Key Points
Strong responses to venetoclax separate T-PLL from other hematologic malignancies in high- throughput drug screening of clinical samples.
Two relapsed and refractory T-PLL patients demonstrated clinical response on venetoclax treatment.
Abstract
T-cell prolymphocytic leukemia (T-PLL) is a rare and aggressive T-lymphoid malignancy usually refractory to current treatment strategies and associated with short overall survival. By applying next-generation functional testing of primary patient-derived lymphoma cells using a library of 106 US Food and Drug Administration (FDA)-approved anticancer drugs or compounds currently in clinical development, we set out to identify novel effective treatments for T-PLL patients. We found that the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax (ABT-199) demonstrated the strongest T-PLL–specific response when comparing individual ex vivo drug response in 86 patients with refractory hematologic malignancies. Mechanistically, responses to venetoclax correlated with protein expression of BCL-2 but not with expression of the BCL-2 family members myeloid cell leukemia 1 (MCL-1) and BCL-XL in lymphoma cells. BCL-2 expression was inversely correlated with the expression of MCL-1. Based on the ex vivo responses, venetoclax treatment was commenced in 2 late-stage refractory T-PLL patients resulting in clinical responses. Our findings demonstrate first evidence of single-agent activity of venetoclax both ex vivo and in humans, offering a novel agent in T-PLL.
Introduction
T-cell prolymphocytic leukemia (T-PLL) is an aggressive T-lymphoid malignancy characterized by proliferation of postthymic prolymphocytes.1,2 Patients typically present with elevated and exponentially rising lymphocyte counts along with splenomegaly, hepatomegaly, lymphadenopathy, and effusions.1 Responses to alkylating agents or polychemotherapy are poor with a median survival of just 7 months.2 The use of the monoclonal anti-CD52 antibody alemtuzumab has improved response rates to 75% or even higher when applied in the first-line setting.3-6 The combination of chemotherapy regimen fludarabine, mitoxantrone, cyclophosphamide (FCM) followed by alemtuzumab increased responses to 90%.7 However, despite high response rates all patients eventually relapse with a median progression-free survival of <12 months.
Cytogenetic abnormalities involve chromosome 14 and also frequently chromosomes 8, 11, and 17, altering activities of oncogenes and tumor suppressors (TCL-1, MTCP-1, ATM, TP-53).8-10 Next-generation sequencing identified additional mutations affecting the JAK-STAT pathway (IL2RG, JAK1, JAK3, STAT5B) and genes encoding epigenetic regulators (EZH2, TET2, BCOR).11-15 However, these recurrent genetic alterations in T-PLL did not correlate with ex vivo responses to specific drug classes.16 To overcome the lack of genomically informed therapy, we here applied direct ex vivo chemosensitivity testing to identify effective agents for T-PLL patients.
Study design
Patients and ex vivo drug screen
Samples were obtained and processed after consenting under protocols EKNr1830/2015 and EKNr2008/2015 approved by the local ethics commission. Venetoclax was provided by AbbVie as part of a single-patient preapproval access program. All patient samples (bone marrow aspirates, peripheral blood, or excised lymph node) were prepared freshly and used for drug-screening analysis within 3 hours of harvest. Resulting single-cell suspensions (in RPMI 1640 plus 10% fetal calf serum) were seeded at 1 × 105 cells per well in 384-well plates containing compound libraries with each drug in 4 concentrations in triplicate (supplemental Table 1, available on the Blood Web site). Drug-concentration range was determined by dose-response experiments in primary cells and cancer cell lines for each compound. Differential cell viability was determined after 72 hours with CellTiter-Glo (Promega) on an EnVision plate reader (PerkinElmer). Dose-response curves and immunoblotting were performed on viably frozen samples. Viability data of these samples after thawing was determined by the CASY Cell Analyzer (OLS) (supplemental Figure 3).
Tissue microarray
Tissue microarrays (TMAs) were generated on a Galileo CK3500 tissue microarrayer (Integrated Systems Engineering) using formalin-fixed paraffin-embedded material (supplemental Table 2).
Data calculation and statistics
Drug responses normalized as percentage of controls were calculated as areas under the curve (AUC) as the sum of all data points for a respective drug in individual patient samples.17 P values were calculated using Wilcoxon, Mann-Whitney, and Kruskal-Wallis tests as indicated.
Results and discussion
High-throughput ex vivo drug screening identifies venetoclax sensitivity of primary T-PLL cells
Pioneering studies provided proof of concept that ex vivo chemosensitivities can match clinical response.16-22 We performed next-generation functional drug profiling on primary cells of 86 patients using 106 compounds (supplemental Table 1). Cell-specific responses were calculated from individual dose-response curves and TMAs were generated for comparative protein-expression profiling (Figure 1A; supplemental Table 2).
Venetoclax shows strongest differential ex vivo response in T-PLL samples and is correlated with BCL-2 expression. (A) Lymph node and liquid biopsies were taken from consenting patients and used for expression profiling via TMAs and ex vivo drug-response profiling (n = 86). (B) Volcano blot, demonstrating mean differential AUCs (ΔAUCs) plotted for individual compounds comparing ex vivo effects in T-PLL vs non-T-PLL samples (n of compounds = 106). Blue circles indicate compounds hitting more specifically T-PLL than non-T-PLL samples (negative ΔAUCs). Gray circles indicate compounds hitting more specifically non-T-PLL than T-PLL samples (positive ΔAUCs). Venetoclax is highlighted in red as the most specific T-PLL compound identified in the drug screening. (C) AUCs of venetoclax in individual ex vivo samples stratified by indication (n = 86). (D) Comparison of AUCs of venetoclax and corresponding BCL-2 expression score by indication (n = 37). (E) Correlation of MCL-1 and BCL-XL expression scores of individual samples (n = 34). B-ALL, B-cell acute lymphoblastic leukemia; T-LBL, T-cell lymphoblastic lymphoma; T-NHL, T-cell non-Hodgkin lymphoma.
Venetoclax shows strongest differential ex vivo response in T-PLL samples and is correlated with BCL-2 expression. (A) Lymph node and liquid biopsies were taken from consenting patients and used for expression profiling via TMAs and ex vivo drug-response profiling (n = 86). (B) Volcano blot, demonstrating mean differential AUCs (ΔAUCs) plotted for individual compounds comparing ex vivo effects in T-PLL vs non-T-PLL samples (n of compounds = 106). Blue circles indicate compounds hitting more specifically T-PLL than non-T-PLL samples (negative ΔAUCs). Gray circles indicate compounds hitting more specifically non-T-PLL than T-PLL samples (positive ΔAUCs). Venetoclax is highlighted in red as the most specific T-PLL compound identified in the drug screening. (C) AUCs of venetoclax in individual ex vivo samples stratified by indication (n = 86). (D) Comparison of AUCs of venetoclax and corresponding BCL-2 expression score by indication (n = 37). (E) Correlation of MCL-1 and BCL-XL expression scores of individual samples (n = 34). B-ALL, B-cell acute lymphoblastic leukemia; T-LBL, T-cell lymphoblastic lymphoma; T-NHL, T-cell non-Hodgkin lymphoma.
To identify specific drug sensitivities for T-PLL, we compared the average ex vivo drug response of T-PLL vs non-T-PLL samples for each drug. The B-cell lymphoma 2 (BCL-2)–selective small-molecule inhibitor venetoclax (ABT-199) demonstrated the strongest differential response for T-PLL (Figure 1B). We also observed strong responses to venetoclax in chronic lymphatic leukemia (CLL) and varying responses in aggressive lymphoma and acute myeloid leukemia (AML) in line with clinical responses and recent reports (Figure 1C).23-25 Ex vivo responses to venetoclax significantly correlated with BCL-2 protein expression scores (Figure 1D, P = .013; negative correlation to AUC, r = −0.403), but not with scores for BCL-2 gene family members BCL-XL and myeloid cell leukemia 1 (MCL-1) (supplemental Figure 2A-B). BCL-XL and MCL-1 expression scores demonstrated a significant correlation (Figure 1E, r = 0.407; P = .017) whereas only MCL-1 appears to be inversely correlated with BCL-2 expression (supplemental Figure 2C-D). T-PLL samples demonstrated strong BCL-2 scores and the most dramatic responses to BCL-2 inhibition by venetoclax (Figure 1D). High expression of BCL-2 and low expression levels of BCL-XL and MCL-1 in T-PLL samples might explain the strong sensitivity toward BCL-2 inhibition (Figure 1D-E; supplemental Figure 2E-F).
Clinical responses of 2 T-PLL patients to treatment with venetoclax
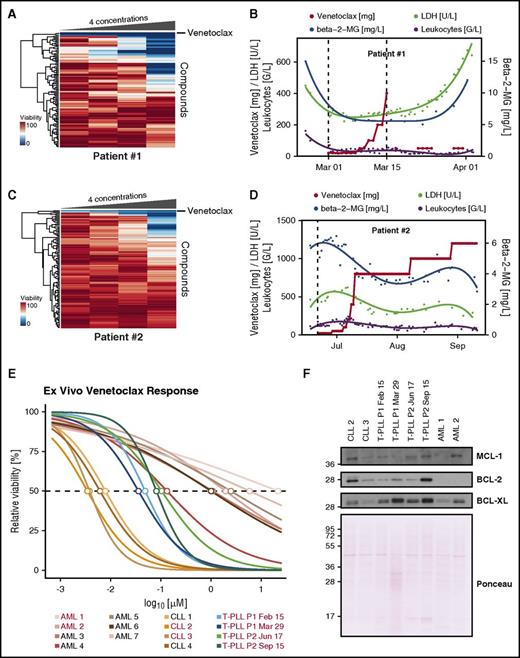
Based on drug and protein expression profiling, venetoclax was administered as an “individual healing attempt” in 2 refractory T-PLL patients. Patient 1, a 58-year-old woman, relapsed within 3 months after 3 treatment lines: (1) FCM, (2) alemtuzumab, and (3) pixantrone, etoposide, bendamustine (PEBen). Fluorescence in situ hybridization revealed deletion 13q and trisomy 12. T-PLL cells presented as clonal prolymphocytes (supplemental Figure 3A). Drug profiling demonstrated strong efficacy of venetoclax (Figure 2A). Before start of venetoclax, the patient presented in poor condition, with splenomegaly (24.7 cm in diameter), lymphocytosis of 161 G/L, and β2-microglobulin (b2MG) and lactate dehydrogenase (LDH) serum levels of 15.1 mg/L and 449 U/L, respectively. Twelve hours upon first venetoclax dosing at 20 mg, lymphocytosis dropped to 28 G/L, b2MG to 7.5 mg/L, LDH to 260 U/L accompanied by laboratory tumor lysis. Overall, venetoclax was tolerated well and resulted in a decrease of splenomegaly from 24.7 cm to 18.3 cm in diameter after 8 days of treatment (supplemental Figure 3B). However, due to severe sepsis, venetoclax had to be stopped after dose ramp-up reached 400 mg after reaching a partial remission as best clinical response (according to criteria described in Dearden1 and Hopfinger et al7 ). Lymphocyte count remained stable but b2MG and LDH serum levels increased after 10 days and the patient died of fulminant sepsis 15 days after completing venetoclax (Figure 2B).
Venetoclax ex vivo response matches clinical response in 2 T-PLL patients. (A) Heatmap of viability measurements for 106 compounds in 4 concentrations in patient 1. The 4 concentrations used for each compound are depicted in supplemental Table 1. Venetoclax clusters on top. (B) Clinical response of T-PLL patient 1. (C) Heatmap of viability measurements for 106 compounds in 4 concentrations in patient 2. Venetoclax clusters on top. (D) Clinical response of T-PLL patient 2. (E) Dose-response curve of venetoclax of patients 1 and 2 before and after venetoclax treatment as well as CLL and AML samples. Concentrations ranging from 0.7 nM to 13 µM in threefold dilutions at 10 concentration points with 8 replicates each. Samples used for western blot in panel F are marked in red. (F) Western blot for BCL-2, BCL-XL, and MCL-1 of samples used in panel E. Total protein stain was performed with Ponceau S. Antibodies used were: BCL-2 (BD Biosciences), MCL-1 (Cell Signaling Technology), BCL-XL (Cell Signaling Technology), horseradish peroxidase (HRP)-conjugated donkey anti-mouse, and HRP-conjugated donkey anti-rabbit (Jackson ImmunoResearch).
Venetoclax ex vivo response matches clinical response in 2 T-PLL patients. (A) Heatmap of viability measurements for 106 compounds in 4 concentrations in patient 1. The 4 concentrations used for each compound are depicted in supplemental Table 1. Venetoclax clusters on top. (B) Clinical response of T-PLL patient 1. (C) Heatmap of viability measurements for 106 compounds in 4 concentrations in patient 2. Venetoclax clusters on top. (D) Clinical response of T-PLL patient 2. (E) Dose-response curve of venetoclax of patients 1 and 2 before and after venetoclax treatment as well as CLL and AML samples. Concentrations ranging from 0.7 nM to 13 µM in threefold dilutions at 10 concentration points with 8 replicates each. Samples used for western blot in panel F are marked in red. (F) Western blot for BCL-2, BCL-XL, and MCL-1 of samples used in panel E. Total protein stain was performed with Ponceau S. Antibodies used were: BCL-2 (BD Biosciences), MCL-1 (Cell Signaling Technology), BCL-XL (Cell Signaling Technology), horseradish peroxidase (HRP)-conjugated donkey anti-mouse, and HRP-conjugated donkey anti-rabbit (Jackson ImmunoResearch).
Patient 2, a 40-year-old man primary refractory after 2 treatment lines ([1] FCM; [2] alemtuzumab) presented with splenomegaly (20 cm in diameter), multiple-stage lymphadenopathy, lymphocytosis (174 G/L), elevated serum levels of LDH (478 U/L), and b2MG (5.1 mg/L). Fluorescence in situ hybridization tested positive for TCL1A translocation, deletion 11q, and trisomy8. Drug profiling indicated response to venetoclax (Figure 2C). The dose ramp-up was well tolerated without tumor lysis. At a daily dose of 800 mg, clinical response could be documented as restitution of splenomegaly, complete regression of lymphadenopathy as evidenced by computed tomography scan, and a significant decrease of all disease-related parameters (Figure 2D; supplemental Figure 3C). After 4 weeks, venetoclax was increased to 1 g for 3 weeks and finally to 1.2 g. However, after 131 days on venetoclax, the patient experienced a relapse-failing salvage treatment with PEBen thereafter. The best clinical response of the patient was a partial remission. High-resolution dose-response curves of venetoclax clearly distinguished CLL, AML, and T-PLL samples (P = .0024) (Figure 2E; supplemental Figure 3D). CLL samples responded at already very-low doses (50% inhibitory concentration [IC50], 3.45-7.89 nM), whereas AML samples responded at relatively high concentrations (IC50, 133 nM to 21.7 µM). Responses of T-PLL samples (IC50, 35.98-1040 nM) were stronger compared with AML samples (P = .0061) but weaker compared with CLL samples (P = .0286). BCL-2 and BCL-XL protein expression was induced upon venetoclax treatment in the 2 patients whereas MCL-1 remained unchanged, thus providing a potential mechanism of venetoclax resistance (Figure 2F). This study demonstrates specific activity of the BCL-2 inhibitor venetoclax in T-PLL. It is noteworthy that a recent ex vivo drug screening also found consistent activities of BCL-2 inhibition in T-PLL, thus confirming these results in an independent cohort.16 As proof of principle, we report the first-in-human treatment using venetoclax in 2 late-stage T-PLL patients. Responses were striking, however potential mechanisms of resistance might develop through BCL-2 and BCL-XL induction. Therefore, studies testing venetoclax with appropriate combination partners in T-PLL are warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to their patients for donating samples and clinical data for this study. The authors thank AbbVie (Emma Ariola, Alexander Dorr) for providing venetoclax, and Elisa Cerri and German Pena for critically reading the manuscript.
This work was supported by Austrian Science Fund (FWF) grants P27132-B20 (P.B.S.), F4701-B20 (S.K.), SFB F4707 (R.M.), and SFB-F06105 (R.M.); the Austrian Federal Ministry of Science, Research and Economy and the National Foundation for Research, Technology and Development (S.K.); and the Anniversary Fund of the Oesterreichische Nationalbank (OeNB) grant P15936 (P.B.S.).
Authorship
Contribution: B.B. and C.K., performed the experiments; E.v.d.K., N.P., L. Kazianka, S.G., B.H., M.E.M., M.P., O.M., and L. Kenner organized clinical samples and data; G. Hoermann, G. Hopfinger, M.-B.A., P.V., I.S.-K., R.M., O.M., L. Kenner, and U.J. provided reagents and intellectual contributions; A.H., W.R.S., U.J., and P.B.S. were responsible for patient treatment and ethical guidelines; S.K. and P.B.S. designed and oversaw the study; B.B., C.K., S.K., and P.B.S. analyzed the data; and B.B. and P.B.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Kubicek, Research Center for Molecular Medicine of the Austrian Academy of Sciences, 1090 Vienna, Austria; e-mail: skubicek@cemm.oeaw.ac.at; and Philipp B. Staber, Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria; e-mail: philipp.staber@meduniwien.ac.at.
References
Author notes
B.B. and C.K. contributed equally.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal