Key Points
Leniolisib, a novel, potent, selective oral PI3Kδ inhibitor was tested in patients with gain-of-function pathogenic variants in PIK3CD.
Treatment was well tolerated and led to improvement in cellular immune dysfunction and reduction of lymphoproliferation.
Abstract
Pathogenic gain-of-function variants in the genes encoding phosphoinositide 3-kinase δ (PI3Kδ) lead to accumulation of transitional B cells and senescent T cells, lymphadenopathy, and immune deficiency (activated PI3Kδ syndrome [APDS]). Knowing the genetic etiology of APDS afforded us the opportunity to explore PI3Kδ inhibition as a precision-medicine therapy. Here, we report in vitro and in vivo effects of inhibiting PI3Kδ in APDS. Treatment with leniolisib (CDZ173), a selective PI3Kδ inhibitor, caused dose-dependent suppression of PI3Kδ pathway hyperactivation (measured as phosphorylation of AKT/S6) in cell lines ectopically expressing APDS-causative p110δ variants and in T-cell blasts derived from patients. A clinical trial with 6 APDS patients was conducted as a 12-week, open-label, multisite, within-subject, dose-escalation study of oral leniolisib to assess safety, pharmacokinetics, and effects on lymphoproliferation and immune dysregulation. Oral leniolisib led to a dose-dependent reduction in PI3K/AKT pathway activity assessed ex vivo and improved immune dysregulation. We observed normalization of circulating transitional and naive B cells, reduction in PD-1+CD4+ and senescent CD57+CD4− T cells, and decreases in elevated serum immunoglobulin M and inflammatory markers including interferon γ, tumor necrosis factor, CXCL13, and CXCL10 with leniolisib therapy. After 12 weeks of treatment, all patients showed amelioration of lymphoproliferation with lymph node sizes and spleen volumes reduced by 39% (mean; range, 26%-57%) and 40% (mean; range, 13%-65%), respectively. Thus, leniolisib was well tolerated and improved laboratory and clinical parameters in APDS, supporting the specific inhibition of PI3Kδ as a promising new targeted therapy in APDS and other diseases characterized by overactivation of the PI3Kδ pathway. This trial was registered at www.clinicaltrials.gov as #NCT02435173.
Introduction
Primary immunodeficiencies (PIDs) are a group of immunological diseases often associated with significant lifelong morbidity and mortality; common variable immunodeficiency, characterized by immunoglobulin defects, comprises a substantial class of PID. Recently, the molecular defect in a subset of patients with a common variable immunodeficiency–like PID was found to be heterozygous, activating mutations in the PIK3CD gene encoding phosphoinositide 3-kinase (PI3K) δ.1-4 This molecularly defined disease has been named “activated PI3Kδ syndrome” (APDS) or “PI3Kδ-activating mutation causing senescent T cells, lymphadenopathy, and immunodeficiency” (PASLI), referred to here as APDS for simplicity. A similar clinical phenotype (also known as APDS2 or PASLI-R1) was described for heterozygous mutations in PIK3R1, which encodes the p85α regulatory subunit that heterodimerizes with p110δ to form PI3Kδ.5-8 PI3K enzymes are lipid kinases that play a role in signal transduction via tyrosine kinase- and G-protein–coupled receptors.9,10 PI3Kδ is a class IA PI3K expressed primarily in hematopoietic cells that converts the substrate phosphatidylinositol (4,5) bisphosphate to the second messenger phosphatidylinositol (3,4,5) trisphosphate, which promotes activation of proteins including the AKT kinase and downstream molecules (eg, mammalian target of rapamycin [mTOR] and S6 kinase). In APDS, mutations in the PIK3CD gene, encoding the p110δ subunit, result in a gain-of-function of PI3Kδ enzyme activity by disrupting inhibitory contacts between p85α and p110δ.11,12 Several distinct p110δ amino acid substitutions have been described in APDS, the most common being E1021K with other sites that include E81K, G124D, N334K, R405C, C416R, E525K, E525A, R929C, and E1021G.1-3,13-17 Approximately 215 APDS1 and APDS2 patients have been described to date, and the number continues to increase.6,12,18 Similar somatic mutations in p110α have been identified in malignancies in which hyperactive PI3K contributes to uncontrolled proliferation.19
The clinical phenotype of APDS typically includes significant nonmalignant lymphoproliferation (including bronchial and intestinal lymphoid hyperplasia and lymphadenopathy/splenomegaly/hepatomegaly), increased risk of malignant lymphoma and immunodysregulation resulting in recurrent oto-sino-pulmonary infections and bronchiectasis, chronic Epstein-Barr virus (EBV) and cytomegalovirus (CMV) viremia, and an increased risk of autoimmune disease including cytopenias.2,6,18,20 In 1 large APDS family, the majority of affected family members died before 30 years of age.1 Current treatment options include stem cell transplantation, immunoglobulin replacement therapy, and empirical treatment such as immunomodulatory, antibiotic, and antiviral therapy for symptom relief. Understanding the genetic etiology of this disease has led to the counterintuitive hypothesis that treating these immunodeficient patients with PI3K pathway inhibitors, which function as putative immunosuppressive drugs, may provide effective and long-term targeted therapy. Notably, some patients treated with the mTOR inhibitor rapamycin have experienced partial reduction of lymphoproliferation.3,18
Leniolisib (CDZ173) is a small-molecule inhibitor of p110δ currently in clinical development (supplemental Table 1 and supplemental Figure 1,21 available on the Blood Web site). It selectively inhibits the p110δ subunit of PI3K, which is hyperactive and drives the clinical manifestations of APDS due to missense mutation in PIK3CD. We hypothesized that leniolisib, used at doses that can dampen the overactive PI3K, will specifically reverse the pathophysiology of APDS in a molecularly targeted manner, thereby providing an effective treatment of this newly described disease of the immune system.22 Moreover, leniolisib may be a new treatment option for patients with pathological activation of the PI3Kδ pathway, as in B-cell malignancies or autoimmune disorders including Sjögren syndrome, systemic lupus erythematosus, or rheumatoid arthritis.
Patients and methods
In vitro studies
Studies in transfected Rat-1 fibroblasts and in primary immune cells isolated from patients with APDS were done to assess the in vitro potency of leniolisib on endogenously activated PI3Kδ (described in supplemental Methods). PIK3CD mutants encoding published forms of p110δ variants were generated by site-directed mutagenesis using human PIK3CD complementary DNA and transiently transfected in mammalian Rat-1 fibroblasts. The effects of leniolisib and mTOR inhibition on endogenous PI3K/AKT pathway activity in the transfectants were evaluated by measuring phosphorylated AKT (pAKT; S473) using homogeneous time-resolved fluorescence.
T-cell blasts from healthy donors as well as APDS patients were generated from isolated T cells by stimulation with anti-CD3 and anti-CD28 antibodies for 3 days. Cells were then incubated with titrated amounts of leniolisib, stimulated with anti-CD3, and the phosphorylation of AKT(S473) and S6(S240/244) was determined by flow cytometry.
Clinical study
Trial design.
The trial was designed as a 12-week, open-label, within-patient, dose-escalation study. After a screening period of up to 50 days that included a washout period of any immunosuppressive/immunomodulatory treatment, all patients received escalating doses of leniolisib (10, 30, and 70 mg twice daily for 4 weeks each). These dose levels were selected based upon in vitro studies as well as on results of the first-in-human study that predicted a pathway suppression (as assessed by pAKT(S473)+ B cells after ex vivo stimulation) of <50% at the lowest dose level and around 90% at the highest dose level.20 The study was conducted in accordance with the Declaration of Helsinki and was approved by health authorities and ethics committees/institutional review boards of the participating hospitals.
Trial participants.
Four male and 2 female patients aged 17 to 31 years with a molecularly identified gain-of-function mutation in the PIK3CD gene and a medical history and clinical symptoms compatible with APDS were enrolled (Table 1). All patients and parents of minors gave written informed consent. There was a 6-week washout period for participants treated with rapamycin prior to enrollment, and no participant received immunosuppressives during the washout or the treatment period. Patients with clinically significant comorbidities unrelated to APDS were excluded from trial participation.
Demographic and clinical characteristics at baseline
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . |
|---|---|---|---|---|---|---|
| Sex | M | F | F | M | M | M |
| Site | NIH, US | NIH, US | NIH, US | NIH, US | Motol University Hospital, CZ | Erasmus MC, NL |
| BMI, kg/m2/ body weight, kg | 22.5/67 | 24.6/67 | 27.2/67 | 18.9/53 | 18.0/57 | 21.4/73 |
| Age of onset/ enrollment, y* | 0.5/17 | 2/24 | 2/17 | 6/20 | 0.5/25 | 0.5/31 |
| Mutation | E525K | E1021K | E1021K | E1021K | E1021K | E1021K |
| Clinically significant cytopenias | No | Thrombocytopenia, neutropenia | No | Lymphopenia, neutropenia, anemia | No | Thrombocytopenia |
| Pulmonary problems | Bronchiectasis, asthma | No | Asthma, recurrent bronchitis | Chronic sinusitis, airway disease, bronchiectasis | Recurrent infections, bronchiectasis, COPD | Chronic sinusitis, bronchial wall thickening |
| Lymphoma | No | 19 y/o, stage IV large BCL, chemotherapy† | No | No | 11 y/o, stage III HL, chemotherapy‡ and radiotherapy | 20 y/o, stage IA, NHL, partial parotidectomy and local radiotherapy |
| Infection history | CMV/EBV | No | EBV | No | No | EBV |
| IgG replacement therapy | SCIG | IVIG | SCIG | None | IVIG | IVIG |
| Previous treatment with mTOR inhibitor§ | Yes | Yes | No | No | Yes | No |
| Reference, patient ID | 3, D.II.1 | 2, F1P1 | — | — | 20, P218 | 17, PI3K-2.2 |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . |
|---|---|---|---|---|---|---|
| Sex | M | F | F | M | M | M |
| Site | NIH, US | NIH, US | NIH, US | NIH, US | Motol University Hospital, CZ | Erasmus MC, NL |
| BMI, kg/m2/ body weight, kg | 22.5/67 | 24.6/67 | 27.2/67 | 18.9/53 | 18.0/57 | 21.4/73 |
| Age of onset/ enrollment, y* | 0.5/17 | 2/24 | 2/17 | 6/20 | 0.5/25 | 0.5/31 |
| Mutation | E525K | E1021K | E1021K | E1021K | E1021K | E1021K |
| Clinically significant cytopenias | No | Thrombocytopenia, neutropenia | No | Lymphopenia, neutropenia, anemia | No | Thrombocytopenia |
| Pulmonary problems | Bronchiectasis, asthma | No | Asthma, recurrent bronchitis | Chronic sinusitis, airway disease, bronchiectasis | Recurrent infections, bronchiectasis, COPD | Chronic sinusitis, bronchial wall thickening |
| Lymphoma | No | 19 y/o, stage IV large BCL, chemotherapy† | No | No | 11 y/o, stage III HL, chemotherapy‡ and radiotherapy | 20 y/o, stage IA, NHL, partial parotidectomy and local radiotherapy |
| Infection history | CMV/EBV | No | EBV | No | No | EBV |
| IgG replacement therapy | SCIG | IVIG | SCIG | None | IVIG | IVIG |
| Previous treatment with mTOR inhibitor§ | Yes | Yes | No | No | Yes | No |
| Reference, patient ID | 3, D.II.1 | 2, F1P1 | — | — | 20, P218 | 17, PI3K-2.2 |
All patients presented with splenomegaly and lymphadenopathy.
BCL, B-cell lymphoma; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CZ, Czech Republic; F, female; HL, Hodgkin lymphoma; ID, identifier; IVIG, IV immunoglobulin; M, male; NHL, non-Hodgkin lymphoma; NIH, National Institutes of Health; NL, The Netherlands; SCIG, subcutaneous immunoglobulin; US, United States; y/o, years old.
Age of onset is the onset of symptoms due to hypogammaglobulinemia.
Six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
Doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide (DBVE-PC).
All patients receiving previous treatment with mTOR inhibitor had a washout period of a minimum 6 weeks prior to start of leniolisib treatment.
Outcome measures.
Patients’ safety was monitored by adverse event registration, physical examination, vital signs, electrocardiography, and safety laboratory (hematology, biochemistry, and urinalysis) testing. These measures were reviewed in safety monitor meetings prior to each dose escalation.
The pharmacokinetics of leniolisib was assessed by analysis of exposure measurements (by liquid chromatography–mass spectrometry/mass spectrometry [LC-MS/MS]).
The primary end point was the pathway suppression as assessed by pAKT+ B cells in the systemic circulation. Whole blood from leniolisib-treated patients was stimulated ex vivo with anti-immunoglobulin M (IgM) and interleukin 4 (IL-4) (as described in supplemental Methods); unstimulated whole blood samples were processed in parallel.
Lymphoproliferation was assessed by diagnostic anatomical imaging by computed tomography (CT) or magnetic resonance imaging (MRI) as described in supplemental Methods.
Markers of immunodeficiency were quantified at baseline and at the end of the 4-week treatment period of each dose level. Lymphocyte subset analysis included naive B cells, transitional B cells, senescent CD57+CD4− T cells, and PD-1+CD4+ T cells.3
Soluble analytes quantified in serum included a panel of cytokines and chemokines, immunoglobulins, and viral load of CMV and EBV. Details of the analytes and methods are provided in supplemental Methods.
Patient and physician global assessment (100-mm visual analog scales) were measured every 4 weeks during the study. Furthermore, a text of patient narrative was provided for each patient by the investigator at the end of the trial.
Statistical analysis.
No formal power calculations were performed to estimate the sample size for this study. Analyses included all available data from all patients. An Emax (maximal effect attributable to the drug) concentration-response model was fitted to link the systemic drug concentration data and pAKT inhibition in the ex vivo–stimulated blood at each measured time point. Individual pharmacokinetic parameters and predicted plasma pharmacokinetic (PK) profiles were obtained using a model-based approach. Details on the models are provided in supplemental Methods. Other data were summarized descriptively.
Results
Leniolisib inhibits p110δ hyperactivity in vitro
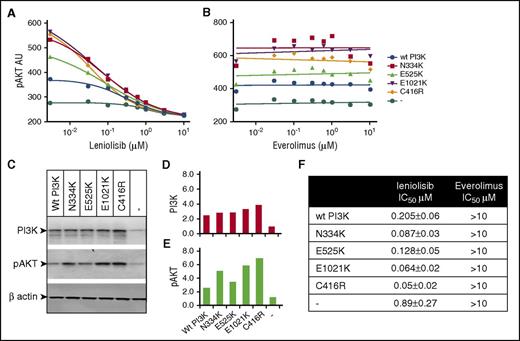
Studies in cell lines overexpressing p110δ mutants and in primary immune cells isolated from patients with APDS were performed to assess the in vitro potency of leniolisib on basal PI3Kδ activity. When any of the 4 PIK3CD mutations resulting in p110δ amino acid substitutions N334K, C416R, E525K, or E1021K described in APDS patients were ectopically expressed in Rat-1 fibroblasts, we observed a twofold to fivefold increase in baseline pAKT levels compared with cells transfected with the wild-type (WT) protein. In a short-term inhibition experiment, leniolisib reduced pAKT levels in a concentration-dependent manner, whereas as expected the mTOR inhibitor everolimus did not (Figure 1). Mapping the p110δ mutations onto the p110δ/p85α/leniolisib x-ray crystallographic structure confirmed that the APDS mutations are in positions distal to, and thus do not interfere with, the binding of leniolisib in the adenosine triphosphate–binding pocket (supplemental Figure 1).
PIK3CD mutant transfectants treated with leniolisib or an mTOR inhibitor. Rat-1 fibroblasts were transfected with human WT (wt PI3K) p110δ or p110δ carrying published APDS mutations N334K, E525K, E1021K, and C416R. The pAKT(S473) levels in the presence of titrated concentrations of leniolisib (A) and the mTOR inhibitor everolimus (B) are shown as individual data points with interpolated data for leniolisib and linear regression for everolimus. (C) Western blot for PI3K, pAKT, and β-actin as loading control. (D-E) Quantification of the western blot from panel C. Arbitrary units of scanning density for PI3K or pAKT were calibrated for loading control by division with the corresponding scanning density of β-actin. Average variation over 6 independent experiments is <5% pAKT arbitrary units (AU) for mutant and wt PI3K. (F) Fifty percent inhibitory concentration (IC50) values with SD derived from 6 independent experiments.
PIK3CD mutant transfectants treated with leniolisib or an mTOR inhibitor. Rat-1 fibroblasts were transfected with human WT (wt PI3K) p110δ or p110δ carrying published APDS mutations N334K, E525K, E1021K, and C416R. The pAKT(S473) levels in the presence of titrated concentrations of leniolisib (A) and the mTOR inhibitor everolimus (B) are shown as individual data points with interpolated data for leniolisib and linear regression for everolimus. (C) Western blot for PI3K, pAKT, and β-actin as loading control. (D-E) Quantification of the western blot from panel C. Arbitrary units of scanning density for PI3K or pAKT were calibrated for loading control by division with the corresponding scanning density of β-actin. Average variation over 6 independent experiments is <5% pAKT arbitrary units (AU) for mutant and wt PI3K. (F) Fifty percent inhibitory concentration (IC50) values with SD derived from 6 independent experiments.
To directly assess the effect of leniolisib in primary patient immune cells, we measured baseline and T-cell receptor stimulation-induced levels of pAKT and the phosphorylation of the ribosomal protein S6 downstream of pAKT in T-cell blasts from patients with APDS, and matched controls with and without leniolisib pretreatment. As expected, APDS patient T cells exhibited markedly higher PI3Kδ signaling compared with healthy controls, and this was robustly inhibited in a dose-dependent manner by leniolisib, regardless of which mutation the patient harbored (Figure 2). Thus, leniolisib potently inhibits hyperactive PI3Kδ in a concentration-dependent manner in vitro despite the amino acid substitutions present in APDS patients.
T-cell blast activation in presence or absence of leniolisib. Peripheral blood mononuclear cells (PBMCs) from healthy subjects (n = 4) or APDS patients (n = 3) with the indicated p110δ mutation were activated with anti-CD3 and anti-CD28 (1 μg/mL each) and maintained in culture media containing IL-2 to generate T-cell blasts. (A) After 30 minutes of preincubation with a dimethyl sulfoxide (DMSO) vehicle control or the indicated concentration of leniolisib, cells were stimulated for 10 minutes with anti-CD3 or were left unstimulated. Then, cells were fixed and stained for intracellular levels of AKT phosphorylated on S473 or S6 phosphorylated on S240/244. A representative healthy subject is shown in panel A. Quantification of cumulative data for median fluorescence intensity (MFI) of pAKT (B) and pS6 (C) stains is shown. The patient with E525K mutation corresponds to study patient 1.
T-cell blast activation in presence or absence of leniolisib. Peripheral blood mononuclear cells (PBMCs) from healthy subjects (n = 4) or APDS patients (n = 3) with the indicated p110δ mutation were activated with anti-CD3 and anti-CD28 (1 μg/mL each) and maintained in culture media containing IL-2 to generate T-cell blasts. (A) After 30 minutes of preincubation with a dimethyl sulfoxide (DMSO) vehicle control or the indicated concentration of leniolisib, cells were stimulated for 10 minutes with anti-CD3 or were left unstimulated. Then, cells were fixed and stained for intracellular levels of AKT phosphorylated on S473 or S6 phosphorylated on S240/244. A representative healthy subject is shown in panel A. Quantification of cumulative data for median fluorescence intensity (MFI) of pAKT (B) and pS6 (C) stains is shown. The patient with E525K mutation corresponds to study patient 1.
Leniolisib is well tolerated in APDS patients
Preclinical studies were extended to humans in a 12-week, open-label, within-patient, dose-escalation study.
Leniolisib was well tolerated at all doses. No significant clinical or laboratory toxicities or side effects limiting the physical activity or well-being of the patient were noted. No significant neutropenia, hypertriglyceridemia, hyperglycemia, gastrointestinal disturbances, skin rashes, or liver toxicity was observed. None of the 6 patients had levels of EBV or CMV that would indicate viremia, and no patient showed an increase in EBV/CMV levels during treatment with leniolisib (data not shown).
A patient global assessment questionnaire describing self-reported APDS-related well-being showed a mean ± standard deviation (SD) increase in well-being of 11 ± 11 mm (range, −3 to 22 mm). A physician global assessment questionnaire also demonstrated less disease activity following 12 weeks of treatment (mean ± SD reduction of 26 ± 16 mm, ranging from 12 to 51 mm reduction). In the textual patient narratives, the investigators described an improvement with treatment, including details on various clinical and quality-of-life improvements. Consistently, increased energy levels and/or decreased fatigue were described in all 6 patients following treatment.
All 6 APDS patients completed the 12-week treatment period as per protocol and were subsequently offered enrollment in an open-label long-term extension study using leniolisib 70 mg twice daily (clinicaltrials.gov #NCT02859727). Five of 6 patients chose to continue into the extension study, and the single patient opting out did so for logistical reasons related to travel.
Leniolisib induces dose-dependent inhibition of PI3Kδ and shows a favorable PK profile
The primary end point was PI3K pathway suppression as assessed by pAKT+ B cells in the systemic circulation. Whole blood from leniolisib-treated patients was ex vivo stimulated with anti-IgM and IL-4, and unstimulated whole-blood samples were processed in parallel. The pharmacokinetics of leniolisib in plasma were assessed by analysis of exposure measurements by LC-MS/MS.
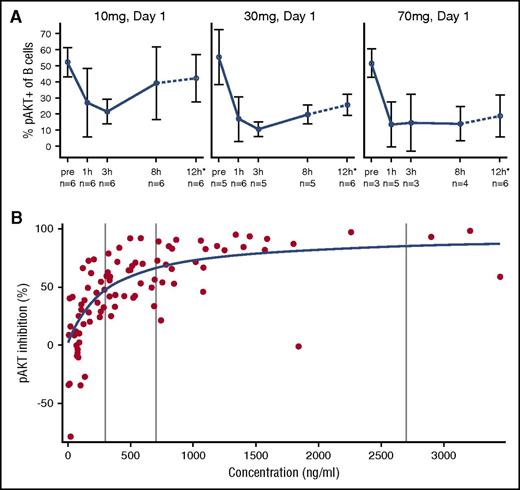
Leniolisib treatment led to potent and concentration-dependent reduction of AKT phosphorylation in ex vivo–stimulated B cells (Figure 3A). Pathway inhibition was characterized by rapid onset and apparent absence of relevant hysteresis, indicating a direct PK/pharmacodynamic (PD) relationship with a half-maximal inhibitory concentration of 300 ng/mL and a 90% effective concentration of 2703 ng/mL corresponding to 0.66 μM and 5.96 μM, respectively (Figure 3B). These observations were further supported by analyzing phosphorylation of S6 after ex vivo stimulation (supplemental Figure 2) as well as without exogenous stimulation (supplemental Figure 3). Unlike lower doses, steady-state drug exposure of 70 mg over the entire dosing interval in a twice-daily regimen was found to reduce PI3K/AKT pathway activity to within normal range of unstimulated pAKT levels in healthy controls. At all doses, no relevant time dependencies in steady-state trough and corresponding pAKT inhibition were seen, indicating absence of tachyphylaxis over the entire duration of the study. Leniolisib was found to be a low-clearance drug (CL/F∼4.2 L per hour [where CL/F is apparent total clearance of the drug from plasma after oral administration]). Linear pharmacokinetics with respect to both dose and time was evident over the entire dose range investigated (supplemental Figure 4). As expected from its rapid absorption (time to maximum concentration, ∼1 hour) and time-averaged disposition half-life (T1/2, ∼5 hours), leniolisib accumulated marginally in reaching steady state, which occurred 2 or 3 days after initiation of each dose increment.
Leniolisib pharmacodynamics: time- and dose-dependent PI3K/AKT pathway activity in B cells. (A) Whole blood from leniolisib-treated patients was stimulated ex vivo at the indicated dose and day with anti-IgM and IL-4 for 20 minutes. Then intracellular levels of pAKT(S473) were quantified by flow cytometry. The dotted line and asterisk indicate that the 12-hour values are mean from pooled data at day 8 and day 15. Data are shown as mean values of the indicated number of patients with SD. (B) Individual observed leniolisib blood exposure and pAKT inhibition in ex vivo–stimulated blood from APDS patients (circles) and results of Emax concentration-response model (line). Data points represent cumulated measurements of all 6 patients at multiple doses and time points. pAKT inhibition is defined as (−1) × percentage change from baseline pAKT value. Vertical lines represent from left to right: half maximal effective concentration (EC50), 70% effective concentration (EC70), and 90% effective concentration (EC90) values.
Leniolisib pharmacodynamics: time- and dose-dependent PI3K/AKT pathway activity in B cells. (A) Whole blood from leniolisib-treated patients was stimulated ex vivo at the indicated dose and day with anti-IgM and IL-4 for 20 minutes. Then intracellular levels of pAKT(S473) were quantified by flow cytometry. The dotted line and asterisk indicate that the 12-hour values are mean from pooled data at day 8 and day 15. Data are shown as mean values of the indicated number of patients with SD. (B) Individual observed leniolisib blood exposure and pAKT inhibition in ex vivo–stimulated blood from APDS patients (circles) and results of Emax concentration-response model (line). Data points represent cumulated measurements of all 6 patients at multiple doses and time points. pAKT inhibition is defined as (−1) × percentage change from baseline pAKT value. Vertical lines represent from left to right: half maximal effective concentration (EC50), 70% effective concentration (EC70), and 90% effective concentration (EC90) values.
Leniolisib ameliorates immune cell derangements
Markers of immunodeficiency were quantified at baseline and at the end of dosing of the 4-week treatment period of each dose level. Lymphocyte subset analysis included naive B cells, transitional B cells, CD57+ T cells, and PD-1+ T cells.3 In all 6 subjects, we confirmed immunological findings described previously in APDS such as abnormal circulating B-cell subset distribution with a relative increase in transitional B cells and reduced naive B cells (Figure 4A,C), increased expression of senescence-associated markers on T cells,3 and disturbed ratio of CD4+ to CD4− cells compared with normal healthy subjects23-26 (supplemental Figure 5). Treatment with leniolisib led to a reduction in the frequency of elevated transitional B cells and a normalization of naive B-cell frequencies. Moreover, the frequency of CD27+CD38+ plasmablasts was also drastically reduced in 4 of 6 patients (supplemental Table 2). The frequencies of PD-1+CD4+ (reflecting either chronic activation/exhaustion or increased circulating follicular helper T cells) and CD57+CD4− T cells (usually associated with senescence) were markedly reduced by leniolisib treatment (Figure 4B-C; supplemental Table 2).27
Changes in immune cell phenotypes in response to leniolisib. (A) Frequencies of transitional (left) and naive (right) B cells. (B) Frequencies of senescent CD57+CD4− (left) and PD-1+CD4+ T cells (right). Data are shown as mean values of indicated number of patients with SD. Vertical dotted lines indicate start of leniolisib dosing. The healthy volunteers’ median reference values for naive B cells, transitional B cells, CD57+CD4− cells, and PD1+CD4+ cells are: 74.7%,24 4.7%,24 17%35 and 13.2%,36 respectively. (C) Representative fluorescence-activated cell sorting (FACS) dot blots for patient 3 from panels A and B at baseline and end of treatment.
Changes in immune cell phenotypes in response to leniolisib. (A) Frequencies of transitional (left) and naive (right) B cells. (B) Frequencies of senescent CD57+CD4− (left) and PD-1+CD4+ T cells (right). Data are shown as mean values of indicated number of patients with SD. Vertical dotted lines indicate start of leniolisib dosing. The healthy volunteers’ median reference values for naive B cells, transitional B cells, CD57+CD4− cells, and PD1+CD4+ cells are: 74.7%,24 4.7%,24 17%35 and 13.2%,36 respectively. (C) Representative fluorescence-activated cell sorting (FACS) dot blots for patient 3 from panels A and B at baseline and end of treatment.
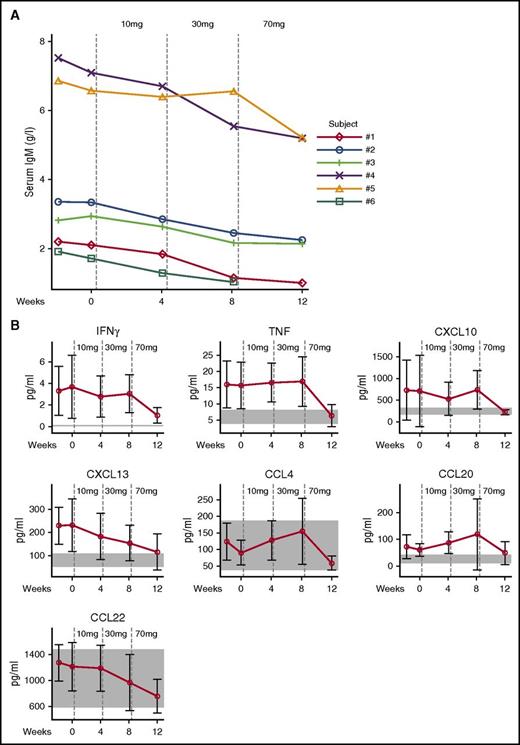
Soluble analytes quantified in serum included immunoglobulins and cytokine and chemokine panels. Details of the analytes and methods are provided in the supplemental Results. Consistent with the normalized transitional B-cell frequencies, the elevated serum levels of IgM declined (Figure 5A). In APDS patients, serum levels of CXCL13, CXCL10, interferon γ (IFNγ), and tumor necrosis factor (TNF) were found to be elevated at baseline and were reduced by leniolisib treatment to levels measured in healthy volunteers. CCL20 was also elevated in APDS, but did not clearly respond to treatment. Leniolisib also reduced serum CCL22 and CCL4, but levels in APDS patients before treatment were within the range of healthy volunteers (Figure 5B). The levels of other chemokines and cytokines assessed were neither different from healthy volunteers nor affected by treatment with leniolisib (data not shown).
IgM antibodies, cytokines, and chemokines. (A) Individual serum IgM levels. Vertical dotted lines indicate start of leniolisib dosing. Normal values in healthy donors aged 16 to 19 years, 0.23 to 2.59 g/L; older than 19 years, 0.40 to 2.30 g/L.37 (B) Soluble serum cytokines and chemokines. Vertical dotted lines indicate start of leniolisib dosing. Data are shown as mean values of 6 patients with SD. Normal values in healthy volunteers are shown as shaded areas.
IgM antibodies, cytokines, and chemokines. (A) Individual serum IgM levels. Vertical dotted lines indicate start of leniolisib dosing. Normal values in healthy donors aged 16 to 19 years, 0.23 to 2.59 g/L; older than 19 years, 0.40 to 2.30 g/L.37 (B) Soluble serum cytokines and chemokines. Vertical dotted lines indicate start of leniolisib dosing. Data are shown as mean values of 6 patients with SD. Normal values in healthy volunteers are shown as shaded areas.
Leniolisib improves lymphadenopathy, splenomegaly, and cytopenias
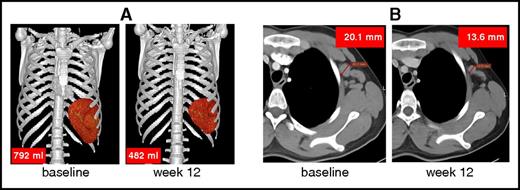
The consequences of lymphoproliferation were assessed by diagnostic anatomical imaging using CT or MRI, as described in the supplemental Results. CT/MRI demonstrated enlarged lymph nodes and spleen, a hallmark of APDS, in all 6 patients at baseline. After 12 weeks of treatment with leniolisib, lymph node sizes (ie, sum of products of diameters) were reduced by 40% (mean; range, 13%-65%) and spleen volumes were reduced by 39% (mean; range, 26%-57%; Table 2; Figure 6). Qualitative assessment of hepatomegaly was positive in 2 of the 6 patients (numbers 1 and 6) at baseline and remained unresolved posttreatment.
Spleen volumes, lymph node size, and liver volumes
| . | Spleen 3D volume . | Lymph node SPD . | Liver 3D volume* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, mm3 . | End of study, mm3 . | ΔBaseline, % . | Baseline, mm2 . | End of study, mm2 . | ΔBaseline, % . | Baseline, mm3 . | End of study, mm3 . | ΔBaseline, % . | |
| Patient 1 | 978 783 | 719 915 | −26 | 2453 | 1194 | −51 | 1 849 588 | 1 650 823 | −11 |
| Patient 2 | 792 042 | 482 188 | −39 | 1926 | 1681 | −13 | 1 438 041 | 1 413 010 | −2 |
| Patient 3 | 385 832 | 166 402 | −57 | 1235 | 427 | −65 | 1 349 165 | 1 442 528 | 7 |
| Patient 4 | 782 213 | 500 841 | −36 | 748 | 503 | −33 | 1 398 929 | 1 332 235 | −5 |
| Patient 5 | 419 843 | 254 634 | −39 | 258 | 133 | −48 | 1 396 210 | 1 359 013 | −3 |
| Patient 6 | 721 831 | 451 208 | −37 | 858 | 589 | −31 | 1 817 801 | 1 904 876 | 5 |
| Mean ± SD | −39 ± 10 | −40 ± 19 | −1 ± 6 | ||||||
| . | Spleen 3D volume . | Lymph node SPD . | Liver 3D volume* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, mm3 . | End of study, mm3 . | ΔBaseline, % . | Baseline, mm2 . | End of study, mm2 . | ΔBaseline, % . | Baseline, mm3 . | End of study, mm3 . | ΔBaseline, % . | |
| Patient 1 | 978 783 | 719 915 | −26 | 2453 | 1194 | −51 | 1 849 588 | 1 650 823 | −11 |
| Patient 2 | 792 042 | 482 188 | −39 | 1926 | 1681 | −13 | 1 438 041 | 1 413 010 | −2 |
| Patient 3 | 385 832 | 166 402 | −57 | 1235 | 427 | −65 | 1 349 165 | 1 442 528 | 7 |
| Patient 4 | 782 213 | 500 841 | −36 | 748 | 503 | −33 | 1 398 929 | 1 332 235 | −5 |
| Patient 5 | 419 843 | 254 634 | −39 | 258 | 133 | −48 | 1 396 210 | 1 359 013 | −3 |
| Patient 6 | 721 831 | 451 208 | −37 | 858 | 589 | −31 | 1 817 801 | 1 904 876 | 5 |
| Mean ± SD | −39 ± 10 | −40 ± 19 | −1 ± 6 | ||||||
Median healthy individual spleen volume, in men (179 000 mm3 [range, 31 000–525 000 mm3]) and in women (134 000 mm3 [range, 26 000–392 000 cm3]).34
3D, 3-dimensional; SPD, sum of product of diameters of preidentified index lymph nodes.
Midclavicular craniocaudal.
Reduction of spleen volume and lymph node size. Representative reduction of (A) an enlarged spleen (patient 1) and (B) lymph node (left axillary lymph node in patient 2). Up to 6 enlarged lymph nodes were identified at baseline and sizes were compared pre- and posttreatment.
Reduction of spleen volume and lymph node size. Representative reduction of (A) an enlarged spleen (patient 1) and (B) lymph node (left axillary lymph node in patient 2). Up to 6 enlarged lymph nodes were identified at baseline and sizes were compared pre- and posttreatment.
All patients had cytopenias at baseline: thrombocytopenia (n = 6), anemia (n = 1), neutropenia (n = 2) and they improved at the end of the 12-week treatment period. Absolute lymphocyte count was the most affected, with nearly all patients (n = 5) normalizing their counts at 12 weeks (supplemental Results; supplemental Table 3).
Discussion
Among PIDs with a defined genetic defect, APDS is a growing cohort of patients worldwide. Currently, patients with APDS receive empirical treatment such as prophylactic antibiotics, immunoglobulin replacement, mTOR inhibitors to suppress lymphoproliferation, chemotherapy for lymphoma, or allogeneic stem cell transplantation. mTOR inhibitors can cause serious and/or debilitating side effects,28 and the only potentially curative treatment of APDS, stem cell transplant, is associated with high morbidity and mortality.15,29 Our study patients differed in a few respects from published cohorts. Three of 6 patients had a history of B-cell lymphoma in contrast to 6% to 22% reported previously.1,3,18 The study subjects were screened for viremia (EBV and CMV) prior to treatment, and contrary to previously reported cohorts with prevalent (24%-100% of patients) herpesvirus infections,1,3,18 no clinically significant viremia was noted in our cohort of 6 patients. Whether this is due to the limited sample size in our study is unclear.
Leniolisib, a selective oral PI3Kδ inhibitor, has been engineered to target the specific kinase that is endogenously mutated and hyperactive in APDS. We observed that leniolisib inhibits not only the WT PI3Kδ but also the mutated, disease-causing kinase in cells transfected with published mutants of p110δ as well as in immune cells derived from APDS patients in vitro. Thus, we hypothesized that leniolisib might be a disease-modifying, targeted treatment of APDS, and therefore, paradoxically, an immunodeficiency could be ameliorated with an immunomodulatory/immunosuppressant drug. The present dose-escalation study of leniolisib indeed showed promising results for APDS patients. We found dramatic regression of splenomegaly and lymphadenopathy, normalization of markers of the disturbed adaptive immune system, improvements in clinical signs and symptoms as well as subjective rating of patients’ well-being including recovery from fatigue.
The observed reduction in transitional B cells and increased naive B-cell frequencies in these patients are consistent with the physiological modulation of PI3Kδ activity necessary during the development and maturation of B cells, though indirect effects through T-cell modulation cannot be excluded. Moreover, PI3K hyperactivation may prevent the expansion of antigen-specific B-cell populations and reduce class switch recombination and somatic hypermutation while increasing transitional B cells.12 The observed decrease in serum IgM levels seen with treatment may be a reflection of the normalization of B-cell subsets and restoration of class switch recombination. We hypothesize that changes in B-cell subpopulations are not a consequence of a decrease in B cells per se, as absolute numbers of circulating B cells were not affected over the course of the 12-week trial or in the extension phase (up to 9 months; data not shown) . However, naive B-cell frequencies increased in all patients (Figure 4). In addition, the percentage of circulating B cells and other B-cell subsets such as nonswitched or isotype-switched memory were either unaffected or only modestly altered by 12 weeks of leniolisib treatment (supplemental Table 2).
Patient 4 discontinued his weekly subcutaneous IgG infusions prior to enrollment and was followed closely for infections due to impaired B-cell function. As noted in supplemental Table 2, whereas 4 of the 6 patients had B cells <1%, the lowest B-cell numbers were observed in this patient. However, he did not have any clinical consequence due to low circulating B cells. As the extension study has progressed, this patient has not required any IgG supplementation. Moreover, IgG supplementation for 2 additional patients enrolled on the extension study was recently stopped by their primary providers.
Leniolisib diminished another pathogenic feature of chronically high PI3Kδ activity, namely increased T-cell senescence. Over the course of the 12-week trial, we did not observe changes in the frequency of naive CD4+ or CD4− T cells. Although rapamycin treatment over a 4-month period partially normalized these values in a previously reported APDS patient,3 the relatively shorter treatment duration with the highest, and most likely, the effective dose (only 4 weeks of the 70 mg twice-daily dose) may explain why this was not observed in the current leniolisib trial.
Abrogation of aberrant PI3K/AKT pathway activity, normalization of immunophenotypes, cytokine/chemokine modulation, and the clinical and subjective response were most notable in the final 4-week dosing period, indicating that 70 mg of leniolisib given twice daily might be the optimal therapeutic dose for APDS patients. This hypothesis is bolstered by the absence of dose-limiting adverse effects. Patients who completed the present study have the option for long-term treatment with leniolisib (70 mg twice daily) in an extension study. All extension-study participants have now received treatment of over 9 months and no patient in the 12-week clinical trial or in the extension study has experienced any significant adverse events. Notably, side effects prevalent with mTOR or other PI3K inhibitors, such as significant neutropenia, hypertriglyceridemia, hyperglycemia, diarrhea/colitis, skin rashes, pyrexia, or liver enzyme elevation,30,31 were not observed in our cohort with leniolisib. Our observation of reduced CCL4 and CXCL13 serum levels after leniolisib treatment are reminiscent of the reduction in chemokine secretion induced by idelalisib in cancer patients.32
It has recently been reported that PI3Kδ inhibition can result in genomic instability by augmenting off-target activity of activation-induced cytidine deaminase.33 Activation-induced cytidine deaminase levels were not monitored in these patients during the 12-week trial. Whether this effect will be observed in APDS patient B cells, which have developmental abnormalities (eg, accumulation of transitional B cells) and elevated baseline PI3Kδ activity, remains to be determined. Importantly, careful long-term surveillance and monitoring for lymphoma, regardless of treatment regimens using leniolisib or other PI3Kδ inhibitors, is paramount in this patient population.
In summary, our study exemplifies the power of precision medicine therapy in a rare disease, and the results present compelling evidence that leniolisib successfully addresses the root cause of APDS1. A 12-week randomized, placebo-controlled trial is now under way to corroborate these results (clinicaltrials.gov NCT02435173).
Given the similarity of the biochemical and clinical phenotype of APDS1 and APDS2, it seems plausible the PI3Kδ inhibition through leniolisib would also be effective in APDS2. Moreover, PI3K pathway modulation may be more broadly applicable to the treatment of other autoimmune and nonmalignant lymphoproliferative disorders in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for their support of this trial. At the National Institutes of Health, the authors thank Thomas Fleisher for facilitating the clinical study, Les Folio, Laura Machado-Pichardo, and Francine Thomas for radiology image processing, Shakuntala Rampertaap for flow cytometry, Pat Aldridge, David Buchbinder, Michael Cellucci, Hey Chong, Jennifer Grossman, Elaine Kulm, Susan Price, Doug Rosing, Morgan Similuk, Elaine Smoot, and Hua Su for clinical support, Peter J. Winkle and Anaheim Clinical Trials for trial support, and Luigi Notarangelo for critically reviewing the manuscript. The authors thank Tomas Milota, Radana Zachova, and Petra Liskova for support of the clinical trial at the Univerzita Karlova in Prague. The authors thank Marianne van der Ent and Kornvalee Meesilpavikkai from Erasmus MC for support of the clinical trial in Rotterdam. At Novartis, the authors thank Bruno Bieth for PK/PD modeling, Andrea Biondani and Louise Mooney for support of the clinical trial, Laurence Colin and Andrew Wright for statistical analysis, Petra Brinkmann for preparation of figures, Daniel d’Orazio for analysis of transfectants, Henrik Moebitz and Sascha Gutmann for x-ray modeling, Frédéric Zécri for synthesis and characterization of leniolisib, Ulrike Sommer, Aurelie Verles, Anais Villamaux, and Stéphanie Kaiser for biomarker analysis, and Luc Alexis Leuthold for pharmacokinetic analysis.
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by Novartis.
Authorship
Contribution: C.L.L. conducted, and M.J.L. supervised, translational studies with APDS patient material; D.G. performed transfectant studies; A.D.C., B.S., D.D.P., and C.B. conceived and designed the clinical study; V.K.R. supervised, and S.W. and A.K. conducted, the trial in the United States; V.A.S.H.D. and P.M.v.H. supervised the trial in The Netherlands; A.Š. supervised the trial in the Czech Republic; B.S. was the global clinical trial leader; S.H. obtained funding; S.D.R. and G.U. recruited participants in the United States and gave scientific input; A.D.J. supervised the performance of imaging measurements; S.d.B. performed the PK analyses; M.C. and J.D. performed the PD analyses; I.P. prepared the statistical analysis; C.K. coordinated the leniolisib project at Novartis; N.S. synthesized and characterized leniolisib; C.L.L., C.B., B.S., and V.K.R. advised on data analysis, interpreted the data, and wrote the report; and all authors revised the report and approved the final draft for publication.
Conflict-of-interest disclosure: C.B., M.C., A.D.C., S.d.B., J.D., D.G., A.D.J., C.K., I.P., D.D.P., B.S., and N.S. are current or former employees of Novartis. The remaining authors declare no competing financial interests.
The current affiliation for A.D.C. is F. Hoffmann-La Roche AG, Basel, Switzerland.
The current affiliation for C.L.L. is Immunobiology Department, Yale University, New Haven, CT.
Correspondence: V. Koneti Rao, Division of Intramural Research, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Dr, Room 12C106, Bethesda, MD 20892-1899; e-mail: koneti@nih.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal