Key Points
Compared with placebo, hydroxyurea did not increase the incidence or severity of malaria events in Ugandan children with SCA.
Hydroxyurea provided significant clinical and laboratory benefits, suggesting it will be safe and effective across sub-Saharan Africa.
Abstract
Hydroxyurea treatment is recommended for children with sickle cell anemia (SCA) living in high-resource malaria-free regions, but its safety and efficacy in malaria-endemic sub-Saharan Africa, where the greatest sickle-cell burden exists, remain unknown. In vitro studies suggest hydroxyurea could increase malaria severity, and hydroxyurea-associated neutropenia could worsen infections. NOHARM (Novel use Of Hydroxyurea in an African Region with Malaria) was a randomized, double-blinded, placebo-controlled trial conducted in malaria-endemic Uganda, comparing hydroxyurea to placebo at 20 ± 2.5 mg/kg per day for 12 months. The primary outcome was incidence of clinical malaria. Secondary outcomes included SCA-related adverse events (AEs), clinical and laboratory effects, and hematological toxicities. Children received either hydroxyurea (N = 104) or placebo (N = 103). Malaria incidence did not differ between children on hydroxyurea (0.05 episodes per child per year; 95% confidence interval [0.02, 0.13]) vs placebo (0.07 episodes per child per year [0.03, 0.16]); the hydroxyurea/placebo malaria incidence rate ratio was 0.7 ([0.2, 2.7]; P = .61). Time to infection also did not differ significantly between treatment arms. A composite SCA-related clinical outcome (vaso-occlusive painful crisis, dactylitis, acute chest syndrome, splenic sequestration, or blood transfusion) was less frequent with hydroxyurea (45%) than placebo (69%; P = .001). Children receiving hydroxyurea had significantly increased hemoglobin concentration and fetal hemoglobin, with decreased leukocytes and reticulocytes. Serious AEs, sepsis episodes, and dose-limiting toxicities were similar between treatment arms. Three deaths occurred (2 hydroxyurea, 1 placebo, and none from malaria). Hydroxyurea treatment appears safe for children with SCA living in malaria-endemic sub-Saharan Africa, without increased severe malaria, infections, or AEs. Hydroxyurea provides SCA-related laboratory and clinical efficacy, but optimal dosing and monitoring regimens for Africa remain undefined. This trial was registered at www.clinicaltrials.gov as #NCT01976416.
Introduction
Sickle cell anemia (SCA) is a life-threatening hematological disorder and among the world’s most prevalent hereditary diseases, with more than 300 000 affected babies born each year.1 The vast majority of these births occur in sub-Saharan Africa, where an estimated 50% to 90% of children with SCA will die by age 5 years, often without an established diagnosis.2 The heterozygous sickle gene mutation confers a strong survival advantage against malaria,3 which explains why the allele frequency is highest in malaria-endemic regions of Africa.1
Hydroxyurea has proven laboratory and clinical efficacy for both children and adults with SCA.4-7 Its mechanisms of action are multiple and incompletely understood, but fetal hemoglobin (HbF) induction in erythroid cells is critical for the inhibition of intracellular sickling. Treatment also has salutary effects on blood cell adhesion, morphology, and rheology.8 Further, hydroxyurea is a safe drug for SCA, with low incidence of treatment-related toxicity and no serious long-term effects observed to date. Hydroxyurea is currently approved by the US Food and Drug Administration for adults with severe symptoms and by the European Medicines Agency for affected adults and children above age 2 years. Based on a large and compelling body of evidence accumulated over the past 30 years, evidence-based guidelines published by the National Heart, Lung and Blood Institute of the National Institutes of Health strongly recommend wider usage, including offering treatment to infants as young as 9 months of age.9
The benefits of hydroxyurea treatment of children with SCA would be greatest in countries within sub-Saharan Africa, if hydroxyurea were found to be safe and have its predicted efficacy in these high-burden areas. However, the effects of hydroxyurea on the presentation and clinical course of malaria must also be considered. Hydroxyurea may directly affect several factors related to the pathogenesis of severe malaria, with potentially deleterious consequences. Some in vitro and animal studies suggest that hydroxyurea increases endothelial intracellular adhesion molecule-1 (ICAM-1) expression,10 which could enhance parasite adhesion to endothelium,11 and also increases tumor necrosis factor α levels,12 both of which are associated with increased malaria severity and death.13 Other studies have challenged these findings,14,15 but definitive human data are lacking. If hydroxyurea induces effects that shift the clinical course in SCA from uncomplicated to severe malaria, this could increase mortality despite benefits for the underlying SCA.16 In addition, in low-resource settings, hydroxyurea may cause neutropenia that could lead to increased severity of the many bacterial infections commonly observed in African children with SCA.17
In contrast to these adverse effects, it is possible that hydroxyurea could have beneficial effects against malaria because HbF, which is increased by hydroxyurea, inhibits Plasmodium falciparum growth in vitro.18 Hydroxyurea treatment also generates nitric oxide,19 which protects against severe malaria in animals20,21 and humans.22 With these contrasting potential mechanisms, the risks and benefits of hydroxyurea in a malaria endemic setting remain unknown, and many critical questions remain regarding the safety, feasibility, and efficacy of hydroxyurea for children with SCA living in malaria-endemic settings within sub-Saharan Africa.
For these reasons, we conducted a prospective randomized double-blinded placebo-controlled clinical trial, the Novel use Of Hydroxyurea in an African Region with Malaria (NOHARM), in young Ugandan children with SCA, to determine the safety and efficacy of hydroxyurea in a malaria-endemic region. NOHARM is registered at www.clinicaltrials.gov as #NCT01976416.
Methods
Study design
A detailed description of the study site, design, and procedures was previously published.23 Briefly, NOHARM was a randomized double-blinded placebo-controlled clinical trial. Because of concerns about the risks of severe malaria and infection with hydroxyurea treatment, the consensus among local experts and the local institutional review board supported a placebo-controlled trial, despite the expected SCA-related treatment benefits in children with SCA. It was agreed, however, that study participants should be given the opportunity to receive subsequent open-label hydroxyurea, if no danger signal was observed from the blinded study treatment. NOHARM was conducted at Mulago Hospital Sickle Cell Clinic in Kampala, Uganda. Kampala has seasonal malaria transmission, with high outpatient and inpatient burdens. Protocol approval was obtained from the institutional review boards of the Makerere University School of Medicine, Indiana University, University of Minnesota, and Cincinnati Children’s Hospital, as well as the Uganda National Drug Authority and Uganda National Council for Science and Technology.
Study participants
Children receiving care at Mulago Hospital Sickle Cell Clinic were eligible if they were 1.00 to 3.99 years of age and living within 50 km of the clinic. Study inclusion criteria included confirmed SCA, weight ≥5.0 kg, and willingness to comply with study procedures. Children with severe malnutrition or known chronic medical conditions, current hydroxyurea treatment, or blood transfusion in the previous 30 days were excluded. Parents of the study participants gave written informed consent.
Randomization and masking
Children meeting inclusion criteria completed enrollment and after screening, were randomized 1:1 to hydroxyurea or placebo by the Data Coordinating Center at Cincinnati Children’s Hospital as described.23 Study allocations were randomly assigned by the statistician, using computer-generated sequences in block files of 8 participants. Study treatments were supplied with A or B labels, and the clinical coordinating center staff, study pharmacists, families and caregivers, and all but 2 members of the Data Coordinating Center were masked to treatment allocation.
Medications
Study treatment (Addmedica, Paris, France) included oral hydroxyurea (Siklos) as 1000-mg scored tablets and 100-mg dispersible tablets, or placebo tablets of identical size and appearance. Treatment was administered once daily at 20 ± 2.5 mg/kg for 12 months, with dose adjustments in both arms for weight gain and hematological toxicities.23
Procedures
All participants received standard care for SCA including folic acid, penicillin prophylaxis, and pneumococcal vaccination. For malaria prophylaxis, children received insecticide-treated mosquito nets and monthly sulphadoxine-pyrimethamine. Study participants were seen for the randomization visit (month 0) and for scheduled visits at 2 weeks posttreatment initiation, monthly from months 1 to 4, and at months 6, 8, 10, and 12 for a total of 10 scheduled visits with monitoring for clinical malaria and toxicities. Caregivers were instructed to return to clinic or hospital whenever the child was unwell; all febrile children received malaria testing. After completing the blinded treatment phase, participants could receive open-label hydroxyurea, as per local Ethics Committee recommendations.
Measurements
Complete blood counts with leukocyte differential and absolute reticulocyte count (ARC) were measured at each visit (Sysmex Corporation, Kobe, Japan) plus blood chemistries using a Cobas 6000 analyzer Model C501 (Roche Diagnostics, Indianapolis, IN). HbF was quantified by capillary electrophoresis (Minicap; Sebia, Paris, France). Malaria was assessed by peripheral blood smear using Giemsa staining: each slide was read by 2 certified microscopists, with a third reading to resolve any discrepancies.
Clinical definitions
All children with measured fever (axillary temperature ≥37.5°C) in the clinic or fever by history were tested for malaria by microscopy. Children with measured fever or a history of fever and detectable Plasmodium species infection of any density on blood smear were diagnosed with clinical malaria, as is standard in this area. Malaria was treated with parenteral artesunate followed by oral artemether-lumefantrine, if the child was hospitalized, or oral artemether-lumefantrine, if the child was not hospitalized. The diagnosis of sickle-related clinical events followed published definitions,24 with modifications such as pneumonia based on clinical rather than radiograph findings. Clinical sepsis was defined as presentation with fever and an unwell appearance, for which IV antibiotics were given. Full definitions for clinical adverse events (AEs) are provided in Table 1.
NOHARM AE definitions
| . | Definition . |
|---|---|
| Clinical AE terminology | |
| Vaso-occlusive pain crisis/dactylitis | Vaso-occlusive pain crisis: acute pain and tenderness in an area of the body, with or without swelling, with no other diagnostic explanation. |
| Dactylitis: vaso-occlusive crisis (acute pain, tenderness, and swelling) localized to hands or feet. | |
| Pneumonia/acute chest syndrome | Pneumonia: history of fever or measured axillary temperature ≥37.5°C, with tachypnea and cough. |
| Acute chest syndrome: signs of pneumonia plus chest pain and/or tenderness. | |
| Clinical sepsis | Measured fever and ill appearance, requiring IV antibiotics. |
| Acute splenic sequestration | Increase in splenic size from last physical examination, accompanied by a decrease in hemoglobin of ≥2 g/dL. |
| Upper respiratory infection | Child with general well appearance with rhinorrhea, nasal congestion, or cough. |
| Gastrointestinal related | Diarrhea, vomiting, constipated, intestinal obstruction. |
| Malaria | Measured fever (axillary temperature ≥37.5°C) or fever by history and Plasmodium species infection on blood smear. |
| Other infection | Other infections, diagnosed clinically. |
| Other (eg, injury) | Other diseases not included above diagnosed during visits for illness. |
| Laboratory AEs* | |
| Anemia | Hemoglobin <6 g/dL |
| Reticulocytopenia | ARC <80 × 109/L and hemoglobin <7 g/dL |
| Neutropenia | Absolute neutrophil count (ANC) <1.0 × 109/L |
| Thrombocytopenia | Platelet count <80 × 109/L |
| Elevated AST/ALT | AST >150 IU/L, ALT >150 IU/L |
| Elevated bilirubin | Total bilirubin >5 mg/dL |
| . | Definition . |
|---|---|
| Clinical AE terminology | |
| Vaso-occlusive pain crisis/dactylitis | Vaso-occlusive pain crisis: acute pain and tenderness in an area of the body, with or without swelling, with no other diagnostic explanation. |
| Dactylitis: vaso-occlusive crisis (acute pain, tenderness, and swelling) localized to hands or feet. | |
| Pneumonia/acute chest syndrome | Pneumonia: history of fever or measured axillary temperature ≥37.5°C, with tachypnea and cough. |
| Acute chest syndrome: signs of pneumonia plus chest pain and/or tenderness. | |
| Clinical sepsis | Measured fever and ill appearance, requiring IV antibiotics. |
| Acute splenic sequestration | Increase in splenic size from last physical examination, accompanied by a decrease in hemoglobin of ≥2 g/dL. |
| Upper respiratory infection | Child with general well appearance with rhinorrhea, nasal congestion, or cough. |
| Gastrointestinal related | Diarrhea, vomiting, constipated, intestinal obstruction. |
| Malaria | Measured fever (axillary temperature ≥37.5°C) or fever by history and Plasmodium species infection on blood smear. |
| Other infection | Other infections, diagnosed clinically. |
| Other (eg, injury) | Other diseases not included above diagnosed during visits for illness. |
| Laboratory AEs* | |
| Anemia | Hemoglobin <6 g/dL |
| Reticulocytopenia | ARC <80 × 109/L and hemoglobin <7 g/dL |
| Neutropenia | Absolute neutrophil count (ANC) <1.0 × 109/L |
| Thrombocytopenia | Platelet count <80 × 109/L |
| Elevated AST/ALT | AST >150 IU/L, ALT >150 IU/L |
| Elevated bilirubin | Total bilirubin >5 mg/dL |
The diagnosis of sickle-related clinical events followed published definitions,24 with modifications such as pneumonia and clinical sepsis.
ALT, alanine transferase; AST, aspartate transferase.
Laboratory AE definitions represent the values necessary for a grade 2 event.
Study outcomes
The primary study outcome was incidence of clinical malaria. Secondary outcomes included the following: (1) a composite of 1 or more SCA-related AEs (pain, dactylitis, acute chest syndrome, splenic sequestration, or transfusion); (2) clinical AEs; and (3) dose-limiting toxicities.
Statistical analysis
We estimated malaria incidence would be between 0.3 and 1.3 malaria episodes per year, based on a study based in Kampala that includes community children (lowest incidence estimate) and children with severe malaria (highest incidence estimate) (C.C.J., unpublished data). With these rates of malaria incidence, and variance to mean ratio 1.7, we estimated that 100 children per treatment arm would provide 90% power (α = 0.05) to detect between 59% and 34% difference in incidence between treatment arms, respectively. Study data were entered into OnCore and analyzed using R (Vienna, Austria; version 3.2.4). The primary analysis included all randomized participants per intention to treat, with P < .05 considered significant. Interim analysis for safety was performed after 100 children completed blinded study treatment. The difference in malaria incidence rate between the hydroxyurea and placebo groups was analyzed using negative binomial regression. For secondary outcomes, P < .01 was considered significant. Continuous measures are described as mean (standard deviation [SD]), and treatment arms were compared using the 2-sample Welch’s t test, or when within the same individuals using a paired Welch’s t test. Categorical measures are described using percent or frequency and compared using the Pearson χ2 test with Yates correction or Fisher’s exact test. Malaria episodes are reported as mean per child (95% confidence interval [CI]). Time to malaria infection is described by Kaplan-Meier curves. Groups were compared for cumulative incidence (ie, time to infection) using Gray’s method, with all-cause death treated as a competing event.25
Results
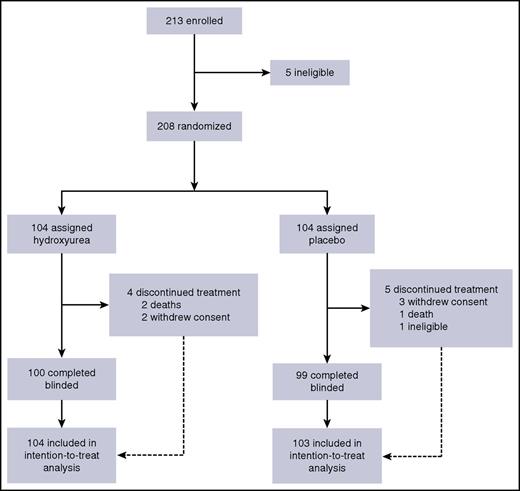
A total of 213 children were enrolled between 24 September 2014 and 2 October 2015; among these, 208 were randomized to either hydroxyurea or placebo (Figure 1). One child was later deemed ineligible because of an elevated baseline ALT, so that child’s data were removed from the analyzed data set. The treatment arms had similar baseline demographic, laboratory, and clinical measures (Table 2). No child had symptomatic or asymptomatic Plasmodium species parasitemia at enrollment.
Baseline demographic, clinical, and laboratory characteristics of the NOHARM randomized cohort
| . | Hydroxyurea (N = 104) . | Placebo (N = 103) . | P . |
|---|---|---|---|
| Demographics, N (%) | |||
| Age at enrollment, mean (SD), y | 2.2 (0.9) | 2.3 (0.9) | .3148 |
| Male | 55 (53) | 57 (55) | .8298 |
| Parent completing secondary school | 32 (31) | 35 (34) | .7614 |
| Running water in the home | 14 (13) | 11 (11) | .6885 |
| Growth measures, mean (SD) | |||
| Height, cm | 85.6 (8.8) | 86.3 (8.2) | .5615 |
| Weight, kg | 11.3 (2.1) | 11.5 (2.1) | .4604 |
| Z score (weight for length/height) | −0.40 (1.06) | −0.39 (1.06) | .9316 |
| Past medical history, N (%) | |||
| Dactylitis | 80 (77) | 82 (80) | .7639 |
| Vaso-occlusive crisis | 88/103 (85) | 81/103 (79) | .3421 |
| Stroke | 0 | 0 | — |
| Splenomegaly | 6/93 (6) | 5/99 (5) | .7619* |
| Acute chest syndrome | 21/103 (20) | 13/100 (13) | .2219 |
| Transfusion | 56 (54) | 57 (55) | .9393 |
| Hospitalization within 1 y of enrollment | 65 (63) | 54 (52) | .1851 |
| Laboratory measures, mean (SD) | |||
| Hemoglobin, g/dL | 7.5 (1.1) | 7.6 (1.0) | .5214 |
| MCV, fL | 79 (9) | 80 (9) | .8248 |
| Fetal hemoglobin [HbF/(HbF + HbS)], % | 14.6 (7.1) | 13.3 (6.0) | .1591 |
| ARC (×109/L) | 380 (122) | 381 (112) | .9623 |
| WBC count, ×109/L | 19.0 (7.2) | 18.7 (5.4) | .7148 |
| ANC, ×109/L | 6.5 (3.1) | 6.2 (2.6) | .4023 |
| Platelets, ×109/L | 358 (171) | 416 (138) | .0075 |
| ALT, U/L | 18 (9) | 19 (9) | .5478 |
| Creatinine, mg/dL | 0.28 (0.09) | 0.28 (0.07) | .6068 |
| . | Hydroxyurea (N = 104) . | Placebo (N = 103) . | P . |
|---|---|---|---|
| Demographics, N (%) | |||
| Age at enrollment, mean (SD), y | 2.2 (0.9) | 2.3 (0.9) | .3148 |
| Male | 55 (53) | 57 (55) | .8298 |
| Parent completing secondary school | 32 (31) | 35 (34) | .7614 |
| Running water in the home | 14 (13) | 11 (11) | .6885 |
| Growth measures, mean (SD) | |||
| Height, cm | 85.6 (8.8) | 86.3 (8.2) | .5615 |
| Weight, kg | 11.3 (2.1) | 11.5 (2.1) | .4604 |
| Z score (weight for length/height) | −0.40 (1.06) | −0.39 (1.06) | .9316 |
| Past medical history, N (%) | |||
| Dactylitis | 80 (77) | 82 (80) | .7639 |
| Vaso-occlusive crisis | 88/103 (85) | 81/103 (79) | .3421 |
| Stroke | 0 | 0 | — |
| Splenomegaly | 6/93 (6) | 5/99 (5) | .7619* |
| Acute chest syndrome | 21/103 (20) | 13/100 (13) | .2219 |
| Transfusion | 56 (54) | 57 (55) | .9393 |
| Hospitalization within 1 y of enrollment | 65 (63) | 54 (52) | .1851 |
| Laboratory measures, mean (SD) | |||
| Hemoglobin, g/dL | 7.5 (1.1) | 7.6 (1.0) | .5214 |
| MCV, fL | 79 (9) | 80 (9) | .8248 |
| Fetal hemoglobin [HbF/(HbF + HbS)], % | 14.6 (7.1) | 13.3 (6.0) | .1591 |
| ARC (×109/L) | 380 (122) | 381 (112) | .9623 |
| WBC count, ×109/L | 19.0 (7.2) | 18.7 (5.4) | .7148 |
| ANC, ×109/L | 6.5 (3.1) | 6.2 (2.6) | .4023 |
| Platelets, ×109/L | 358 (171) | 416 (138) | .0075 |
| ALT, U/L | 18 (9) | 19 (9) | .5478 |
| Creatinine, mg/dL | 0.28 (0.09) | 0.28 (0.07) | .6068 |
A total of 208 children were randomized to either hydroxyurea (N = 104) or placebo (N = 104). One participant was later deemed ineligible, and those data were removed from the data set. Values are shown as the mean (SD) or the number of study participants with the measure/total tested for that measure (% affected).
HbS, sickle hemoglobin; MCV, mean corpuscular volume; WBC, white blood cell.
Fisher's exact test because of the low frequency.
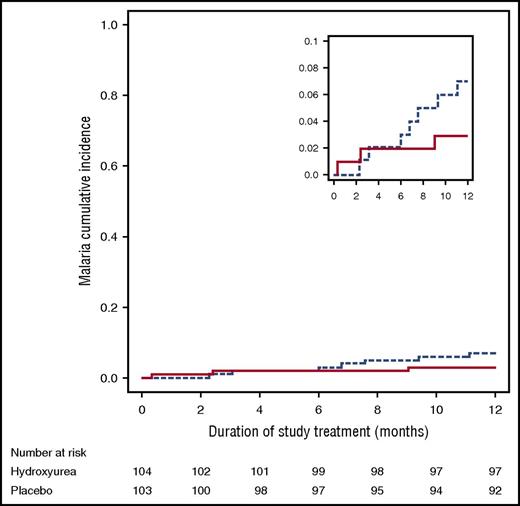
Malaria occurred at a low rate throughout the study, perhaps reflecting excellent adherence to malaria prophylaxis. Over the 1-year study period, malaria testing was done for 235 episodes of measured fever or history of fever in study participants, with only 12 fever episodes because of malaria. Malaria incidence did not differ between children prescribed hydroxyurea (0.05 episodes per child per year, 95% CI [0.02, 0.13]) vs placebo (0.07 episodes per child per year [0.03, 0.16]); the hydroxyurea/placebo malaria incidence rate ratio was 0.7 ([0.2, 2.7], P = .61). Time to infection also did not differ significantly between hydroxyurea and placebo (Figure 2). All 12 malaria episodes were because of P. falciparum; 1 child had coinfection with P. malariae. Three children on hydroxyurea had a total of 5 malaria episodes, compared with 7 children on placebo with a total of 7 malaria episodes (Table 3). The median parasite density was 26 410 parasites per µL (minimum, 1064; maximum, 193 705). Six of the 12 episodes of malaria were severe, requiring hospitalization (2 with hemoglobin concentration <5 g/dL, 1 with impaired consciousness, 1 with hemoglobin concentration <5 g/dL and impaired consciousness, and 2 unable to take oral medication). All 10 study participants with malaria recovered. In the 12 confirmed cases of malaria, there were 4 episodes with concomitant clinical AE: vaso-occlusive pain crisis (N = 3) and acute chest syndrome/pneumonia (N = 1). In addition, there were 2 episodes of malaria with concomitant SAE: splenic sequestration (N = 1) and bacteremia (N = 1).
Incidence of malaria events in the NOHARM trial, with no statistical difference observed between the blinded treatment arms (P = .19). Comparisons of the hydroxyurea to placebo group were calculated using Gray’s test for competing events, treating death as a competing event. Solid line represents hydroxyurea; dashed line represents placebo. The smaller inset diagram is identical to the larger graph but has a different scale.
Incidence of malaria events in the NOHARM trial, with no statistical difference observed between the blinded treatment arms (P = .19). Comparisons of the hydroxyurea to placebo group were calculated using Gray’s test for competing events, treating death as a competing event. Solid line represents hydroxyurea; dashed line represents placebo. The smaller inset diagram is identical to the larger graph but has a different scale.
AEs in the NOHARM study population
| . | Hydroxyurea (N = 104) . | Placebo (N = 103) . | P . | ||
|---|---|---|---|---|---|
| Events . | Participants . | Events . | Participants . | ||
| SAEs | 6 | 6 | 6 | 6 | |
| Bacteremia/sepsis | 2 | 2 | 2 | 2 | 1.0* |
| Acute chest syndrome/pneumonia | 1 | 1 | 2 | 2 | .62* |
| Vaso-occlusive crisis | 0 | 0 | 1 | 1 | .50* |
| Acute splenic sequestration | 2 | 2 | 0 | 0 | .50* |
| Anemia | 0 | 0 | 1 | 1 | .50* |
| Sudden death | 1 | 1 | 0 | 0 | 1.0* |
| SCA-related events (composite) | |||||
| Vaso-occlusive pain crisis, dactylitis, acute chest syndrome, splenic sequestration, or blood transfusion | 47 | 71 | .001 | ||
| Clinical AEs | 232 | 76 | 308 | 88 | |
| Vaso-occlusive pain crisis/dactylitis | 58 | 38 | 106 | 59 | .004 |
| Acute chest syndrome/pneumonia | 24 | 21 | 32 | 24 | .71 |
| Clinical sepsis | 8 | 6 | 16 | 13 | .14 |
| Acute splenic sequestration | 0 | 0 | 0 | 0 | — |
| Upper respiratory tract infection | 107 | 54 | 108 | 62 | .29 |
| Gastrointestinal related | 15 | 13 | 15 | 12 | 1.0 |
| Malaria | 5 | 3 | 7 | 7 | .21* |
| Other infections | 8 | 7 | 14 | 14 | .16 |
| Others (eg, injury) | 7 | 7 | 10 | 9 | .78 |
| Clinical interventions | 34 | 14 | 53 | 32 | |
| Transfusion | 14 | 12 | 18 | 17 | .41 |
| Hospitalization | 20 | 12 | 35 | 28 | .007 |
| Laboratory AEs | 57 | 31 | 78 | 46 | |
| Anemia | 40 | 25 | 65 | 42 | .015 |
| Reticulocytopenia | 3 | 3 | 6 | 5 | .50* |
| Neutropenia | 2 | 2 | 0 | 0 | .50* |
| Thrombocytopenia | 12 | 11 | 4 | 4 | .11 |
| Elevated AST/ALT | 0 | 0 | 2 | 1 | .50* |
| Elevated bilirubin | 0 | 0 | 1 | 1 | .50* |
| Dose-limiting toxicities | 21 | 15 | 17 | 13 | |
| Anemia | 6 | 4 | 8 | 8 | .36 |
| Reticulocytopenia | 1 | 1 | 5 | 5 | .12* |
| Neutropenia | 2 | 2 | 0 | 0 | .50* |
| Thrombocytopenia | 12 | 11 | 4 | 4 | .11 |
| . | Hydroxyurea (N = 104) . | Placebo (N = 103) . | P . | ||
|---|---|---|---|---|---|
| Events . | Participants . | Events . | Participants . | ||
| SAEs | 6 | 6 | 6 | 6 | |
| Bacteremia/sepsis | 2 | 2 | 2 | 2 | 1.0* |
| Acute chest syndrome/pneumonia | 1 | 1 | 2 | 2 | .62* |
| Vaso-occlusive crisis | 0 | 0 | 1 | 1 | .50* |
| Acute splenic sequestration | 2 | 2 | 0 | 0 | .50* |
| Anemia | 0 | 0 | 1 | 1 | .50* |
| Sudden death | 1 | 1 | 0 | 0 | 1.0* |
| SCA-related events (composite) | |||||
| Vaso-occlusive pain crisis, dactylitis, acute chest syndrome, splenic sequestration, or blood transfusion | 47 | 71 | .001 | ||
| Clinical AEs | 232 | 76 | 308 | 88 | |
| Vaso-occlusive pain crisis/dactylitis | 58 | 38 | 106 | 59 | .004 |
| Acute chest syndrome/pneumonia | 24 | 21 | 32 | 24 | .71 |
| Clinical sepsis | 8 | 6 | 16 | 13 | .14 |
| Acute splenic sequestration | 0 | 0 | 0 | 0 | — |
| Upper respiratory tract infection | 107 | 54 | 108 | 62 | .29 |
| Gastrointestinal related | 15 | 13 | 15 | 12 | 1.0 |
| Malaria | 5 | 3 | 7 | 7 | .21* |
| Other infections | 8 | 7 | 14 | 14 | .16 |
| Others (eg, injury) | 7 | 7 | 10 | 9 | .78 |
| Clinical interventions | 34 | 14 | 53 | 32 | |
| Transfusion | 14 | 12 | 18 | 17 | .41 |
| Hospitalization | 20 | 12 | 35 | 28 | .007 |
| Laboratory AEs | 57 | 31 | 78 | 46 | |
| Anemia | 40 | 25 | 65 | 42 | .015 |
| Reticulocytopenia | 3 | 3 | 6 | 5 | .50* |
| Neutropenia | 2 | 2 | 0 | 0 | .50* |
| Thrombocytopenia | 12 | 11 | 4 | 4 | .11 |
| Elevated AST/ALT | 0 | 0 | 2 | 1 | .50* |
| Elevated bilirubin | 0 | 0 | 1 | 1 | .50* |
| Dose-limiting toxicities | 21 | 15 | 17 | 13 | |
| Anemia | 6 | 4 | 8 | 8 | .36 |
| Reticulocytopenia | 1 | 1 | 5 | 5 | .12* |
| Neutropenia | 2 | 2 | 0 | 0 | .50* |
| Thrombocytopenia | 12 | 11 | 4 | 4 | .11 |
All serious adverse events (SAEs), AEs, and laboratory AEs were compared between treatment arms. Proportions of affected participants were compared by χ2 tests with Yates correction. Full definitions of AEs are provided in Table 1.
Fisher's exact test instead of χ2 test, because of the low expected frequency.
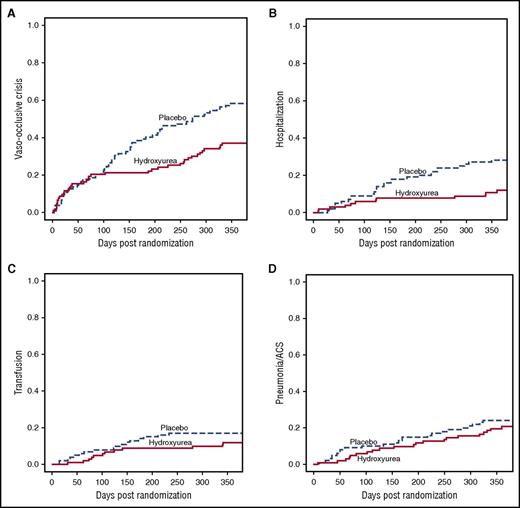
A per protocol composite clinical outcome included 1 or more SCA-related clinical events (vaso-occlusive painful crisis, dactylitis, acute chest syndrome/pneumonia, splenic sequestration, or blood transfusion). The proportion of children with this outcome was significantly lower in the hydroxyurea arm than the placebo arm (45% vs 69%; Table 3, P = .001). For individual clinical events, vaso-occlusive pain and hospitalizations were significantly less frequent with hydroxyurea than placebo (Table 3; Figure 3). This difference in hospitalization rate was largely driven by differences in vaso-occlusive crises, which accounted for 42% of all hospitalizations. The number needed to treat to prevent 1 hospitalization was 6.4, whereas the number needed to treat to prevent a SCA-related event was 2.5. No strokes occurred in either treatment arm during the 1-year treatment period. Clinical sepsis was more frequent with placebo (12.6%) than hydroxyurea (5.8%), but this difference was not statistically significant (P = .14). Blood cultures were positive in only 2 episodes, both for Staphylococcus aureus, 1 in each treatment arm.
Cumulative incidence of sickle-related AEs over 12 months, by blinded treatment arm. (A) Painful vaso-occlusive crisis; P = .004. (B) Hospitalization; P = .002. (C) Transfusion; P = .27. (D) Pneumonia/acute chest syndrome; P = .51.
Cumulative incidence of sickle-related AEs over 12 months, by blinded treatment arm. (A) Painful vaso-occlusive crisis; P = .004. (B) Hospitalization; P = .002. (C) Transfusion; P = .27. (D) Pneumonia/acute chest syndrome; P = .51.
SAEs, defined as death, an acute life-threatening event, or hospitalization for >7 days, occurred equally with 6 in each treatment arm (Table 3). During the blinded treatment phase, 3 children died (2 hydroxyurea, 1 placebo) and 5 others withdrew from the study. Causes of death were presumed sepsis (1 hydroxyurea, 1 placebo) and sudden death, cause unknown (hydroxyurea). In 2 of the SAEs listed in Table 3, 2 participants had malaria concomitant with bacteremia or splenic sequestration, as noted previously.
The 2 treatment arms had similar counts of laboratory AEs except anemia (Table 3). Specifically, low hemoglobin (<6.0 g/dL) occurred more frequently in children receiving placebo than hydroxyurea, whereas the frequencies of neutropenia, thrombocytopenia, and reticulocytopenia did not differ significantly between treatment arms. Similarly, the frequency of protocol-defined dose-limiting hematological toxicities did not differ between treatment arms (Table 3). Neutropenia was notably rare, occurring only once in only 2 participants over the entire study.
Children receiving hydroxyurea had significant treatment-associated increases in hemoglobin concentration, MCV, and HbF compared with those receiving placebo (Table 4). Substantial increases in HbF were observed for both children below and above the median enrollment age, despite starting with different baseline HbF levels. Conversely, children on hydroxyurea had significant decreases in WBC count, ANC, ARC, and platelets compared with children receiving placebo (Table 4). The treatment arms did not differ in changes in ALT or creatinine. Medication adherence, assessed by careful questioning at each clinic visit, was deemed excellent in both treatment arms.
Selected laboratory characteristics of the NOHARM randomized population
| Laboratory measures . | Month 12 . | Change from baseline . | ||||
|---|---|---|---|---|---|---|
| Hydroxyurea . | Placebo . | P . | Hydroxyurea . | Placebo . | P . | |
| Hemoglobin, g/dL | 8.7 (1.3) | 7.4 (1.0) | <.001 | 1.2 (1.2) | −0.1 (0.9) | <.001 |
| MCV, fL | 88 (9) | 81 (8) | <.001 | 9 (7) | 1 (5) | <.001 |
| Fetal hemoglobin [HbF/(HbF + HbS)], % | 22.9 (8.6) | 10.4 (4.8) | <.001 | 8.5 (6.7) | −3.1 (3.3) | <.001 |
| Enrollment age below median | 24.1 (8.5) | 12.1 (4.8) | <.001 | 7.9 (7.4) | −4.1 (3.5) | <.001 |
| Enrollment age above median | 21.4 (8.7) | 8.8 (4.2) | <.001 | 9.3 (5.6) | −2.2 (2.8) | <.001 |
| ARC, ×109/L | 247 (107) | 391 (122) | <.001 | −144 (119) | 11 (119) | <.001 |
| WBC count, ×109/L | 13.7 (5.1) | 18.0 (5.1) | <.001 | −5.4 (5.8) | −0.7 (5.1) | <.001 |
| ANC, ×109/L | 5.2 (2.5) | 6.6 (2.6) | <.001 | −1.3 (3.0) | 0.4 (3.0) | <.001 |
| Platelets, ×109/L | 371 (166) | 446 (143) | <.001 | 12 (184) | 28 (152) | .50 |
| ALT, U/L | 19 (8) | 18 (8) | .59 | 0.8 (11) | −0.9 (10) | .28 |
| Creatinine, mg/dL | 0.32 (0.11) | 0.31 (0.08) | .64 | 0.04 (0.13) | 0.04 (0.10) | .92 |
| Laboratory measures . | Month 12 . | Change from baseline . | ||||
|---|---|---|---|---|---|---|
| Hydroxyurea . | Placebo . | P . | Hydroxyurea . | Placebo . | P . | |
| Hemoglobin, g/dL | 8.7 (1.3) | 7.4 (1.0) | <.001 | 1.2 (1.2) | −0.1 (0.9) | <.001 |
| MCV, fL | 88 (9) | 81 (8) | <.001 | 9 (7) | 1 (5) | <.001 |
| Fetal hemoglobin [HbF/(HbF + HbS)], % | 22.9 (8.6) | 10.4 (4.8) | <.001 | 8.5 (6.7) | −3.1 (3.3) | <.001 |
| Enrollment age below median | 24.1 (8.5) | 12.1 (4.8) | <.001 | 7.9 (7.4) | −4.1 (3.5) | <.001 |
| Enrollment age above median | 21.4 (8.7) | 8.8 (4.2) | <.001 | 9.3 (5.6) | −2.2 (2.8) | <.001 |
| ARC, ×109/L | 247 (107) | 391 (122) | <.001 | −144 (119) | 11 (119) | <.001 |
| WBC count, ×109/L | 13.7 (5.1) | 18.0 (5.1) | <.001 | −5.4 (5.8) | −0.7 (5.1) | <.001 |
| ANC, ×109/L | 5.2 (2.5) | 6.6 (2.6) | <.001 | −1.3 (3.0) | 0.4 (3.0) | <.001 |
| Platelets, ×109/L | 371 (166) | 446 (143) | <.001 | 12 (184) | 28 (152) | .50 |
| ALT, U/L | 19 (8) | 18 (8) | .59 | 0.8 (11) | −0.9 (10) | .28 |
| Creatinine, mg/dL | 0.32 (0.11) | 0.31 (0.08) | .64 | 0.04 (0.13) | 0.04 (0.10) | .92 |
Results are shown only for participants who had data for the measure both at baseline and at 12 mo, as mean (SD) or the percentage of participants affected. The median age at enrollment was 2.2 y. Treatment group differences at month 12 and changes from baseline were tested by Welch’s t tests.
Discussion
In this prospective randomized double-blinded placebo-controlled trial of young children with SCA living in Uganda, hydroxyurea therapy was both safe and efficacious. The 2 treatment arms did not differ in the incidence, severity, or other outcomes from malaria infection; in the incidence of clinical sepsis or bacteremia; or in the number of laboratory AEs or dose-limiting toxicities, including neutropenia. In contrast, the previously described laboratory and clinical benefits of hydroxyurea therapy were clearly observed in this young population. The increases in hemoglobin concentration and HbF levels, along with decreases in neutrophils and reticulocytes, were similar to values observed in US-based trials,4-7,26 and the rates of important clinical SCA-related events such as vaso-occlusive painful crisis and hospitalizations were similarly reduced. Taken together, these data suggest that hydroxyurea should be strongly considered as an important therapeutic option for young children with SCA living in malaria endemic areas.
NOHARM represents the first randomized trial of hydroxyurea in Africa. The study design captured all possible malaria events, with blood smear testing for all episodes of current and recent fever. The incidence of malaria in our cohort was low, suggesting effective protection by insecticide-treated bed nets and monthly oral malaria prophylaxis with sulfadoxine-pyrimethamine provided to all study participants, or possibly a decreased risk of clinical malaria in children with SCA. Some form of malaria prophylaxis for children with SCA is a standard recommendation in most African countries,27 and insecticide-treated bed net use for children <5 years of age in malaria endemic areas of Africa has increased dramatically over the past decade, to an average of >60% coverage,28 so the preventive measures used in this study are similar to those in many other malaria endemic areas where children with SCA live. However, adherence to these recommendations is highly variable, so our study findings may not apply to children with SCA not on malaria prophylaxis and/or not using insecticide-treated bed nets. Because malaria incidence was low in our study, the range of relative differences in malaria incidence between hydroxyurea and placebo is broad (95% CI for incidence rate ratio 0.2 to 2.7), but the absolute differences in episodes is small (95% CI, 2-13 episodes per 100 children per year with hydroxyurea vs 3-16 episodes per 100 children per year with placebo). Hydroxyurea did not worsen malaria severity, because hospitalizations for malaria did not differ between treatment arms, and no child died of malaria. Because of the low malaria incidence, this study does not provide definitive guidance regarding the safety of hydroxyurea in all malaria endemic areas, but the lack of increased risks of malaria incidence, severity, and outcome is reassuring. The incidence of malaria in these children with SCA further suggests that absolute differences in malaria risk with hydroxyurea will be very small. However, risks of hydroxyurea may differ in areas of higher malaria transmission, and genetic or environmental factors in other areas could affect risk of hydroxyurea toxicity or malaria-hydroxyurea interactions. In light of the present study data showing efficacy of hydroxyurea against pain crises and hospitalizations, with no evidence of increased malaria or infection risk in this area of low malaria transmission, it may be difficult to justify additional placebo-controlled trials in areas of higher malaria transmission. If additional placebo-controlled studies are not conducted, future studies of open-label hydroxyurea in other malaria endemic regions should carefully document the rates and complications of malaria during hydroxyurea treatment. These data will help determine the long-term safety profile of hydroxyurea for children living in malaria-endemic regions.
All 5 of the study children hospitalized with severe malaria (1 child was hospitalized twice) survived, in contrast to an earlier study from Kenya in which 4 of 5 children with SCA hospitalized for severe malaria died (80% mortality).16 The presence of malaria parasites on peripheral blood smear also did not increase the risk of death during hospitalization, contrasting with a Tanzanian study of children with SCA, which reported increased mortality in hospitalized children with parasitemia.29 In contrast, another study from Kenya reported no deaths among 38 children with SCA and severe malarial anemia, the most common form of severe malaria affecting these children.30 Similarly, in a recent study in Kampala conducted by our group, none of 22 children with SCA who developed severe malarial anemia died.31 Together, these study findings cast doubt on the contention that severe malaria causes high rates of mortality in children with SCA. In the first 2 studies cited, which described increased mortality from malaria in children with SCA, malaria prophylaxis was either not given routinely16 or consisted of chloroquine.29 The sulfadoxine-pyrimethamine prophylaxis given in the present study is likely more effective than chloroquine prophylaxis. In addition, the 2 studies were conducted during a period of much lower bed net coverage, and a significant proportion of children in these earlier study cohorts received chloroquine or sulfadoxine-pyrimethamine for treatment of uncomplicated malaria, instead of the more effective current standard of care, artemisinin-combination therapy. Together, these factors may have played a role in the higher mortality seen with malaria in these prior cohorts.
Increased infection in children with drug-induced neutropenia was an additional concern regarding hydroxyurea treatment in an area where invasive bacterial infection is common in children. However, neutropenia was rare in NOHARM and did not differ on hydroxyurea vs placebo treatment, and episodes of pneumonia, clinically defined sepsis, and bacteremia did not differ between hydroxyurea or placebo treatment arms. These data confirm findings observed in the United States5 and should help allay safety concerns about hydroxyurea and infection risk related to neutropenia in low-resource settings.
Hydroxyurea was also associated with significantly fewer SCA-related clinical events, specifically vaso-occlusive crises, dactylitis, and hospitalizations. Other laboratory outcomes of long-term clinical importance for children with SCA, including increases in hemoglobin concentration and HbF, as well as decreases in leukocyte, neutrophil, and reticulocyte counts, were all more favorable in children receiving hydroxyurea than placebo, further supporting the drug's efficacy in this study population. Despite the differences in nutritional status from US-based populations, as evidenced by the low baseline Z scores, the hydroxyurea responses were remarkably similar to published results in the BABY HUG trial for both clinical and laboratory effects.5 The potential benefits of hydroxyurea treatment on growth in NOHARM are also important to assess but have not yet undergone formal analysis.
After the double-blinded treatment phase, the entire NOHARM cohort was offered open-label hydroxyurea as per the study design, and all but 1 family opted for treatment. Future analysis can thus compare the effects of early initiation and the longer-term effects of treatment. Because hydroxyurea escalated to maximum tolerated dose is standard in the United States,7,25 additional studies should assess how SCA-related AEs and hematological responses compare for children receiving fixed-dose treatment vs dose escalation regimens. Outcomes were excellent in the present study with a safe and relatively easily administered fixed dose (20 mg/kg), so it will be important to investigate how treatment benefits and risks differ with different dosing schemes of hydroxyurea for children with SCA living in malaria-endemic areas. Children in African countries often have limited access to health care facilities, and those facilities typically have limited laboratory testing capability. Less frequent laboratory monitoring would allow wider implementation of hydroxyurea treatment.
Additional studies that include longer duration of hydroxyurea in malaria-endemic areas will help determine optimal drug dosing, the range of AEs, and malaria incidence in areas with higher malaria transmission. Until then, the present study documents that with adequate monitoring, mosquito nets, and malaria prophylaxis, fixed-dose hydroxyurea treatment of children with SCA in malaria-endemic areas is safe with no increased incidence of malaria, sepsis, bacteremia, or SAEs. In combination with the observed clinical efficacy (fewer painful events and hospitalizations), the NOHARM study findings support the wider use of hydroxyurea for children with SCA living in malaria-endemic regions across sub-Saharan Africa.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to thank the Mulago Hospital Sickle Cell Clinic and Global Health Uganda staff for conducting study work, the Data Coordinating Center staff at Cincinnati Children’s Hospital Medical Center for setup of the study database and monitoring of study data, Fogarty and Doris Duke Charitable Foundation trainees who did operational work on the study, members of the Data and Safety Monitoring Board, and especially the study participants and their caregivers. Addmedica Inc. (Paris, France) donated both hydroxyurea and placebo study treatments.
This work was supported by a grant from the Doris Duke Charitable Foundation (ICRA 2013139), and Cincinnati Children’s Research Foundation provided financial support for the NOHARM Data Coordinating Center.
The Doris Duke Charitable Foundation and Addmedica Inc. did not participate in the following aspects of the research: (1) study design; (2) data collection, analysis, or interpretation; (3) writing the report; or (4) decision to submit the publication.
Authorship
Contribution: R.O.O., R.E.W., and C.C.J. designed the study, supervised the trial, analyzed the results, and wrote the first draft of the manuscript; T.S.L. helped coordinate many critical aspects of the trial to ensure its safe and successful operational execution; C.M.N., H.A.H., and P.K. enrolled patients, collected data, and helped interpret the results; A.L. and J.S.H. performed statistical analyses for the trial; and all authors participated in the editing of the manuscript and approved the final version.
Conflict-of-interest disclosure: R.E.W. is a consultant for Global Blood Therapeutics and Nova Laboratories, is on an advisory board for Agios Pharmaceuticals, receives research support from Bristol-Myers Squibb, and serves on a Data and Safety Monitoring Board for the US Food and Drug Administration. None of these disclosures are relevant to the results and conclusions of the NOHARM trial. The remaining authors declare no competing financial interests.
Correspondence: Russell E. Ware, Division of Hematology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: russell.ware@cchmc.org.
References
Author notes
R.E.W. and C.C.J. are joint senior authors.