Key Points
Tisagenlecleucel (CTL019) has demonstrated clinical efficacy in relapsed/refractory B-cell ALL and CLL.
The cellular kinetic profile of tisagenlecleucel was consistent across the 2 diseases, with higher exposure in responding vs nonresponding patients.
Abstract
Tisagenlecleucel (CTL019) is an investigational immunotherapy that involves reprogramming a patient’s own T cells with a transgene encoding a chimeric antigen receptor to identify and eliminate CD19-expressing cells. We previously reported that CTL019 achieved impressive clinical efficacy in patients with relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL), including the expansion and persistence of CTL019 cells, which correlates with response to therapy. Here, we performed formal cellular kinetic analyses of CTL019 in a larger cohort of 103 patients treated with CTL019 in 2 different diseases (ALL and CLL). CTL019 was measured in peripheral blood and bone marrow, using quantitative polymerase chain reaction and flow cytometry. CTL019 levels in peripheral blood typically peaked at 10 to 14 days postinfusion and then declined slowly over time. Patients with complete response (CR)/CR with incomplete count recovery had higher levels of CTL019 in peripheral blood, with greater maximal concentration and area under the curve values compared with nonresponding patients (P < .0001 for each). CTL019 transgene levels were measurable up to 780 days in peripheral blood. CTL019 trafficking and persistence were observed in bone marrow and cerebrospinal fluid. CTL019 expansion correlated with severity of cytokine release syndrome (CRS) and preinfusion tumor burden in pediatric ALL. The results described here are the first detailed formal presentation of cellular kinetics across 2 diseases and highlight the importance of the application of in vivo cellular kinetic analyses to characterize clinical efficacy and CRS severity associated with CTL019 therapy.
Introduction
Tisagenlecleucel (CTL019) is an investigational genetically modified autologous T-cell immunotherapy cancer therapy that involves reprogramming a patient’s own T cells with a transgene encoding a chimeric antigen receptor (CAR) via a lentiviral vector. The CAR is specific for the B-cell antigen CD19, allowing CTL019 cells to identify and eliminate CD19-expressing malignant and normal B cells. The CAR comprises a murine single-chain anti-CD19 antibody fragment and 4-1BB and CD3-ζ intracellular signaling domains.1 The CD19 antigen recognition domain is responsible for binding of the CAR T cell to CD19. CD3-ζ is critical for initiating T-cell activation and antitumor activity, as measured by cytotoxicity and cytokine production,2 and the 4-1BB co-stimulatory signaling enhances proliferation, antitumor activity, oxidative metabolism, central memory differentiation, and persistence of the CTL019 cells both ex vivo and in animal models.1,3,4
Early results from clinical studies of CTL019 in patients with CD19+ relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia (ALL) showed promising and durable antitumor efficacy. Recent studies demonstrated an overall response rate of 82% (68% complete response [CR], 14% CR with incomplete blood count recovery [CRi]) to 93% (all CR)5,6 in pediatric patients with R/R ALL, and 53% (35% CR, 18% partial response [PR]) in patients with CLL.7,8 CTL019 and other CAR T-cell therapies have been associated with adverse events, including cytokine release syndrome (CRS).9-11 CRS is associated with high levels of circulating proinflammatory cytokines during CAR T-cell expansion and target engagement. CRS can be managed with supportive care and, if needed, antibodies that block interleukin 6 (IL-6) receptor signaling, such as tocilizumab; in some cases, limited corticosteroid treatment is used to further control CRS.10,12-14
This is the first publication to characterize the kinetics in vivo of a CAR T-cell therapy across multiple diseases. Cellular kinetics differ greatly from the pharmacokinetics of conventional molecules. Pharmacokinetic components applicable to small and large molecules, including distribution, metabolism, and excretion, are not directly applicable to CTL019 because it is a replicating, cell-based product. In contrast to conventional pharmacokinetics, levels of CTL019 transgene result from the cell product administered, as well as in vivo proliferation of CTL019 cells. Therefore, the term “cellular kinetics” refers to the in vivo characterization of cell-based therapies such as CAR T cells. Here, we present the first formal analysis of the cellular kinetics of CTL019 and its relationship to efficacy and safety in ALL and CLL.
Methods
Patients and clinical trial design
Three studies (NCT01626495 [pediatric and young adult B-cell ALL (pediatric B-ALL)], NCT01747486 [adult CLL], and NCT01029366 [adult ALL and CLL]; supplemental Table 1, available on the Blood Web site) were conducted after review and approval by the Children’s Hospital of Philadelphia and University of Pennsylvania institutional review boards. All patients provided written informed consent. The studies were initiated by the University of Pennsylvania and Children's Hospital of Pennsylvania and completed through a collaboration between the University of Pennsylvania and Children's Hospital of Pennsylvania with Novartis. Details were published previously.12,15,16 Cellular kinetic analysis was a primary or secondary objective for all 3 trials. Data are pooled across the studies and presented by indication (pediatric B-ALL, adult ALL, CLL). Patients received either a single dose of CTL019 or 2 to 3 fractionated doses within the first 28 days. A minority of patients received additional infusions beyond day 28 for R/R disease or loss of detectable transgene; these data have been excluded from this analysis because they may have biased cellular kinetic parameter estimation.
Cellular kinetics
The cellular kinetics of CTL019 were determined from individual concentration-time profiles of circulating CTL019 T cells and characterized using noncompartmental methods as applied in conventional pharmacokinetic analysis in peripheral blood (PB), bone marrow (BM), and cerebrospinal fluid (CSF; supplemental Table 2). The cellular kinetic parameters currently summarized exclude cellular kinetic data associated with reinfusion beyond day 28. Quantitative polymerase chain reaction (qPCR) and flow cytometry were used to measure CTL019 transgene and CD3+ CTL019 levels, respectively. Two terms used to describe the cellular kinetic profile of CTL019 are “expansion” and “persistence.” Expansion describes the maximal level of gene-modified cells in vivo after CTL019 infusion and is synonymous with the conventional pharmacokinetic parameter maximal concentration (Cmax) and persistence (measured by Tlast and Clast) indicates the duration that CTL019 cells are present in PB and tissues (Tlast) and the last measurable level of transgene present (Clast). Tmax is the time when maximal expansion occurs; after Tmax, circulating levels of CTL019 cells (eg, CD3+CAR+ cells or transgene) decline over time but remain detectable in the persistence phase. The area under the curve (AUC) represents the total presence of cells in both the overall expansion (up to 28 days) and persistence (up to 84 days) phases after infusion. AUC0-28d is a relevant parameter to evaluate differences in expansion between responding and nonresponding patients because the first clinical disease response assessment is at day 28. Several parameters are used to describe persistence, including the half-life (T1/2), Clast, and Tlast.
Quantitative polymerase chain reaction
CTL019 levels were also measured by qPCR, using transgene-specific primers and reported as transgene copies per microgram of genomic DNA.12 Cellular kinetics of CTL019 were determined in PB, BM, and CSF. Genomic DNA was isolated, and the transgene was quantified using TaqMan technology (Applied Biosystems). A validated assay17 was used to detect the integrated CD19 CAR transgene sequence, using, where available, 200 ng genomic DNA per time in PB, BM, and CSF samples. To control for assay quality, a parallel amplification was performed using 20 ng genomic DNA and a primer/probe combination specific for a nontranscribed sequence upstream of the cyclin-dependent kinase inhibitor 1A (p21) gene.18 These amplifications generated a correction factor to adjust for calculated vs actual DNA input; the lower limit of quantification was 25 copies/µg. The percentage of CTL019 cells in CSF was estimated using 1 ng genomic DNA, equivalent to 158.7 cells; data presented only included samples with a DNA yield for assay specification.
Flow cytometry
CTL019 T cells, including %CD3+CAR+ cells and CD8+ and CD8− subsets, were measured by flow cytometry in both PB and BM, using an exploratory assay described previously.12 The flow data shown as CD3+CTL019+ cells can be used interchangeably with CD3+CAR+ cells.
Data analysis
The cellular kinetics of CTL019 were calculated using noncompartmental analyses with Phoenix WinNonLin 6.4 (Pharsight): Cmax, Tmax, AUC0-28d, AUC0-84d, T1/2, Clast, and Tlast. AUC was calculated using the linear trapezoidal rule. T1/2 was calculated as ln2/λz, for which λz was estimated by linear regression of the terminal log-linear portion of the concentration-time curve. Cellular kinetic parameters are derived based on doses administered within the first 28 days and summarized by clinical response category. Derived parameters were pooled across the 3 studies by indication. The concordance between AUC0-28d and Cmax from qPCR and flow cytometry was evaluated using a linear regression model. Data visualization was performed using the R lattice package (http://www.R-project.org/). Summary statistics were determined using SAS (SAS Institute) and R and are presented by clinical response category. Kinetic parameters derived by flow cytometry should be interpreted with caution, given the exploratory nature of the assay.
Clinical response assessments
As previously reported,12,19 leukemia response assessments were initially determined on the basis of the day 28 assessment after CTL019 infusion. In pediatric B-ALL and adult ALL, response categories were CR, CRi, and no response (NR).12,20 In CLL, response categories were CR, PR, PR with incomplete blood count recovery (PRi), NR, and progressive disease (PD).15,21
Results
Patient characteristics
Cellular kinetic data were obtained from 3 studies in pediatric (age 1-24 years) and adult (age 25-71 years) ALL and in CLL (age 50-77 years) after a single dose or multiple fractionated doses (supplemental Table 1), as previously detailed.12,22,23 Data from 103 patients (55 pediatric B-ALL, 6 adult ALL, and 42 CLL) were pooled and examined by indication. Baseline demographic and disease characteristics, which were previously reported,12,15,19,23 were updated with additional patients and are summarized in supplemental Table 3; 66% of pediatric patients had prior allogeneic stem cell transplant, whereas no adult patients with ALL and only 1 patient with CLL had prior transplant. The number of prior therapies was 1 to 9 (median, 4 for pediatric B-ALL and CLL and 3 for ALL).
Kinetics of CTL019 in peripheral blood
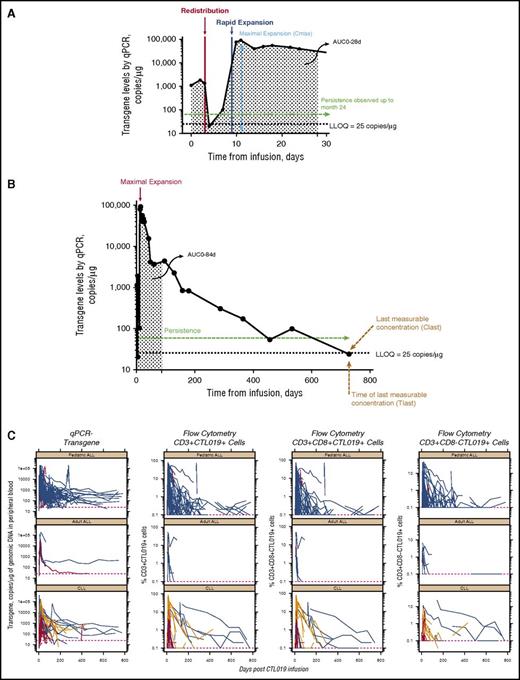
Representative individual patient profiles of circulating CTL019 levels (by qPCR) over time are shown in Figure 1A (first 28 days) and Figure 1B (full kinetic profile in the same patient with pediatric B-ALL). Immediately after peak infusion levels, there was a transient decline in circulating CTL019 transgene, which can possibly be attributed to the distribution of cells throughout the PB, BM, and other tissues12,24-26 ; this is followed by rapid in vivo expansion of circulating CTL019 cells. Figure 1C shows individual CTL019 levels measured by qPCR and flow cytometry, including measurements of CD3+CAR+, CD8+CAR+, and CD8−CAR+ T cells. A correlation was observed between transgene level by qPCR and cell surface expression of CAR by flow cytometry in PB, summarized both by individual patient AUC0-28d (r2 = .5518) and Cmax (r2 = .6461) values (supplemental Figure 1A-B), indicating an overall concordance between the 2 methods. Cmax was achieved within 2 weeks, followed by multiexponential decline during the next few weeks and months; in some patients, cells were detectable beyond 2 years, supporting the prolonged persistence of CTL019 in vivo in pediatric B-ALL, adult ALL, and CLL. Although heterogeneity was seen, responding patients (CR, CRi, or PR) had higher cumulative exposure compared with nonresponding patients with pediatric B-ALL or CLL (Figure 2; Table 1). With flow cytometry, a several-fold greater expansion was observed in patients with pediatric B-ALL with CR/CRi vs patients with NR/PD (Figure 2A). Similarly, greater expansion was observed in patients with CLL with either CR/CRi or PR/PRi compared with patients with NR/PD. When data were pooled for patients with ALL and CLL, those with CR/CRi/PR/PRi had higher AUC0-28d and Cmax than patients with NR/PD (P < .0001 for each) by both qPCR (Table 1) and flow cytometry (supplemental Table 4). Tocilizumab administration for the management of CRS did not appear to affect the expansion of CTL019 (supplemental Appendix).
CTL019 cellular kinetics. CTL019 cellular kinetics from a representative profile from a patient with pediatric B-ALL showing the initial expansion phase to day 28 (A) and day 780 (B). (C) Time course of levels of CTL019 transgene by qPCR and %CD3+ CTL019+ by flow cytometry for 3 patient groups: adult ALL, CLL, and pediatric B-ALL. LLOQ, lower limit of quantitation. CR/CRi, blue lines; PR/PRi, gold lines; NR/PD, red lines.
CTL019 cellular kinetics. CTL019 cellular kinetics from a representative profile from a patient with pediatric B-ALL showing the initial expansion phase to day 28 (A) and day 780 (B). (C) Time course of levels of CTL019 transgene by qPCR and %CD3+ CTL019+ by flow cytometry for 3 patient groups: adult ALL, CLL, and pediatric B-ALL. LLOQ, lower limit of quantitation. CR/CRi, blue lines; PR/PRi, gold lines; NR/PD, red lines.
Relationship between exposure and expansion of CTL019 cells and response category in pediatric B-ALL, adult ALL, and CLL. (A) AUC0-28d. (B) Cmax. Dots represent the mean, and whiskers represent the standard error.
Relationship between exposure and expansion of CTL019 cells and response category in pediatric B-ALL, adult ALL, and CLL. (A) AUC0-28d. (B) Cmax. Dots represent the mean, and whiskers represent the standard error.
Summary of cellular kinetic parameters in peripheral blood by qPCR
| . | Pediatric B-ALL . | Adult ALL . | CLL . | Pooled ALL and CLL* . | |||||
|---|---|---|---|---|---|---|---|---|---|
| CR/CRi . | NR/PD . | CR/CRi . | NR/PD . | CR/CRi . | PR/PRi . | NR/PD . | CR/CRi/PR/PRi . | NR/PD . | |
| AUC0-28d, copies/µg × d | |||||||||
| n | 52 | 3 | 5 | 1 | 9 | 7 | 19 | 73 | 23 |
| Geometric mean | 342 731.8 | 104 566.7 | 485 033.4 | 172 471.2 | 168 375.4 | 424 663.4 | 5012.1 | 328 212.0† | 8 688.0 |
| CV% | 158.5 | 1170 | 206.6 | — | 559.5 | 395.4 | 1108.6 | 208.5 | 1 910.2 |
| AUC0-84d, copies/µg × d | |||||||||
| n | 43 | — | 3 | 1 | 7 | 7 | 10 | 60 | 11 |
| Geometric mean | 436 905.1 | — | 324 558.5 | 184 897.5 | 556 256.1 | 669 577.4 | 11 530.5 | 465 373.0 | 14 838.9 |
| CV% | 145.9 | — | 148.1 | — | 389 | 356.1 | 1600.3 | 178.2 | 1 721.1 |
| Cmax, copies/µg | |||||||||
| n | 50 | 3 | 5 | 1 | 8 | 7 | 22 | 70 | 26 |
| Geometric mean | 47 988.4 | 17 193.1 | 54 248.9 | 26 405.1 | 27 371.3 | 37 336.2 | 577.7 | 44 276.4† | 989.9 |
| CV% | 132.3 | 779.4 | 164.9 | — | 477.5 | 259.8 | 743.9 | 167.0 | 1 470.3 |
| Tmax, d | |||||||||
| n | 50 | 3 | 5 | 1 | 8 | 7 | 22 | 70 | 26 |
| Median | 11 | 13 | 11 | 8 | 14 | 16 | 15 | 11 | 15 |
| Range | 2-31 | 8-16 | 10-22 | — | 4-32 | 1-22 | 1-32 | 1-32 | 1-32 |
| T1/2, d | |||||||||
| n | 37 | 3 | — | — | 5 | 4 | 5 | 46 | 8 |
| Median | 14.2 | 1.9 | — | — | 41.3 | 21.6 | 10.1 | 18.8 | 8.8 |
| Range | 0.7-400 | 1.2-2.9 | — | — | 8.5-81.9 | 14.7-33.1 | 8.1-11.8 | 0.7-400 | 1.2-11.8 |
| Clast, copies/µg | |||||||||
| n | 33 | 1 | 5 | 1 | 8 | 7 | 22 | 53 | 24 |
| Geometric mean | 291.8 | 26.4 | 389.9 | 27.3 | 122.6 | 196.3 | 121.7 | 249.6 | 107.3 |
| CV% | 360.5 | — | 2443.3 | — | 186.1 | 188.1 | 187.4 | 355.3 | 193.6 |
| Tlast, d | |||||||||
| n | 33 | 1 | 5 | 1 | 8 | 7 | 22 | 53 | 24 |
| Median | 196 | 23 | 65 | 458 | 352 | 176 | 28.5 | 192 | 28.5 |
| Range | 18-780 | — | 59-742 | — | 56-772 | 79-429 | 1-177 | 18-780 | 1-32 |
| . | Pediatric B-ALL . | Adult ALL . | CLL . | Pooled ALL and CLL* . | |||||
|---|---|---|---|---|---|---|---|---|---|
| CR/CRi . | NR/PD . | CR/CRi . | NR/PD . | CR/CRi . | PR/PRi . | NR/PD . | CR/CRi/PR/PRi . | NR/PD . | |
| AUC0-28d, copies/µg × d | |||||||||
| n | 52 | 3 | 5 | 1 | 9 | 7 | 19 | 73 | 23 |
| Geometric mean | 342 731.8 | 104 566.7 | 485 033.4 | 172 471.2 | 168 375.4 | 424 663.4 | 5012.1 | 328 212.0† | 8 688.0 |
| CV% | 158.5 | 1170 | 206.6 | — | 559.5 | 395.4 | 1108.6 | 208.5 | 1 910.2 |
| AUC0-84d, copies/µg × d | |||||||||
| n | 43 | — | 3 | 1 | 7 | 7 | 10 | 60 | 11 |
| Geometric mean | 436 905.1 | — | 324 558.5 | 184 897.5 | 556 256.1 | 669 577.4 | 11 530.5 | 465 373.0 | 14 838.9 |
| CV% | 145.9 | — | 148.1 | — | 389 | 356.1 | 1600.3 | 178.2 | 1 721.1 |
| Cmax, copies/µg | |||||||||
| n | 50 | 3 | 5 | 1 | 8 | 7 | 22 | 70 | 26 |
| Geometric mean | 47 988.4 | 17 193.1 | 54 248.9 | 26 405.1 | 27 371.3 | 37 336.2 | 577.7 | 44 276.4† | 989.9 |
| CV% | 132.3 | 779.4 | 164.9 | — | 477.5 | 259.8 | 743.9 | 167.0 | 1 470.3 |
| Tmax, d | |||||||||
| n | 50 | 3 | 5 | 1 | 8 | 7 | 22 | 70 | 26 |
| Median | 11 | 13 | 11 | 8 | 14 | 16 | 15 | 11 | 15 |
| Range | 2-31 | 8-16 | 10-22 | — | 4-32 | 1-22 | 1-32 | 1-32 | 1-32 |
| T1/2, d | |||||||||
| n | 37 | 3 | — | — | 5 | 4 | 5 | 46 | 8 |
| Median | 14.2 | 1.9 | — | — | 41.3 | 21.6 | 10.1 | 18.8 | 8.8 |
| Range | 0.7-400 | 1.2-2.9 | — | — | 8.5-81.9 | 14.7-33.1 | 8.1-11.8 | 0.7-400 | 1.2-11.8 |
| Clast, copies/µg | |||||||||
| n | 33 | 1 | 5 | 1 | 8 | 7 | 22 | 53 | 24 |
| Geometric mean | 291.8 | 26.4 | 389.9 | 27.3 | 122.6 | 196.3 | 121.7 | 249.6 | 107.3 |
| CV% | 360.5 | — | 2443.3 | — | 186.1 | 188.1 | 187.4 | 355.3 | 193.6 |
| Tlast, d | |||||||||
| n | 33 | 1 | 5 | 1 | 8 | 7 | 22 | 53 | 24 |
| Median | 196 | 23 | 65 | 458 | 352 | 176 | 28.5 | 192 | 28.5 |
| Range | 18-780 | — | 59-742 | — | 56-772 | 79-429 | 1-177 | 18-780 | 1-32 |
A single patient (patient 117; supplemental Figure 3) had a Cmax value of 178 481.7 copies/µg, whereas the other 2 NR patients had values of 4694 and 6066 copies/µg. Although patient 117 had very high expansion by qPCR, limited expansion was observed by flow cytometry.
Data were pooled because there were insufficient data for statistical testing for each population separately. Although there were disease-specific differences between ALL and CLL, the trend for higher exposure among patients with CR/CRi and PR/PRi vs those with NR/PD across both diseases was the rationale for pooling the data.
P < .0001 (CR/CRi/PR/PRi vs NR/PD).
Pediatric ALL
In pediatric B-ALL, the CTL019 transgene was detected in PB immediately after infusion. Median Tmax occurred 11 days after infusion in CR/CRi patients (n = 50) and 13 days in nonresponding patients (n = 3; Table 1). Geometric mean (CV%) maximal expansion (Cmax) was 48 000 copies/µg genomic DNA (132) in CR/CRi patients and 17 200 copies/µg genomic DNA (779) in NR patients.
Cmax (CV%) was 31.8% (90%) in CR/CRi patients (n = 51) and 0.3% (91%) in NR patients (n = 2) for %CD3+/CTL019+ cells by flow cytometry (supplemental Table 4), suggesting that at maximal expansion, 31.8% (range, 2.7% to 80.1%) of circulating CD3+ cells, on average, carried the CTL019-expressing CAR surface protein, in contrast to a negligible amount of CAR-expressing CD3+ cells in NR patients. In the typical CR/CRi patient (Figure 1), initially undetectable CTL019 transgene levels rose to 90 800 copies/µg genomic DNA from infusion (day 1) to Tmax (day 11), signifying a multilog expansion in vivo from baseline to Cmax (Figure 1C). Cmax and AUC0-28d were 90- and 12-fold greater, respectively, in responding patients vs nonresponding patients by flow cytometry of CD3+/CTL019+ T cells and were lower when measured by qPCR (eg, 2.8-fold higher Cmax in CR/CRi vs NR patients; Table 1).
CTL019 was measurable in patients with pediatric B-ALL with CR/CRi for a median Tlast of 196 days (range, 18-780 days) by qPCR (Table 1). Tlast was reported only in 1 NR patient and was excluded for the other 2 NR patients because they received a CTL019 dose beyond 28 days. In addition to poor persistence, NR patients had shorter Tlast because of shorter follow-up and study discontinuation for lack of response. Patients with a CR/CRi had a median T1/2 of 14.2 days (Table 1). Tlast and T1/2 values are influenced by observation time; with longer follow-up, Tlast values are expected to become higher.
Cellular kinetic profiles by duration event-free survival (EFS) categories (supplemental Figure 2) suggest that patients with longer CTL019 persistence maintained longer EFS (supplemental Appendix).
Adult ALL
The cellular kinetics of CTL019 in PB of 6 adult patients with ALL by qPCR and flow cytometry are presented in Table 1 and supplemental Table 4, respectively. Cmax and AUC0-28d were higher and T1/2 was longer in CR/CRi patients (n = 5) vs the 1 NR patient. Overall, the expansion and cellular kinetic profile in adult ALL appeared consistent with that in pediatric B-ALL.
Chronic lymphocytic leukemia
In patients with CLL, median T1/2 and Tlast of the CTL019 transgene were longer in patients with CR/CRi vs PR/PRi vs NR/PD (Table 1); however, median Tmax was similar across response groups. Higher exposure to circulating CTL019 T cells was observed in responding patients (CR/CRi and PR/PRi) compared with nonresponding patients (NR/PD). Although the sample size of the NR/PD group was small, there was a clear trend for higher expansion in patients with CR/CRi relative to NR/PD by both transgene levels and flow cytometry (Figure 2; supplemental Appendix).
Persistence of CTL019 in peripheral blood
Time-matched Tlast and Clast are presented for each individual patient (supplemental Figure 4). Clast decreased with longer duration of CAR-positive cells; however, Clast also depended on the frequency and duration of sampling. Transgene was measurable beyond 400 days in some patients. This suggests that persistence of CTL019 cells was generally maintained at detectable levels and slowly declined during a long period.
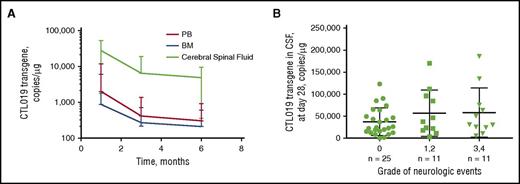
Trafficking to bone marrow and cerebrospinal fluid
CTL019 cells have been shown to distribute extensively to BM and CSF.22,23,27 Transgene levels in BM in pediatric B-ALL were approximately 60% to 70% of PB levels at months 3 and 6 (Table 2). Maximal CTL019 transgene level occurred in BM at the initial time after infusion (generally day 28), followed by a decline over time, similar to what was observed in PB (supplemental Figure 5). Input DNA was consistent for BM and PB (≈ 200 ng), suggesting that the high degree of partitioning is a true measure of transgene trafficking. In pediatric B-ALL, adult ALL, and CLL, CTL019 transgene and CD3+/CAR+ cells were present in BM at higher levels and for a longer time in responding patients compared with nonresponding patients, and were detected >2 years after infusion. Although there are limited BM data available in patients with nonresponding pediatric B-ALL, the geometric mean transgene level in PB was 340 copies/µg (n = 3) and 104 copies/µg (n = 1) in BM at day 28, indicating that nonresponding patients had lower transgene levels in both PB and BM.
Peripheral blood and bone marrow CTL019 transgene levels in pediatric B-ALL
| Time point summary . | PB (n = 52) . | BM (n = 52) . | CSF (n = 55) . |
|---|---|---|---|
| Geometric mean, copies/µg (CV%), n | |||
| Day 28 | 2010 (495.9), 51 | 876 (592.7), 49 | 40 556.07 (85.15), 52 |
| Month 3 | 413 (234.2), 42 | 272 (164.7), 44 | 3 966.668 (96.72), 16 |
| Month 6 | 304 (202.6), 29 | 212 (185.6), 33 | 2 493.821 (122.76), 17 |
| Time point summary . | PB (n = 52) . | BM (n = 52) . | CSF (n = 55) . |
|---|---|---|---|
| Geometric mean, copies/µg (CV%), n | |||
| Day 28 | 2010 (495.9), 51 | 876 (592.7), 49 | 40 556.07 (85.15), 52 |
| Month 3 | 413 (234.2), 42 | 272 (164.7), 44 | 3 966.668 (96.72), 16 |
| Month 6 | 304 (202.6), 29 | 212 (185.6), 33 | 2 493.821 (122.76), 17 |
Higher CTL019 transgene levels were detectable in CSF compared with PB at day 28 and months 3 and 6 when expressed per microgram of genomic DNA; however, low absolute numbers of cells were observed in CSF (0-63 663 CTL019 cells/mL) relative to the cellularity of BM and PB (Table 2; Figure 3). Penetration of CTL019 in CSF may be an important clinical finding because no central nervous system relapses have been observed in patients with pediatric B-ALL.27 No significant relationship between Cmax and grade of neurological events was observed (P > .05 for each category; Figure 3).
Mean (CV%) CTL019 transgene. (A) Copies of CTL019 transgene in PB, BM, and CSF during the first 6 months after infusion. (B) CTL019 transgene in CSF at day 28 by transient neuropsychiatric event. α for 1-way analysis of variance multiple comparisons test was 0.05. Grade 0 vs grade 1/2 neurological events, P = .5770; grade 0 vs grade 3/4 neurological events, P = .3591; grade 1/2 vs grade 3/4 neurological events, P = .9457.
Mean (CV%) CTL019 transgene. (A) Copies of CTL019 transgene in PB, BM, and CSF during the first 6 months after infusion. (B) CTL019 transgene in CSF at day 28 by transient neuropsychiatric event. α for 1-way analysis of variance multiple comparisons test was 0.05. Grade 0 vs grade 1/2 neurological events, P = .5770; grade 0 vs grade 3/4 neurological events, P = .3591; grade 1/2 vs grade 3/4 neurological events, P = .9457.
Effect of tumor burden on cell expansion, CRS, and neurological events
Preinfusion tumor burden (defined by percentage BM blasts) has emerged as an important parameter predicting CRS severity after CTL019 therapy in ALL.28,29 An analysis was performed to evaluate the relationships among expansion, preinfusion tumor burden, and safety (including CRS and neurological events). Higher Cmax and AUC0-28d were seen in patients with pediatric B-ALL with high tumor burden (≥50% blast count in BM; Figure 4A-B; supplemental Figure 6). There is increased expansion in patients with higher preinfusion tumor burden, and a significant difference was seen between patients with more than 50% blasts compared with those with less than 0.01% (P = .0006), and also between patients with less than 0.01% blasts vs those with 5% to 50% (P = .018). These analyses also show an association for higher CRS grade (Figure 4A) and neurological events (Figure 4B) in patients with greater tumor burden and higher expansion.
Relationship between maximal CTL019 expansion, preinfusion tumor burden, and grade of CRS and neurologic events in pediatric B-ALL. (A) CRS grade. (B) Neurologic event grade. The numbers presented represent the highest grade of CRS or neurologic events observed (0/1/2/3/4). Gray dots represent the mean, and whiskers represent the standard error.
Relationship between maximal CTL019 expansion, preinfusion tumor burden, and grade of CRS and neurologic events in pediatric B-ALL. (A) CRS grade. (B) Neurologic event grade. The numbers presented represent the highest grade of CRS or neurologic events observed (0/1/2/3/4). Gray dots represent the mean, and whiskers represent the standard error.
Cellular kinetics and serum cytokine levels
Serum cytokine levels were measured in patients with ALL, including responders (CR/CRi or PR/PRi) and nonresponders (NR/PD); patients with CLL were omitted because of insufficient cytokine data. Levels of cytokines such as interferon γ and IL-6 increased after infusion and remained elevated in patients experiencing CRS. There was a correlation between peak CTL019 transgene and cytokine levels during the first 28 days (interferon γ: Spearman r = 0.4304 [P = .001]; IL-6: Spearman r = 0.63 [P < .001] (supplemental Figure 7). Nonresponding patients generally had lower AUC0-28d and Cmax and corresponding lower levels of cytokines.
Discussion
To our knowledge, this is the first comprehensive cellular kinetic analysis of pediatric and adult patients with ALL and CLL receiving CAR T-cell therapy. We found high expansion of CTL019 using 2 different bioanalytical methods, with persistence measurable beyond 2 years in adults and children. There was a strong correlation between flow cytometry and qPCR in measuring levels of circulating CD3+CAR+ cells. CAR-T cells are a living drug that display a kinetic profile that is uniquely different from conventional therapeutics (ie, small molecules and biologics), as the cells undergo rapid expansion followed by persistence measured in months to years. Despite these differences, traditional pharmacokinetic analysis methods can be applied to characterize expansion and persistence.
It is important to note that flow cytometry measures circulating CAR+ cells with CAR protein expressed on the cell surface, whereas qPCR quantitates integration of the transgene into the T-cell genome, and therefore does not measure expression of the inserted CAR. The agreement between the 2 assays highlights the concordance between cell surface expression and integration into genomic DNA of the CAR transgene; both are important for evaluating whether the observed persistence data include functional CTL019 cells. Although there was high concordance between the time-matched cellular kinetic data and summarized exposure parameters of circulating CTL019 cells by qPCR against flow cytometry (supplemental Figure 1), the flow-based detection assay data presented here underscore the importance of flow cytometry as a potential tool to better predict response in patients treated with CTL019. In several patients, expansion was observed by qPCR, but flow cytometry indicated that the high levels of transgene present in the patient were not a result of functional CAR T cells. The lack of functional expansion in these patients resulted in poor disease control. Moreover, the high variability in exposure (Cmax and AUCs) could be attributed to the variable levels of proliferation that CTL019 cells undergo in these patients and may be affected by tumor burden, antitumor activity, and many other factors in this highly individualized therapy. It was previously reported that patients with B-cell aplasia may have detectable transgene levels without detectable cells by flow cytometry.12 In this analysis, transgene and CD3+CTL019 T cells were present for several years, although qPCR appeared to be a more sensitive assay because functional persistence of CD3+CTL019 measured by flow cytometry fell below the limit of detection in several patients. However, functional persistence was confirmed because the patients continued to have B-cell aplasia.30
Across 2 diseases, including both pediatric and adult patients, CTL019 expanded to a higher degree in responding patients compared with nonresponding patients. This finding is consistent with the mechanism of action of CTL019 because more CAR T-cell activity against target cells may enhance effector T-cell responses and stimulate CAR T-cell proliferation. Furthermore, CTL019 has a high degree of trafficking, penetration, and persistence in BM and can traffic to the central nervous system and persist for 6 months or longer. Importantly, levels of CTL019 in CSF did not correlate with rates of grade 3/4 neurological events.
Pediatric patients with B-ALL with higher baseline tumor burden have greater expansion and higher CRS grades (day −1 tumor burden was not determined for adult patients with ALL, and therefore this association could not be determined). This relationship is expected based on the mechanism of action of CTL019; CRS is related to the effector activity of CAR T cells, and higher preinfusion levels of target antigen will stimulate greater T-cell activity and cytokine release. This finding has not been confirmed in CLL or non-Hodgkin lymphomas because disease burden and location differ from those of ALL. A relationship between IL-6 and interferon γ levels and expansion was not identified, which may be a result of the different times of sample collection for cellular kinetic vs cytokine analysis.
In ALL, limited long-term persistence data are available for nonresponding patients because these patients typically discontinue the study when nonresponse is determined. However, in CLL, there is longer follow-up in nonresponding patients; a clear separation in exposures is seen in CR/CRi patients compared with PD/NR patients over time, whereby CR/CRi patients maintain higher exposure for prolonged periods (Figure 1C).15 These differences are more pronounced with flow-based measurements. There may be disease-specific factors influencing the cellular kinetics (eg, preinfusion tumor burden or extramedullary disease). In pediatric B-ALL, baseline tumor burden has an important effect on expansion profile, but it is unclear whether a similar phenomenon occurs in CLL because disease burden and etiology differ. Consistent cellular kinetic data were obtained across both disease states, indicating that the kinetics of transgene and %CD3+CTL019+ cells are related to the antigen-driven T-cell proliferation characteristic of the mechanism of action of CTL019.
Patients with ALL or CLL demonstrated comparable cellular kinetics of CTL019, with an initial delay and rapid expansion during the first 1 to 2 weeks, followed by a slower exponential decline and lower level of persistence over months to years. However, some differences were also apparent. Patients with responding ALL (CR/CRi) initially had higher Cmax than patients with responding CLL (CR/PR). However, patients with responding CLL demonstrated delayed Tmax and longer T1/2 compared with patients with responding ALL with higher AUC0-84d values. In both ALL and CLL, blood and marrow morphological leukemia is rapidly cleared within 28 days in responding patients; however, extramedullary disease (liver, spleen, lymph nodes) resolves more slowly over several months in some patients with CLL.15 The delayed and extended CTL019 kinetic expansion may reflect this delayed clearance of extramedullary disease sites as a result of differences in local microenvironmental conditions, overall larger tumor burden in CLL, and/or the known defects described in CLL T-cell function.31 Although the patterns and trends of CAR T-cell expansion and persistence show some general similarities between CLL and ALL, there may be attributes of the product or tumor that contribute to differences in T-cell quality, including disease type or location and treatment history. In pediatric and young adult B-ALL, the relationship between cell viability and cellular kinetics was evaluated (supplemental Figure 9). These analyses showed no relationship between cell viability and in vivo expansion.
Patients who received tocilizumab had higher tumor burden in pediatric B-ALL and had higher CRS grades, which potentially contributed to greater expansion. Comparisons of CTL019 cellular kinetics between NR patients treated with tocilizumab for management of grade 3/4 CRS and NR patients not treated with tocilizumab were not feasible because of the small sample size. However, tocilizumab did not abrogate CTL019 expansion, an important finding given the use of tocilizumab to safely manage patients with severe CRS (Table 3).
Summary of PB cellular kinetic parameters for CTL019 transgene by day 28 response and use of tocilizumab
| Parameter . | Received tocilizumab . | No tocilizumab . | ||
|---|---|---|---|---|
| CR/CRi (n = 15) . | NR (n = 0) . | CR/CRi (n = 37) . | NR (n = 3) . | |
| AUC0-28d, copies/µg × d | ||||
| Geometric mean (CV%), n | 893 000 (76.3), 15 | — | 232 000 (137.8), 37 | 105 000 (1170), 3 |
| Cmax, copies/µg | ||||
| Geometric mean (CV%), n | 92 300 (58.8), 15 | — | 36 300 (138), 35 | 17 200 (779.4), 3 |
| Tmax, d | ||||
| Median (range), n | 11 (7-18), 15 | — | 10 (2-31), 35 | 13 (8-16), 3 |
| Parameter . | Received tocilizumab . | No tocilizumab . | ||
|---|---|---|---|---|
| CR/CRi (n = 15) . | NR (n = 0) . | CR/CRi (n = 37) . | NR (n = 3) . | |
| AUC0-28d, copies/µg × d | ||||
| Geometric mean (CV%), n | 893 000 (76.3), 15 | — | 232 000 (137.8), 37 | 105 000 (1170), 3 |
| Cmax, copies/µg | ||||
| Geometric mean (CV%), n | 92 300 (58.8), 15 | — | 36 300 (138), 35 | 17 200 (779.4), 3 |
| Tmax, d | ||||
| Median (range), n | 11 (7-18), 15 | — | 10 (2-31), 35 | 13 (8-16), 3 |
Data on a similar CAR T-cell therapy with 4-1BB and CD3-ζ signaling domains were reported by Turtle et al,10 describing its expansion and persistence in adult patients with B-ALL. A visual inspection of the PB profile of CD19 CAR T cells suggested similar initial expansion kinetics as the CTL019 transgene and %CD3+CTL019+, with peak levels observed around day 10, but the reported persistence was shorter than presented here for CTL019.10 In addition, they also showed that blood levels were higher in patients with high tumor burden and that the severity of CRS and neurological events correlated with higher peak levels (Cmax) of circulating CD4+ and CD8+ CAR T cells
The relationship between EFS and persistence was evaluated and presented in supplemental Figure 2 for pediatric patients with B-ALL and suggests an association between longer persistence and longer EFS. The relationship between the effect of detectable transgene on duration of remission was not formally assessed because patients received re-infusion or alternative anticancer therapy, and hence would require censoring. However, we have evaluated the relationship between expansion and response and have seen significantly higher expansion in responding patients compared with nonresponding patients based on the day 28 response assessment (P < .001). In pediatric B-ALL, we have previously reported that short persistence, marked by early B-cell recovery before 3 months, is associated with an increased risk for relapse.30 Finally, in CLL, some patients with long-term remissions (beyond 5 years) were found to have sustained persistence.15 The cellular kinetic relationships observed here with CTL019 in patients with ALL and CLL may not apply to other diseases, such as non-Hodgkin lymphoma, because of disease-specific characteristics.
The presence and expansion of functional CAR transduced T cells is required for initial response, which demonstrates the importance of CAR T cellular kinetics (cell presence) and also the functionality of CAR T cells. Maintenance of response (at least in terms of preventing CD19+ recurrence) seems to be associated with CTL019 transgene persistence. In addition, evolving responses at later times (ie, 3-6 months) in diffuse large B-cell lymphoma likely rely on functional persistence of the infused cells as determined by B-cell levels.32 However, there may be additional factors not captured by cellular kinetics assays that could affect this relationship in individual patients and in different disease settings. Such factors might include different functional capacity of a patient’s own T cells (eg, T cells in CLL are known to have impaired function), characteristics of a patient’s tumor cells, the location of the tumor, and the tumor environment. All these factors may affect T-cell-mediated cell killing. For example, in patients with predominantly extramedullary disease involvement (eg, CLL and NHL), the tumor microenvironment could potentially modulate CAR T-cell effectiveness in these locations. Further research will provide the opportunity to integrate such parameters into the assessment and prediction of response.
To summarize, the cellular kinetic profile of CTL019 was consistent across disease categories (pediatric B-ALL, adult ALL, and CLL), with maximal expansion occurring within the first 2 weeks. Responding patients typically experienced greater expansion, as shown by the clear separation in cellular kinetic parameters using flow cytometry, and longer persistence of CTL019 in both PB and BM compared with nonresponding patients. Our data confirm early pharmacokinetic studies of CTL019 and describe the importance of baseline factors such as tumor burden for predicting the degree of CAR T-cell expansion and potential for CRS. This article highlights the importance of expansion and persistence of CAR T cells in responding patients and elucidates the relationships among CRS, tumor burden, and cell expansion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michael Zhang and Yanqui Weng, both Novartis employees, for assistance with statistical analyses presented herein. Editorial assistance was provided by Judith Murphy (ArticulateScience LLC), and was funded by Novartis Pharmaceuticals Corporation.
Authorship
Contribution: S.L.M., D.L.P., N.F., B.L.L., J.J.M., S.A.G., C.H.J., and S.F.L. enrolled patients, performed research, and contributed to data collection and interpretation; K.T.M., X.H., E.W., and R.A. performed cellular kinetic and/or statistical analyses and contributed to data interpretation; P.W. and A.C. contributed to data interpretation; K.T.M. wrote the first draft; and all authors were involved in revising the manuscript and approved the final version.
Conflict-of-interest disclosure: E.W. and R.A. are employees of Novartis; K.T.M., P.W., X.H., and A.C. are employees of and own equity in Novartis. S.L.M. received consultancy fees from and participated on advisory boards for Novartis. D.L.P. received research support from Novartis, participated on advisory boards for Servier, has a spouse that is an employee of and owns equity in Genentech, and has a patent related to the submitted work. N.F. received research support from Novartis. B.L.L. received grants from Novartis and personal fees from GE Healthcare and holds patents related to the submitted work. J.J.M. received research support from Novartis and holds patents related to the submitted work. S.A.G. received grants and consultancy fees from Novartis and holds patents related to the submitted work. C.H.J. received grants from Novartis and holds patents related to the submitted work. S.F.L. received grants from Novartis and speaking fees from Genentech.
Correspondence: Karen Thudium Mueller, Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, NJ 07936; e-mail: karen.thudium@novartis.com.