Abstract
Warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome is a genetic disease characterized by neutropenia, lymphopenia, susceptibility to infections, and myelokathexis, which describes degenerative changes of mature neutrophils and hyperplasia of bone marrow myeloid cells. Some patients present with hypogammaglobulinemia and/or refractory warts of skin and genitalia. Congenital cardiac defects constitute uncommon manifestations of the disease. The disorder, which is inherited as an autosomal dominant trait, is caused by heterozygous mutations of the chemokine receptor CXCR4. These mutations lead to an increased sensitivity of neutrophils and lymphocytes to the unique ligand CXCL12 and to an increased accumulation of mature neutrophils in the bone marrow. Despite greatly improved knowledge of the disease, therapeutic choices are insufficient to prevent some of the disease outcomes, such as development of bronchiectasis, anogenital dysplasia, or invasive cancer. The available therapeutic measures aimed at preventing the risk for infection in WHIM patients are discussed. We critically evaluate the diagnostic criteria of WHIM syndrome, particularly when WHIM syndrome should be suspected in patients with congenital neutropenia and lymphopenia despite the absence of hypogammaglobulinemia and/or warts. Finally, we discuss recent results of trials evaluating plerixafor, a selective antagonist of CXCR4, as a mechanism-oriented strategy for treatment of WHIM patients.
Introduction
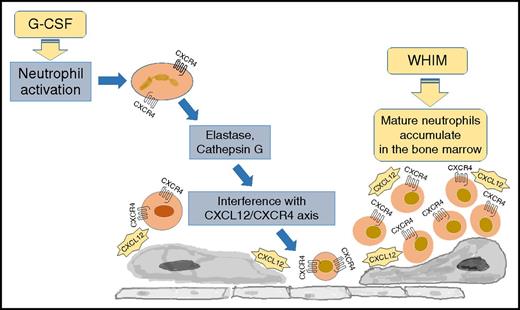
Warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome (OMIM 193670) is a primary immunodeficiency caused by heterozygous mutations in the CXCR4 gene.1-4 Located on chromosome 2q22, CXCR4 encodes for a G-protein–coupled chemokine receptor that is highly expressed by various stem and progenitor cells from the embryonic stage, thus orchestrating hematopoietic, cardiovascular, nervous system, and reproductive development. Previously characterized because of its role as a coreceptor for the human immunodeficiency virus, CXCR4 binds to its only chemokine ligand, CXCL12, to regulate bone marrow homeostasis and leukocyte trafficking to and from the peripheral blood. Heterozygous mutations of CXCR4 result in an impaired internalization and prolonged response of the receptor to CXCL12 and neutrophil accumulation in the bone marrow (Figure 1).3,5-7
The pathogenesis of neutropenia in WHIM syndrome. WHIM syndrome is characterized by neutropenia, lymphopenia, and monocytopenia despite hypercellular bone marrow (myelokathexis). The disease is caused by heterozygous mutations of CXCR4 resulting in a carboxy-terminus truncation of the receptor. Because of the peptide truncation, the receptor becomes hyperresponsive to the ligand CXCL12, resulting in the accumulation of mature neutrophils in the bone marrow. The treatment with granulocyte colony-stimulating factor (G-CSF) results in the downregulation of CXCR4 and a decreased cell responsiveness to CXCL12, promoting neutrophil mobilization from the bone marrow to the peripheral blood.
The pathogenesis of neutropenia in WHIM syndrome. WHIM syndrome is characterized by neutropenia, lymphopenia, and monocytopenia despite hypercellular bone marrow (myelokathexis). The disease is caused by heterozygous mutations of CXCR4 resulting in a carboxy-terminus truncation of the receptor. Because of the peptide truncation, the receptor becomes hyperresponsive to the ligand CXCL12, resulting in the accumulation of mature neutrophils in the bone marrow. The treatment with granulocyte colony-stimulating factor (G-CSF) results in the downregulation of CXCR4 and a decreased cell responsiveness to CXCL12, promoting neutrophil mobilization from the bone marrow to the peripheral blood.
The purpose of the present article is to discuss the treatment approach and review the clinical, immunological, and molecular phenotypes of the syndrome. Where evidence is available, it is cited; suggestions are otherwise based on our experience and opinions.
WHIM syndrome diagnosis
Case 1
The patient presented with tetralogy of Fallot and underwent surgical correction at the age of 2 years. On that occasion, neutropenia (absolute neutrophil count [ANC]: 300 cells/mcL) and leukopenia (1510 cells/mcL) were observed. She suffered from bronchitis, and when she had her first pneumonia at the age of 4 years, she was started on regular antibiotic prophylaxis (amoxicillin/clavulanate) and G-CSF (3 μg/kg daily). Bone marrow aspirate revealed myelokathexis. When she was 5 years old, she developed hypogammaglobulinemia (immunoglobulin G [IgG]: 377 mg/dL, normal range 633-1916 mg/dL; IgA: 5 mg/dL, normal range 41-315 mg/dL; IgM: 44 mg/dL, normal range 56-261 mg/dL). She responded well to the antitetanus booster vaccine, but the protection declined within 1 year. She had a pneumococcal pneumonia while on treatment, so G-CSF was stopped at the age of 8 years, and antibiotic prophylaxis was stopped at the age of 10 years. In her adolescence, she suffered from 2 severe episodes of pneumonia that required intravenous antibiotics, intravenous immunoglobulins (IVIG), and G-CSF. Since the age of 18 years, she has been receiving regular IVIG (400 mg/kg every 28 days), which is aimed at controlling pulmonary infections. She had a foot wart when she was 7 years old that resolved spontaneously. However, she developed new human papillomavirus (HPV) lesions on her legs, which were treated with topical salicylic acid.
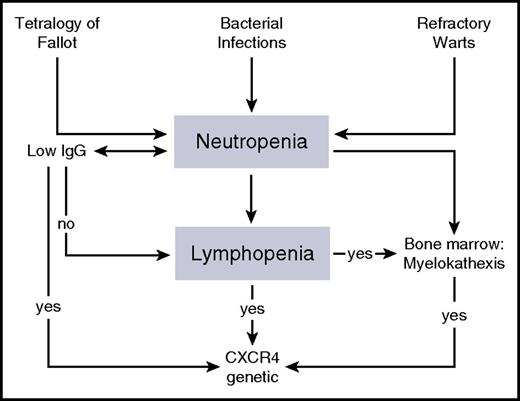
As observed in our case, the incidental discovery of lymphopenia and neutropenia is suggestive of WHIM syndrome. WHIM should also be suspected when neutropenia associates with hypercellularity of the bone marrow or with myelokathexis (kathexis means “retention”). However, it should also be suspected in any case of neutropenia with lymphopenia and/or monocytopenia, as “myelokathexis” may be overlooked by less experienced cytologists8 or bone marrow aspirate may not be always available (Figure 2). In addition, detection of typical CXCR4 mutations in patients with neutropenia and lymphopenia does not necessarily require bone marrow aspirate for a disease diagnosis. Indeed, the evaluation of bone marrow smears requires a well-experienced cytologist to diagnose myelokathexis. The latter consists in the failure of neutrophil release from the marrow, resulting in degenerative changes in mature cells1,4,9 (hypersegmentated pycnotic nucleus with long filaments connecting the lobes and cytoplasmic vacuolization). Immunoglobulin levels may be normal or reduced, vaccine responses may be protective or poor, immunophenotyping may show B-cell and T-cell lymphopenia, but the percentage distribution of lymphocyte subpopulations may remain in the normal range.
A diagnostic flowchart of WHIM syndrome. WHIM syndrome should be suspected in patients with bacterial infections, refractory warts, or congenital heart defects, such as tetralogy of Fallot. The analysis of leukocyte counts in subjects with possible WHIM syndrome can reveal severe neutropenia (ANC reduced to below 500 cells per µL), monocytopenia, and lymphopenia, while immunoglobulin levels are not affected in all of the patients. Although myelokathexis can be observed in the majority of WHIM patients, CXCR4 genetic analysis should be performed in any patient with lymphopenia and neutropenia, despite whether the patient has a normal bone marrow morphology.
A diagnostic flowchart of WHIM syndrome. WHIM syndrome should be suspected in patients with bacterial infections, refractory warts, or congenital heart defects, such as tetralogy of Fallot. The analysis of leukocyte counts in subjects with possible WHIM syndrome can reveal severe neutropenia (ANC reduced to below 500 cells per µL), monocytopenia, and lymphopenia, while immunoglobulin levels are not affected in all of the patients. Although myelokathexis can be observed in the majority of WHIM patients, CXCR4 genetic analysis should be performed in any patient with lymphopenia and neutropenia, despite whether the patient has a normal bone marrow morphology.
The association between neutropenia and monocytopenia can also be observed in GATA binding protein 2 (GATA2) deficiency, but bone marrow aspirate in GATA2-deficient patients usually reveals myelodysplasia. Myelokathexis is not pathognomonic of WHIM syndrome, as it was observed in neutropenia linked to mutations of the gene encoding of glucose-6-phosphatase catalytic subunit 3 (G6PC3)10,11 in mice with Cxcr2 loss-of-function mutations12 or in 1 subject with leukocytosis and gastric cancer.13 When neutropenia is manifested in the first year of life, a differential diagnosis should also consider other causes of congenital lymphopenia and neutropenia, including leaky severe combined immunodeficiency, reticular dysgenesia, and DNA repair defects. In the presented case 1, tetralogy of Fallot was the first manifestation. This association was reported in 10% of WHIM syndrome patients,8,14,15 suggesting that the diagnostic assessment of infants with congenital heart defect should include a full blood count to screen for neutropenia (Table 1). Lack of neutropenia in at least 3 or more complete blood counts in the absence of acute or recent infection rules out the hypothesis of WHIM syndrome. Warts, no matter how severe and associated with hypogammaglobulinemia, may suggest other immune defects, including epidermodysplasia verruciformis and CD4 lymphopenia.16
The diagnostic workup and follow-up are summarized
| Time . | Planned analyses . |
|---|---|
| At clinical suspicion | Detailed investigation of the infections |
| Physical examination | |
| Laboratory tests: | |
| Full blood count | |
| Serum immunoglobulins (IgG, IgA, and IgM) | |
| Tetanus antitoxoid antibodies | |
| Lymphocyte subset absolute counts: CD3, CD4, CD8, CD19, and CD16 | |
| Cultures of sputum or nose-pharyngeal suction secretion | |
| Bone marrow aspirate | |
| At diagnosis | Laboratory tests: |
| Full blood count | |
| Serum immunoglobulins (IgG, IgA, and IgM) | |
| Extended T- and B-lymphocyte subsets (absolute counts): CD3; CD4; CD8; CD19; CD16; naïve and memory T cells; naïve, memory, and switched memory B cells | |
| Response to booster vaccine if antitoxoid antibodies unprotective | |
| Full blood count during acute infection to confirm normalization of the neutrophil count | |
| If suspected skin/anogenital lesions, consider a dermatologist evaluation and viral DNA analysis. | |
| If history of recurrent pulmonary infections, consider a pulmonologist evaluation and chest computed tomography (CT) scan. | |
| Every 6 mo | Physical examination |
| Consider a dermatologist and/or pulmonologist evaluation | |
| Pulmonary functional tests | |
| Laboratory tests: | |
| Full blood count* | |
| Serum immunoglobulins (IgG, IgA, and IgM)† | |
| Lymphocyte subsets (CD3, CD4, CD8, and CD19) | |
| Culture of sputum or nose-pharyngeal suction secretion | |
| Chest CT scan (on the basis of clinical manifestations) |
| Time . | Planned analyses . |
|---|---|
| At clinical suspicion | Detailed investigation of the infections |
| Physical examination | |
| Laboratory tests: | |
| Full blood count | |
| Serum immunoglobulins (IgG, IgA, and IgM) | |
| Tetanus antitoxoid antibodies | |
| Lymphocyte subset absolute counts: CD3, CD4, CD8, CD19, and CD16 | |
| Cultures of sputum or nose-pharyngeal suction secretion | |
| Bone marrow aspirate | |
| At diagnosis | Laboratory tests: |
| Full blood count | |
| Serum immunoglobulins (IgG, IgA, and IgM) | |
| Extended T- and B-lymphocyte subsets (absolute counts): CD3; CD4; CD8; CD19; CD16; naïve and memory T cells; naïve, memory, and switched memory B cells | |
| Response to booster vaccine if antitoxoid antibodies unprotective | |
| Full blood count during acute infection to confirm normalization of the neutrophil count | |
| If suspected skin/anogenital lesions, consider a dermatologist evaluation and viral DNA analysis. | |
| If history of recurrent pulmonary infections, consider a pulmonologist evaluation and chest computed tomography (CT) scan. | |
| Every 6 mo | Physical examination |
| Consider a dermatologist and/or pulmonologist evaluation | |
| Pulmonary functional tests | |
| Laboratory tests: | |
| Full blood count* | |
| Serum immunoglobulins (IgG, IgA, and IgM)† | |
| Lymphocyte subsets (CD3, CD4, CD8, and CD19) | |
| Culture of sputum or nose-pharyngeal suction secretion | |
| Chest CT scan (on the basis of clinical manifestations) |
Close monitoring of neutrophil count in patients on G-CSF therapy.
Regular monitoring of serum immunoglobulin levels in case the treatment reaches optimal IgG serum levels (at least above 500 mg/dL).
CXCR4 mutations reported in WHIM patients consist of premature stop codons or frameshifts and result in the loss of 10 to 19 residues of the cytoplasmic tail of the receptor. The CXCR4 intracellular tail mediates its negative regulation through G-protein–coupled receptor kinase (GRK)–mediated phosphorylation and β-arrestin–mediated internalization2,3,14,17 (Table 2). Consequently, the mutated receptor displays an impaired ligand-dependent downregulation and prolonged responses of CXCR4 mutants to CXCL12 (ie, gain of function causing an excessive accumulation of mature neutrophils, lymphocytes, and monocytes in the bone marrow and accounting for the symptoms of the disease3,5,6,18,19 ). In addition, the decreased numbers of circulating hematopoietic stem and progenitor cells in WHIM patients, as a consequence of impaired CXCR4 desensitization, might also lead to lymphopenia.20 One WHIM patient has been identified as lacking CXCR4 mutations but displaying a decreased expression of GRK3, which normally mediates CXCR4 desensitization.6 In addition, Liu et al reported a novel missense, nontruncating C-terminus mutation (E343K) that is associated with the impaired CXCR4 downregulation and reduced CXCR4 desensitization, although to a smaller extent than other reported mutations17 (Table 2). Moreover, a heterozygous 5-bp deletion (nucleotides 986-990) CXCR4 (L329fs) frameshift variant resulting in substitution of the final 24 predicted amino acids of the carboxy-terminal domain of the receptor with 12 missense amino acids has been recently described in a WHIM patient21 (Table 2). Interestingly, mutations affecting the intracellular tail of CXCR4 can occur as somatic mutations, which is shown in patients with Waldenström macroglobulinemia, suggesting that the C-tail of CXCR4 has an important regulatory function for B-cell lymphomagenesis.
Summary of the reported CXCR4 mutations in the WHIM patients
| Nucleotide change . | Mutation . | Amino acid change . | Patients (n) . | References . |
|---|---|---|---|---|
| g.1000C>T | Nonsense | p.R334X | 33 | 3,-5, 8, 14, 15, 27, 32, 37, 39, 44, 45, 49,,,,-54 |
| g.1006G>T | Nonsense | p.G336X | 2 | 8 |
| g.1013C>G | Nonsense | p.S338X | 8 | 5, 8, 14, 15, 38, 55 |
| g.1027G>T | Nonsense | p.E343X | 2 | 3, 5 |
| g.1027G>A | Nonsense | p.E343K | 4 | 17 |
| g.1016-17delCT | Deletion | p.S339fs342X | 4 | 3, 5, 8 |
| g.1021delT | Deletion | p.S341fs365X | 1 | 8 |
| c.969_970insG | Insertion | p.G323fs343X | 2 | 14 |
| c.986-990del TCTCC | Frameshift | p.L329fs | 1 | 21 |
| Wild type | — | No CXCR4 mutation detected | 3 | 3, 14 |
| Nucleotide change . | Mutation . | Amino acid change . | Patients (n) . | References . |
|---|---|---|---|---|
| g.1000C>T | Nonsense | p.R334X | 33 | 3,-5, 8, 14, 15, 27, 32, 37, 39, 44, 45, 49,,,,-54 |
| g.1006G>T | Nonsense | p.G336X | 2 | 8 |
| g.1013C>G | Nonsense | p.S338X | 8 | 5, 8, 14, 15, 38, 55 |
| g.1027G>T | Nonsense | p.E343X | 2 | 3, 5 |
| g.1027G>A | Nonsense | p.E343K | 4 | 17 |
| g.1016-17delCT | Deletion | p.S339fs342X | 4 | 3, 5, 8 |
| g.1021delT | Deletion | p.S341fs365X | 1 | 8 |
| c.969_970insG | Insertion | p.G323fs343X | 2 | 14 |
| c.986-990del TCTCC | Frameshift | p.L329fs | 1 | 21 |
| Wild type | — | No CXCR4 mutation detected | 3 | 3, 14 |
Treating neutropenia and myelokathexis
In case 1, neutropenia is in the moderate to severe range (ie, an ANC reduced below 500 cells per µL). Neutropenia represents the most highly expressed finding at onset and is typically associated with myelokathexis. However, WHIM patients generally do not present with overwhelming neutropenic infections. This is likely because functional neutrophils are rapidly released from the bone marrow during stress or infections. Despite neutropenia and bone marrow abnormalities, neither leukemia nor myelodysplastic syndrome have ever been reported. More interestingly, neutropenia usually associates with profound monocytopenia and lymphopenia, thus significantly increasing the risk for infection.
Use of G-CSF for WHIM patients
In the sole placebo-controlled clinical trial on the use of G-CSF in patients with neutropenia, the daily administration of G-CSF has been shown to be effective in children and adults with severe chronic neutropenia of various etiologies,10,22-24 resulting in a sustained increase in the neutrophil count and decrease in the infection-related morbidity. As this randomized trial did not include WHIM patients, the use of G-CSF treatment in WHIM patients is an extrapolation of the indication in other subtypes of severe congenital neutropenia patients. As observed in case 1 and reported in many WHIM patients, the continuous treatment with G-CSF results in a correction of neutropenia, but there is no evidence that this treatment can reduce the number of infectious episodes.14 Moreover, G-CSF treatment does not correct lymphopenia and monocytopenia and does not affect warts in WHIM patients. Furthermore, infectious episodes do not seem to directly correlate to ANC, and WHIM patients generally do not suffer from manifestations that are observed in severe congenital neutropenia patients, such as chronic stomatitis. In addition, as stated previously, neutropenia usually normalizes during infection, thus limiting the risk for sepsis. The extended review of literature shows that the efficacy of G-CSF is controversial and that many patients withdrew from the treatment because of the lack of efficacy for controlling the frequency of infections (Figure 3). Thus, there is actually no strong evidence to recommend prophylactic G-CSF, but further clinical studies are needed to directly address specific end points in WHIM patients. However, in WHIM patients at high risk for bacterial infections, including those who fail to normalize ANCs during infectious episodes, G-CSF should be recommended. In contrast to most congenital neutropenias, no secondary leukemias have been reported in WHIM patients. However, 4 cases of lymphoma were reported in WHIM syndrome, including a lethal B-cell lymphoma in a 54-year-old female, a fatal Epstein-Barr virus (EBV)–related intestinal lymphoma in a 26-year-old female, a primary cutaneous follicle center lymphoma in a 32-year-old male, and a nonlethal B-cell lymphoma in a 31-year-old male, which was followed in the same patient with maxillary HPV-related carcinoma.8,25-28
Treatment regimens used in WHIM patients. An exhaustive literature review of journal articles published since the first description of the disorder in 1964 identified 70 cases of WHIM syndrome. In 57 cases, CXCR4 mutations were reported, and 3 cases were defined as having the wild-type CXCR4 (see Table 2 for details). The other 10 cases were clinically diagnosed as WHIM before 2003. The treatments that were used include G-CSF or, rarely, granulocyte-macrophage colony-stimulating factor (GM-CSF), immunoglobulin replacement therapy (intravenously [IVIG] or subcutaneously [scIG]), and antibiotic prophylaxis. The association of these therapeutic strategies is reported. Occasionally, G-CSF was used for a short course during acute infection, to the study neutrophils response, or as a premedication before chemotherapy for lymphoma.
Treatment regimens used in WHIM patients. An exhaustive literature review of journal articles published since the first description of the disorder in 1964 identified 70 cases of WHIM syndrome. In 57 cases, CXCR4 mutations were reported, and 3 cases were defined as having the wild-type CXCR4 (see Table 2 for details). The other 10 cases were clinically diagnosed as WHIM before 2003. The treatments that were used include G-CSF or, rarely, granulocyte-macrophage colony-stimulating factor (GM-CSF), immunoglobulin replacement therapy (intravenously [IVIG] or subcutaneously [scIG]), and antibiotic prophylaxis. The association of these therapeutic strategies is reported. Occasionally, G-CSF was used for a short course during acute infection, to the study neutrophils response, or as a premedication before chemotherapy for lymphoma.
Use of the CXCR4 receptor antagonist in targeted therapy in patients with WHIM syndrome
As the CXCR4 chemokine receptor is more sensitive to CXCL12 activation in WHIM syndrome cells,5-7 the pharmaceutical exposition of the receptor to an antagonist may restore normal CXCR4 chemokine receptor function and thereby reestablish a physiological pathway. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.5-7 Consequently, treatment with a CXCR4 antagonist has the potential to restore normal circulating blood cells levels (the levels of both neutrophils and lymphocytes)29,30 and might impact the efficacy of the treatment of HPV infection. Indeed, the first human trial shows promising short-term effects: in ∼1 week, the therapy with plerixafor dramatically restored neutrophil and lymphocyte counts in a total of 9 patients.31,32 More recently, a phase 1 study of subcutaneously self-injected plerixafor at 0.01 mg/kg twice per day in 3 WHIM patients for 6 months appeared to be both safe and efficient.33 In particular, this treatment led to durable increases in the leukocyte and lymphocyte counts, even though the leukocyte and lymphocyte counts oscillated during the treatment and exhibited large differences between the peak and trough values. Although the monocyte and neutrophil counts were less variable, both results were likely related to the rapid pharmacokinetics of plerixafor (T1/2 = 5 hours).33 Notably, plerixafor received the orphan drug designation status by the European Medicine Agency.
Treatment of hypogammaglobulinemia
Despite the term hypogammaglobulinemia being part of the acronym that identifies the disease, low immunoglobulin levels have been reported in 20% to 89% of patients, which is a value that varies depending on the study. Although Kawai et al34 reported in 2009 that 89.6% (26/29) of patients presented hypogammaglobulinemia, this was not confirmed by the Italian and the French cohorts, in which only 30% (3/10) and 20% (2/10) of the patients, respectively, presented low immunoglobulin levels. As observed in case 1, hypogammaglobulinemia is generally mild to moderate, affects 1 or more immunoglobulin classes (IgG, IgA, and IgM are variably affected), and associates with a poor vaccine response. Patients normally respond to booster vaccines, but the protective antibody response is not sustained over time (ie, 1 year).8 This is probably related to the abnormal trafficking of B cells out of the bone marrow and throughout the germinal center environment, thus leading to abnormal isotype switching and defective B-cell maturation and function. Despite normal immunoglobulin levels or a mild reduction in the immunoglobulin levels, WHIM subjects display B-cell lymphopenia with a reduced number of switched memory (CD27+) B cells and B-cell oligoclonality, likely contributing to infection.5,35 Moreover, the inefficient plasma cell differentiation and maintenance that was shown in mice may account for the defective humoral immunity observed in WHIM patients.36
Indications for the use of immunoglobulin therapy for WHIM
Since the detection of hypogammaglobulinemia, WHIM patients have often been started with monthly intravenous (IVIG) or weekly subcutaneous immunoglobulin therapies, even though this symptomatic treatment is expensive, requires good patient adherence, and has an efficacy that has not been properly proved (Figure 3). At present, reports of WHIM patients treated with immunoglobulin prophylaxis show a significantly reduced incidence of respiratory tract infections, and this is particularly evident when treatment is started early in life.2,14,34,37 Patients who are not started on prophylactic immunoglobulin therapy are at a high risk for developing severe bronchiectasis.34,38 Consequently, early immunoglobulin replacement therapy should be recommended in all WHIM patients, regardless of whether they manifest hypogammaglobulinemia (Table 3). However, immunoglobulin replacement does not affect the manifestations of HPV.
Summary of the disease-oriented treatment of patients with WHIM syndrome
| . | Recommended . | Optional/experimental . |
|---|---|---|
| Infections Hypogammaglobulinemia | Immunoglobulin replacement treatment | |
| IV: 400 mg/kg monthly | ||
| SC: 100 mg/kg weekly | ||
| Neutropenia | None | G-CSF (1-2 μg/kg daily) |
| Warts | Imiquimod (topical) | Plerixafor (0.01-0.02 mg/kg SC twice daily) |
| Other dermatological therapies (ie, laser therapy and cryotherapy) |
| . | Recommended . | Optional/experimental . |
|---|---|---|
| Infections Hypogammaglobulinemia | Immunoglobulin replacement treatment | |
| IV: 400 mg/kg monthly | ||
| SC: 100 mg/kg weekly | ||
| Neutropenia | None | G-CSF (1-2 μg/kg daily) |
| Warts | Imiquimod (topical) | Plerixafor (0.01-0.02 mg/kg SC twice daily) |
| Other dermatological therapies (ie, laser therapy and cryotherapy) |
SC, subcutaneously.
Treatment of infections (other than HPV) in WHIM patients
Case 2
A 2.5-year-old girl with upper respiratory tract infections and otitis occurring since the first year of life was hospitalized for orbital cellulitis, severe neutropenia (ANC: <100 cells/mcL) and leukopenia (white blood cell count: 500 cells/mcL) but normal immunoglobulin levels. Then, she had 3 episodes of pneumonia that required intravenous antibiotics and IVIG and G-CSF treatments. At the age of 4 years, she was regularly administered antibiotic prophylaxis (amoxicillin/clavulanate) and G-CSF (3.5 μg/kg daily), but the latter stopped because of anemia and piastrinopenia. Despite antibiotic prophylaxis, she developed recurrent pneumonia and subsequently hypogammaglobulinemia (IgG: 413 mg/dL, normal range 462-1710 mg/dL; IgM: 54 mg/dL, normal range 62-257 mg/dL). At the age of 6 years, she started IVIG (400 mg/kg every 28 days) and respiratory physiotherapy. A chest CT scan revealed pulmonary atelectasis. On treatment, she had only 1 episode of pneumonia, which was at the age of 9 years, and her pulmonary function tests improved. Antibiotic prophylaxis was stopped and IVIG was continued. A second bone marrow aspirate analysis was consistent with the finding of myelokathexis. Thereafter, she was vaccinated for HPV.
In case 2, infections refer to bacterial events that are typically manifested since early childhood. Bacterial infections in WHIM patients may include numerous upper respiratory infections, bouts of pneumonia, pansinusitis, middle ear infections, cellulitis of skin, cutaneous abscesses, suppurative adenitis, urinary tract infections, enteric tract infections, progressing periodontal disease, and, more rarely, osteoarthritis, mastoiditis, meningitis, and sepsis. Pathogens that are frequently identified include,2,34 but are not limited to, Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus, which are commonly detected in the sputum, and Escherichia coli, Proteus mirabilis, and Salmonella typhi murium. Opportunistic agents such as Pseudomonas aeruginosa, Moraxella catharralis, Klebsiella pneumoniae, and Burkholderia cepacia are not frequent but are detected mainly in patients with chronic lung disease. Beaussant et al reported an atypical Mycobacterium infection (M gordonae isolation) in 2 WHIM patients.14
It is common that most infections are minor, are fully responsive to oral antibiotics, and run a relatively benign course, particularly during childhood. This is in part explained by the observation that mature neutrophils are released from the bone marrow to the peripheral blood during infections, leading to normal neutrophil counts. A single case of sepsis-related mortality39 and a possible Mycobacterium-related fatal liver failure14 were recently reported, both of which were in adults. Severe caries, periodontitis, and dental abscesses may lead to premature tooth loss. As it is expected for other primary immunodeficiencies, the recurrence of pneumonia may lead to progressive lung damage manifested with the development of diffuse bronchiectasis.2 Following the breakdown of airways caused by the infection-related inflammatory response, bronchi wall-thickening and/or enlargement impairs the clearance of pulmonary secretions, thus enhancing the colonization with opportunistic bacteria and increasing the risk for more severe lung infections.
In addition to HPV infections, childhood viral infections (ie, varicella) normally run a benign course without complications. WHIM patients mostly suffer from herpes virus family infections (herpes simplex virus 1 [HSV1] stomatitis and genital HSV2).2,14 Severe recurrent HSV and varicella-zoster virus infections are reported.37 Moreover, an adult WHIM patient with cytomegalovirus infection14 and, as previously mentioned, 2 cases of EBV-induced lymphoma have been observed,25,26 the latter suggesting the need for the careful monitoring of EBV infection. In WHIM patients, there is no evidence of an increased incidence of fungal infections: Beaussant et al reported isolations of Candida albicans from the intestinal and respiratory tracts and 1 case of onychomycosis, all of which were in adult patients.14 However, the use of antifungal prophylaxis has never been reported.
Treatment of acute infections
During acute infection, WHIM patients spontaneously normalize leukocyte counts. Therefore, the use of G-CSF is not mandatory in WHIM patients, but it is recommended that it be routinely given to patients who fail to normalize ANC during infections and in cases of surgery (Table 3). Moreover, it seems reasonable to use intravenous antibiotic infusions and immunoglobulin replacement therapy during acute infections.
Prevention and treatment of chronic obstructive pulmonary disease
Considering the increased risk for developing chronic obstructive pulmonary disease, the evaluation of respiratory function should be recommended for each WHIM patient at diagnosis. Investigations should include a culture of the sputum or of the nose-pharyngeal suction secretion, pulmonary function tests, and chest imaging. If a history of recurrent chest infections and/or symptoms are suggestive of bronchiectasis, the first assessment should include a high-resolution CT of the chest. During long-term follow-up, spirometry may be regularly used as a reproducible marker to monitor the progression and/or intercurrent exacerbations. During early childhood, the prevention of progressive lung disease can be pursued by reducing the frequency of pulmonary infections. In that, the prompt starting of immunoglobulin therapy has shown clinical benefit. Recently, preliminary results with plerixafor seem promising in terms of controlling the risk for infections, including respiratory events.33 Meanwhile, the prompt diagnosis and antibiotic treatment of any bronchial infection is strongly recommended. When bronchiectasis is present at diagnosis, treatment may be centered on the prevention of exacerbations, prompt management of pulmonary infections, guidance by sputum culture results, and optimization of the clearance of airway secretions with regular chest physiotherapy.40,41 Recent evidence supports chronic low-dose macrolide therapy as a therapeutic option for non–cystic fibrosis bronchiectasis to control exacerbations.42 In a recently published meta-analysis, Yang et al state that for patients with non–cystic fibrosis bronchiectasis, long-term inhaled antibiotics, such as aminoglycosides, can effectively reduce the sputum bacterial density, increase Pseudomonas aeruginosa eradication, and attenuate the risk for exacerbation. However, these outcomes are accompanied by higher risks for wheeze and bronchospasm.42
Antimicrobial prophylaxis in WHIM patients
Various primary immunodeficiencies require antibiotic prophylaxis (ie, cotrimoxazole prevents Pneumocystis jiroveci infection in patients with CD4+ lymphopenia, and asplenic patients need penicillin prophylaxis because of their high risk for encapsulated bacterial infection). When recurrent bacterial infections cause moderate to severe morbidity and impair the quality of life of patients, the current standard therapeutic approach often refers to oral antibiotic prophylaxis to control infections, but evidence of its efficacy in WHIM patients is lacking. In this disorder, Pneumocystis jiroveci infection has never been reported, and sepsis is unusual. However, daily prophylaxis with cotrimoxazole (and, alternatively, amoxicillin or azithromycin) may prevent staphylococcal infection, which is similar for patients with chronic granulomatous disease (CGD). Currently, azithromycin prophylaxis seems to be efficient in reducing pulmonary exacerbations in patients with cystic fibrosis and in those with non–cystic fibrosis bronchiectasis.40 The prompt diagnosis and treatment of every acute bacterial infection is strongly recommended to prevent complications. Regarding antiviral prophylaxis, WHIM patients may suffer from herpetic infections, but they are commonly without a severe course and complications. Thus, prophylaxis with acyclovir is not routinely recommended. However, acyclovir prophylaxis has been reported as being effective in preventing further attacks in a WHIM patient suffering from a severe form of recurrent herpetic infection.37 Consequently, antiviral prophylaxis might be considered in selected cases.
Clinical manifestations and current symptomatic treatments of warts
Case 3
A female patient presented manifestations of disease in her first year of life with pneumonia, neutropenia (ANC: 315 cells/mcL), and leukopenia (1800 cells/mcL). A bone marrow aspirate revealed a result of myelokathexis. Since the age of 6 years, she has suffered from recurrent pulmonary infections, had P aeruginosa and S aureus detected in her sputum, and suffered from severe otitis complicated with bilateral hypoacusia. In her adult age, she has had episodes of pneumonia despite treatment with G-CSF and had a varicella-zoster virus infection. When she was 22 years old, she developed severe cutaneous warts (on the hands, feet, elbows, and face) refractory to laser treatment, and genital HPV complicated with intraepithelial neoplasia, which required vulvectomy with perinectomy. At the age of 33 years, she was diagnosed with anorectal carcinoma, which resolved after undergoing radiotherapy plus chemotherapy. Additionally, she had a noncomplicated pregnancy.
This case reveals the extreme severity of the increased and rather selective susceptibility of WHIM patients to HPV infections. Infectious lesions vary from skin and/or anogenital warts to the degeneration of premalignant genital dysplasia and invasive cancer. Skin verrucosis typically affects the extremities (hands/feet) and to a lesser extent, the face, and it is manifested from the first or the second decade of life in ∼70% of WHIM patients.2,14 The appearance of anogenital warts, or condylomata acuminata, is present in 23% to 28% of cases14 and marks a turning point in the natural course of the disease, leading to repeated aggressive surgical removals, as these lesions may progress to intractable multifocal dysplasia and invasive cancer with an early fatal course.
The severity of warts is variable: in most patients, warts may be few in number and may recover spontaneously,37 whereas the presence of multiple warts (>10 lesions) on exposed areas may greatly impair patient’s quality of life, and there is evidence of a correlation between the age of onset of skin warts and the severity of wart proliferation.14 The identification of HPV types from skin lesion biopsies is not routinely performed, but the characterization of low-risk (ie, 6 and 11) and high-risk (ie, 16 and 18) types might not necessarily correlate to the prognosis in the WHIM patient population. In the French cohort, low-risk HPV-type 6 was detected in a genital lesion, which progressed to invasive cancer, albeit this type is considered benign in the general population.14 HPV-positive oral squamous cell carcinoma has also been reported.28 The susceptibility to HPV infections of WHIM patients is not related to an impaired lymphocyte proliferation, as this is observed in many primary immunodeficiencies. In contrast, current knowledge suggests the roles of abnormal homeostasis and trafficking of plasmacytoid dendritic cells in the susceptibility to warts.43 This hypothesis is further supported by studies performed on a 58-year-old patient who underwent complete remission of WHIM syndrome with a resolution of warts and sustained spontaneous corrections of neutropenia, monocytopenia, dendritic cell deficiency, and myelokathexis, despite maintaining deficiencies of B and naïve T lymphocytes. The recovery of this patient was because of chromothriptic deletions of 1 copy of chromosome 2, including the deletion of the CXCR4 allele carrying the R334X mutation, thus resulting in lymphoid/myeloid mosaicism with a complete recovery of cells belonging to the myeloid linage, while the lymphoid cells retained the CXCR4 mutation.44 This study further supports the notion that myeloid-derived cells are critical for the control of HPV infection in WHIM patients.
Current feasible treatment of warts and HPV lesions
In WHIM patients, warts are typically refractory to conventional treatments (ie, salicylic acid and cryotherapy), aggressive surgical removal, and pharmacological agents (ie, imiquimod, antiviral agents, and interferons)2,14 (Table 3). Currently, the quadrivalent HPV vaccine (Gardasil) cannot be considered as curative, as its protection is restricted to only 4 HPV serotypes (high-risk mucosal HPV 16 and 18 and low-risk mucosal HPV 6 and 11). Its prophylactic administration to young WHIM patients not suffering from cutaneous or anogenital warts (at the time of presentation) was shown to be associated with protective immunity,45 and these patients have remained wart-free a few years later.14 No data are available on the use of the newer generation of the anti-HPV vaccine that is directed against a larger spectrum of HPV serotypes (Gardasil-9) and the other HPV vaccine (Cervarix) in WHIM patients.46 Overall, these observations and the evidence that complications of any kind have never been reported with live vaccines, such as the varicella vaccine and Bacillus Calmette–Guérin (BCG) vaccine, in patients diagnosed after vaccination, suggest that HPV vaccines may be routinely used as early prophylactic treatment of young WHIM patients. Meanwhile, it has to be considered that the long-term efficacy of vaccines is reduced in WHIM patients because of defective development or maintenance of memory B cells35 and the early decline of an antibody response against vaccine antigens.8,45 Consequently, additional HPV vaccinations (ie, after 5 years) may represent a reasonable approach to further augment the HPV antibody titers, as the protective efficacy of the vaccine in immunocompetent individuals was shown to last a minimum of 5 years.47 Encouraging results have recently been obtained by McDermott et al in their phase 1 clinical trial based on the long-term (6 months) administration of low doses (0.01 to 0.02 mg/kg, subcutaneously and twice daily) of the anti-CXCR4 plerixafor, which, in combination with imiquimod, resulted in effectively improving warts.33 The frequent and aggressive evaluation by a dermatologist is strongly recommended for any suspicious skin lesion in WHIM patients of any age.
Final considerations
Based on the long-term outcome of WHIM patients, no deaths before the age of 20 years have been reported in this patient population. As was previously mentioned, the causes of deaths were lymphoma in 2 cases (patients who were 26 and 54 years of age), bacterial meningitis in 1 case (a patient who was 31 years of age), probable mycobacteriosis with liver failure in 1 case (a patient who was 40 years of age), and an HPV-induced genital cancer in 1 case (a patient who was 40 years of age), suggesting that the overall cancer risk in this population is quite high after the third decade of life.
Current therapeutic options for WHIM patients include immunoglobulin replacement therapy, G-CSF, and antibiotic prophylaxis. The use of G-CSF treatment, which appears to be expensive and lacks proven evidence of having efficacy in the prevention of infectious episodes in these rare patients, should only be recommended in patients who fail to normalize ANC counts during infections, while the treatment with immunoglobulins might be helpful for protecting children from the development of bronchiectasis. Despite the fact that the supportive treatments described previously may limit the course of severe infections, they fail to control HPV and Mycobacterium infections, which leads to a potential lethal outcome. An 8-year-old WHIM patient successfully received umbilical cord blood stem cell transplantation from an HLA-matched sibling donor.48 However, because of the risks related to the transplantation procedure, this treatment might not be suitable for all WHIM patients. Since 2011, trials using plerixafor, the selective reversible antagonist of the CXCR4 chemokine receptor, have showed promising results. Consequently, our current recommendations to improve the survival and quality of life for these patients include the early recognition of WHIM in patients on the basis of a more sensitive diagnostic criteria (Figure 2). In particular, although the European Society for Immunodeficiencies criteria suggest the need for myelokathexis for the diagnosis of WHIM syndrome, we strongly support the notion that the association of neutropenia with diseases causing mutations of CXCR4 are sufficient for a diagnosis. Moreover, we suggest that WHIM syndrome should be suspected in any patient with congenital neutropenia and lymphopenia, despite the absence of hypogammaglobulinemia and/or warts. In addition, we suggest immunoglobulin replacement in patients with a history of infections and/or hypogammaglobulinemia and an active detection and prompt treatment of HPV-related lesions. Finally, the promising results of the plerixafor trials suggest that drugs targeting CXCR4 could become the most effective treatment option for WHIM patients in the future.
Acknowledgments
The authors would like to thank Lucia D. Notarangelo, Vassilios Luogaris, Cristoph Klein, and Francoise Bachelerie for their critical discussions.
Authorship
Contribution: R.B. and J.D. discussed the therapeutic options that address the complications of the disease; R.B. wrote the manuscript; and J.D. contributed to writing of the manuscript and critically revised the manuscript.
Conflict-of-interest disclosure: R.B. and J.D. have received consulting fees from X4 Pharmaceuticals.
A list of members of the WHIM research group appears in “Appendix.”
Correspondence: Raffaele Badolato, Istituto di Medicina Molecolare “Angelo Nocivelli,” Università di Brescia, c/o Spedali Civili, 25123 Brescia, Italy; e-mail: raffaele.badolato@unibs.it.
Appendix: study group members
The members of the WHIM research group are: Laura Dotta (Dipartimento di Scienze Cliniche e Sperimentali and A. Nocivelli Institute of Molecular Medicine, Brescia, Italy) and Sarah Beaussant Cohen (Pediatric Hematology Oncology Department, Centre Hospitalo-Universitaire de Besançon, Université de Franche Comté, France).

![Figure 3. Treatment regimens used in WHIM patients. An exhaustive literature review of journal articles published since the first description of the disorder in 1964 identified 70 cases of WHIM syndrome. In 57 cases, CXCR4 mutations were reported, and 3 cases were defined as having the wild-type CXCR4 (see Table 2 for details). The other 10 cases were clinically diagnosed as WHIM before 2003. The treatments that were used include G-CSF or, rarely, granulocyte-macrophage colony-stimulating factor (GM-CSF), immunoglobulin replacement therapy (intravenously [IVIG] or subcutaneously [scIG]), and antibiotic prophylaxis. The association of these therapeutic strategies is reported. Occasionally, G-CSF was used for a short course during acute infection, to the study neutrophils response, or as a premedication before chemotherapy for lymphoma.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/23/10.1182_blood-2017-02-708552/4/m_blood708552f3.jpeg?Expires=1769086604&Signature=qCTQw-wvBA6hUT~uelu56JoseuwQE0XmnD0RcBK1Za0lk7GleG5Sd2vFo5u4c6N0O91sHsP-aZ3Cd~endI6vxmyMtUgpHNoHJwnxS~YptMXj9zXtJxNI~AM4yEhUg1~O4tppcIQn9bkphYAEb3GIVPMYTuUw1yeerJHOzifcFduo11bkSzWJdJe7VwcivjHNF5JjvPGufUy9Cl2GBJTRppaKuw4kPaBfTstNtrAB97vBhF9YVRQG1Y5puTz5tS134KgnjWDz9KMzc~ZhwVwyoOesUxoZgF37fdksX1d4Qwgf1yWP7AJr7XgpT2F0W5BlRc4Q0Y2qZMHcqkDc4Foj~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal