κ light chain.21 Targeting κ-expressing cells spares λ-expressing B lymphocytes.21 In a phase 1 study, 7 patients with MM were treated with κ-targeted CAR-Ts.21 All 7 MM patients had active disease at the time of CAR-T infusion and had 1 or more lines of prior chemotherapy; 6 of 7 patients had an ASCT in the past.21 Three patients received salvage chemotherapy within 4 weeks prior to infusion of CAR-Ts.21 Those who did not have prior chemotherapy within 4 weeks of CAR-T infusion or who did not have an absolute lymphocyte count <500/μL received 12.5 mg/kg cyclophosphamide prior to infusion of CAR-Ts.21 Four patients had SD lasting 6 weeks to 24 months, and 3 patients had no response to κ CAR-T infusion.21
Signaling lymphocyte–activating molecule F7
One of the most promising CAR targets for MM is signaling lymphocyte–activating molecule F7 (SLAMF7), a product of the SLAMF7 gene and part of a SLAM-related receptor family; SLAMF7 is also known as CD319 or CS1.88-90 In normal tissues, it is expressed on plasma cells, NK cells, some CD8+ T cells, activated B cells, activated monocytes, and dendritic cells.88,90 In contrast to some other myeloma markers like CD138, SLAMF7 is not expressed on nonhematologic organs.88,89 In 1 cohort of MM patients, SLAMF7 messenger RNA was expressed in >90% of patient samples regardless of cytogenetic abnormalities.88 Importantly, SLAMF7 expression is maintained on MM cells regardless of disease relapse or prior chemotherapeutic treatments and is not expressed on hematopoietic stem cells.88,89 Elotuzumab, a humanized monoclonal antibody that binds SLAMF7, has activity against MM, especially when given in combination with immunomodulatory agents.91 In phase 2 and 3 trials evaluating elotuzumab in combination with bortezomib and dexamethasone or in combination with lenalidomide and dexamethasone, addition of elotuzumab was associated with improvements in PFS with limited toxicities.91,92
Preclinical work has demonstrated that anti-SLAMF7 CAR-T therapy can efficiently eradicate MM cells in vitro and in vivo.89,93 Wang et al developed 2 second-generation CARs, 1 targeting B-cell maturation antigen (BCMA) and another targeting SLAMF7.94 Although both CAR-Ts exhibited similar in vitro effector function, the anti-SLAMF7-CAR-T exhibited better antitumor activity than the anti-BCMA CAR-T in a mouse model.94 Whether expression of SLAMF7 on NK cells and CD8+ cells will be a limiting factor for widespread use will be evaluated in clinical trials.
B-cell maturation antigen
BCMA or CD269 is a member of the tumor necrosis factor superfamily; it is expressed on some B cells, normal plasma cells, and on MM cells.95,96 BCMA is not expressed by hematopoietic stem cells or nonhematologic cells.9,95,96 BCMA was uniformly expressed in most cases of MM by immunohistochemistry.9 By flow cytometry, cell-surface BCMA was expressed at varying levels by almost all cases of MM.9 Our group designed CARs with scFvs derived from murine anti-BCMA monoclonal antibodies.9,95 T cells expressing these CARs could specifically recognize BCMA, kill primary myeloma cells in vitro, and eradicate BCMA+ tumors in mice.95
This preclinical work led to the first in-human clinical trial of CAR-Ts targeting BCMA at the National Cancer Institute (NCI).9 An anti-BCMA CAR containing a murine scFv, a CD8α hinge and transmembrane region, a CD28 costimulatory domain, and a CD3ζ T-cell activation domain was created by synthesizing the appropriate DNA and ligating it into a γ-retroviral backbone.9 Patients who had MM with uniform BCMA expression by either immunohistochemistry or flow cytometry were enrolled.9 All participants received conditioning chemotherapy prior to infusion of CAR-Ts with a goal of depleting endogenous leukocytes to reduce regulatory T cells and increase levels of cytokines such as IL-15 and IL-7.5,97,98 The chemotherapy regimen used was cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 given daily on the same 3 days followed by CAR-T infusion 2 days after the end of the chemotherapy.9 Administration of chemotherapy or radiation therapy to deplete recipient leukocytes has been shown to enhance the antitumor activity of adoptively transferred T cells in mice.99-101
Twelve patients with MM were enrolled in this study at the NCI; patients had a median of 7 prior lines of therapy.9 Toxicities were mild in patients receiving lower doses of CAR-Ts (0.3 × 106-3.0 × 106 CAR-Ts per kilogram).9 As the dose of CAR-T escalated, patients began to have more symptoms of CRS, including fever and tachycardia.9 Two patients treated at the highest dose level (9 × 106 CAR+ T cells per kilogram) experienced CRS and a variety of grade 3 and 4 toxicities including neutropenia, thrombocytopenia, hypotension, and acute kidney injury that were managed without long-lasting complications.9
Of the 10 patients treated with lower doses of anti-BCMA CAR-Ts, 1 patient experienced a very-good PR (VGPR) that lasted 8 weeks, 8 patients had SD that lasted from 2 to 12 weeks, and 1 patient had a transient PR.9 Of 2 patients treated at the highest dose level, 1 patient achieved stringent CR (sCR) that lasted for 17 weeks, and 1 patient obtained a VGPR that lasted 66 weeks.9 The myeloma of the patient obtaining the CR was highly chemotherapy-resistant prior to CAR-T therapy as demonstrated by relapse 3 months after ASCT. Prior to anti-BCMA CAR-T therapy, 90% of this patient’s bone marrow cells were plasma cells.9 The patient obtaining the 66-week VGPR had bone marrow that was 80% replaced by MM prior to CAR-T therapy.9 Partial loss of BCMA expression on MM cells was demonstrated in 1 patient.9
A common concern raised with CAR-T therapy is whether soluble antigen may hinder efficacy. In the NCI trial, serum BCMA protein did not eliminate CAR-T activity against MM; moreover, serum BCMA levels dropped significantly in patients who obtained responses to anti-BCMA CAR-Ts.9 In agreement with preclinical work, anti-BCMA CAR-T did not damage nonhematopoietic organs after infusions of T cells.9,95 The responses achieved in patients with chemotherapy-resistant, heavily pretreated refractory myeloma has led to a clinical trial expansion cohort with ongoing accrual.
Cohen et al have published their initial findings from a phase 1 trial of anti-BCMA CAR-Ts without conditioning chemotherapy in patients with relapsed or refractory MM.37 One patient was treated with 1.8 × 108 CAR-Ts, 2 with 2 × 108 CAR-Ts, and 3 patients at the highest dose level of 5 × 108 CAR-Ts.37 Toxicities were similar to those published by Ali et al,9 except for an episode of grade 4 posterior reversible encephalopathy syndrome that manifested as severe delirium, seizures, obtundation, and cerebral edema.37 This was treated with corticosteroids, antiepileptics, and, interestingly, cyclophosphamide; there was no long-term neurological dysfunction.37 Reported results showed that 1 patient had an ongoing sCR at 7 months with minimal residual disease–negative bone marrow by flow cytometry.37 One patient who had pleural and dural MM involvement was found to have CAR-Ts in pleural fluid and cerebrospinal fluid and achieved VGPR with resolution of extramedullary disease; this patient’s VGPR lasted 5 months, and progression was associated with loss of BCMA expression on MM cells.37 Four patients had modest to minimal in vivo CAR-T proliferation and minimal to no antimyeloma response.37 Of note, the trial reported by Cohen et al used an anti-BCMA CAR with fully human antibody variable regions; whether utilizing a construct without murine components will mitigate immunological rejection will be important to assess.
A third anti-BCMA CAR-T clinical trial is a multicenter trial being conducted by Bluebird Bio.102 Twenty-one patients with relapsed/refractory MM have been infused with bb2121, an anti-BCMA CAR-T that expresses a CAR with the same scFv as the anti-BCMA CAR used at the NCI.9 In contrast to the NCI anti-BCMA CAR, bb2121 has a 4-1BB costimulatory motif, and it is encoded by a lentivirus.102 bb2121 is administered after cyclophosphamide plus fludarabine lymphocyte-depleting chemotherapy.102 To date, the authors report that the safety profile of bb2121 has been favorable through doses as high as 800 × 106 CAR-Ts with no dose-limiting toxicity observed.102 Seventy-one percent of patients have had CRS that was generally mild.102 Efficacy of bb2121 in this study has been promising. Of 3 patients receiving a CAR-T dose of 50 × 106 CAR-Ts, 1 patient obtained a PR, but no patients obtained responses of VGPR or CR.102 Among 18 patients receiving CAR-T doses >50 × 106 CAR-Ts, 15 patients have reached at least 60 days of follow-up, and 11 of 15 patients with at least 60 days of follow-up have obtained best responses of VGPR or CR.102 All patients with at least 60 days of follow-up receiving a cell dose of >50 × 106 CAR-Ts have obtained at least a PR.102
Fan et al have reported early results from an ongoing phase 1 anti-BCMA CAR trial that is enrolling patients with relapsed or refractory MM.103 The CAR used in this study is called LCAR-B38M. Among 19 patients reported, there was a 100% overall response rate.103 Eighteen patients obtained a sCR or a VGPR after receiving anti-BCMA CAR-Ts.103 CRS occurred in 74% of patients but was noted to be mild in most patients.103
Future directions
As the general field of CAR-T technology evolves, improvements in CAR-T therapies for MM are anticipated. It is important to optimize CAR design by assessing different costimulatory, hinge, and transmembrane domains, and by minimizing the immunogenicity of CARs. BCMA continues to show promise as a target antigen with a favorable expression profile and promising early clinical trial results, so more anti-BCMA CAR-T clinical trials are forthcoming.9,95 Because of concern for antigen escape, it will be important to continue to identify new targets for CAR-T therapy; evaluation of known targets such as SLAMF7 and identification of new MM targets is critical. Future studies are needed to elucidate the phenotype of the putative myeloma stem cell, which will aid in designing CAR-T therapies aimed at the fraction of MM cells most able to survive and proliferate. Studies assessing combinations of CAR-Ts with other agents, including immunomodulatory drugs, are needed.94 Initial results showed that addition of lenalidomide to anti-CS1 CAR T cells significantly improved CAR-T persistence and antimyeloma activity in preclinical experiments.94
Conclusions
CAR-Ts are a promising new approach for treating MM. Several patients have now obtained objective responses of MM after infusions of anti-BCMA CAR-T.9,37,102,103 Cytokine-release toxicity has been severe in some patients receiving anti-BCMA CAR-T, but the toxicities have generally been reversible and short-lived.9 The most exciting aspect of CAR-T therapy for MM is that it offers a novel approach that might be able to kill MM cells that are resistant to standard therapies. Because CAR-T therapies for MM are at an early stage of development, significant improvements are probable. Results obtained so far should encourage further research on CAR-T therapies for MM (Table 2).
Clinical trials of CAR-Ts for MM with published results
| Target . | Institution . | CAR construct . | Conditioning chemotherapy . | Response . | Reference . |
|---|---|---|---|---|---|
| CD138 | Chinese People's Liberation Army General Hospital | Lentivirus; CD28 costimulatory molecule; murine scFv | Varied, all patients received some type of chemotherapy shortly before cell infusion | 5 patients treated; best responses: 4 SD, 1 PD | 36 |
| CD19 | University of Pennsylvania | Lentivirus 4-1BB costimulatory molecule; murine scFv | Melphalan 140-200 mg/m2 prior to ASCT | 10 patients treated; median PFS 185 d (range, 42-479 d) | 10, 35 |
| Immunoglobulin κ light chain | Baylor College of Medicine | γ- retrovirus; CD28 costimulatory molecule; murine scFv | Salvage chemotherapy by referring physician (3 patients) or 12.5 mg/kg cyclophosphamide 4 days prior to CAR-T infusion (4 patients) | 7 patients treated: 4 SD, 3 no response | 21 |
| BCMA | National Cancer Institute | γ-retrovirus; CD28 costimulatory molecule; murine scFv | 3 doses of 300 mg/m2 cyclophosphamide and 3 doses of 30 mg/m2 fludarabine | 12 patients treated; best responses: 1 sCR; 2 VGPR; 1 PR; 8 SD | 9 |
| BCMA | Multicenter; Bluebird Bio | Lentivirus; 4-1BB costimulatory molecule; murine scFv | 3 doses of 300 mg/m2 cyclophosphamide and 3 doses of 30 mg/m2 fludarabine | Among 18 patients with at least 2 mo follow-up, currently, best responses are 4 CR, 7 VGPR, 5 PR, 2 SD | 102 |
| BCMA | University of Pennsylvania | Lentivirus; 4-1BB costimulatory molecule; human scFv | None | 6 patients treated; 1 sCR; 1 VGPR; 4 with minimal or no response | 37 |
| BCMA | The Second Affiliated Hospital of Xi’an Jiaotong University | Not published | Not published | Among 19 patients there was a 100% response rate with 18 patients obtaining sCR or VGPR | 103 |
| Target . | Institution . | CAR construct . | Conditioning chemotherapy . | Response . | Reference . |
|---|---|---|---|---|---|
| CD138 | Chinese People's Liberation Army General Hospital | Lentivirus; CD28 costimulatory molecule; murine scFv | Varied, all patients received some type of chemotherapy shortly before cell infusion | 5 patients treated; best responses: 4 SD, 1 PD | 36 |
| CD19 | University of Pennsylvania | Lentivirus 4-1BB costimulatory molecule; murine scFv | Melphalan 140-200 mg/m2 prior to ASCT | 10 patients treated; median PFS 185 d (range, 42-479 d) | 10, 35 |
| Immunoglobulin κ light chain | Baylor College of Medicine | γ- retrovirus; CD28 costimulatory molecule; murine scFv | Salvage chemotherapy by referring physician (3 patients) or 12.5 mg/kg cyclophosphamide 4 days prior to CAR-T infusion (4 patients) | 7 patients treated: 4 SD, 3 no response | 21 |
| BCMA | National Cancer Institute | γ-retrovirus; CD28 costimulatory molecule; murine scFv | 3 doses of 300 mg/m2 cyclophosphamide and 3 doses of 30 mg/m2 fludarabine | 12 patients treated; best responses: 1 sCR; 2 VGPR; 1 PR; 8 SD | 9 |
| BCMA | Multicenter; Bluebird Bio | Lentivirus; 4-1BB costimulatory molecule; murine scFv | 3 doses of 300 mg/m2 cyclophosphamide and 3 doses of 30 mg/m2 fludarabine | Among 18 patients with at least 2 mo follow-up, currently, best responses are 4 CR, 7 VGPR, 5 PR, 2 SD | 102 |
| BCMA | University of Pennsylvania | Lentivirus; 4-1BB costimulatory molecule; human scFv | None | 6 patients treated; 1 sCR; 1 VGPR; 4 with minimal or no response | 37 |
| BCMA | The Second Affiliated Hospital of Xi’an Jiaotong University | Not published | Not published | Among 19 patients there was a 100% response rate with 18 patients obtaining sCR or VGPR | 103 |
Authorship
Contribution: J.N.K. and L.M. researched published papers and abstracts on CAR-T therapies for MM and drafted and edited the manuscript.
Conflict-of-interest disclosure: J.N.K. receives research funding from cooperative research and development agreements between the National Cancer Institute (NCI) and Kite Pharma Inc, and between the NCI and Bluebird Bio Inc, and also has multiple patent applications related to CARs. L.M. declares no competing financial interests.
Correspondence: James N. Kochenderfer, National Cancer Institute, 9000 Rockville Pike, 10 Center Dr, CRC Room 3-3888, Bethesda, MD 20892; e-mail: kochendj@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal