Key Points
Characterization of the kinetics and risk factors for severe CRS after CD19 CAR T cells will facilitate preemptive therapy and management.
Severe CRS is characterized by endothelial activation.
Abstract
Lymphodepletion chemotherapy followed by infusion of CD19-specific chimeric antigen receptor–modified (CAR) T cells has produced impressive antitumor responses in patients with refractory CD19+ B-cell malignancies but is often associated with cytokine release syndrome (CRS). Our understanding of CRS continues to evolve, and identification of the kinetics of CRS and predictive clinical and laboratory biomarkers of severity are needed to evaluate strategies to mitigate toxicity. We report the clinical presentation of and identify biomarkers of severe CRS in 133 adult patients who received CD19 CAR T cells. CRS developed in 70% of patients, including 62.5% with grade 1 to 3 CRS (grade 1, 26%; grade 2, 32%; grade 3, 4.5%), 3.8% with grade 4, and 3.8% with grade 5. A majority of cases of grade ≥4 CRS occurred during CAR T-cell dose finding. Multivariable analysis of baseline characteristics identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS. Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. Angiopoietin-2 and von Willebrand factor, which are biomarkers of endothelial activation, were increased during severe CRS and also before lymphodepletion in patients who subsequently developed CRS. We describe a classification-tree algorithm to guide studies of early intervention after CAR T-cell infusion for patients at high risk of severe CRS. These data provide a framework for early intervention studies to facilitate safer application of effective CD19 CAR T-cell therapy.
Introduction
Lymphodepletion chemotherapy followed by infusion of T cells that are engineered to express CD19-specific chimeric antigen receptor (CAR) T cells has shown remarkable efficacy in patients with relapsed and/or refractory CD19+ B-cell malignancies. Reported complete response (CR) rates are as high as 93% in B-cell acute lymphoblastic leukemia (B-ALL) along with overall response rates of 77% in chronic lymphocytic leukemia (CLL) and 82% in non-Hodgkin lymphoma (NHL).1-13 Durable CRs without subsequent antitumor therapy have been observed in a subset of patients who received CD19 CAR T-cell therapy, which demonstrates the potential of this approach to improve survival in otherwise refractory patients.1,2,8
After adoptive transfer, CAR T cells are activated by encounter with CD19+ tumor or normal B cells, which results in proliferation of CAR T cells, lysis of the target cell, and cytokine secretion that can be associated with the clinical evidence of cytokine release syndrome (CRS) and neurotoxicity. CRS after CD19 CAR T-cell therapy presents with fever, hypotension, coagulopathy, and capillary leak and has been reported to occur in 54% to 91% of patients, including severe CRS in 8.3% to 43%.1,2,7-10,14-16 The increased availability of CD19 CAR T-cell therapies in multicenter trials highlights the need to provide clinicians who treat patients with B-ALL, CLL, or NHL with a detailed description of the clinical symptoms of CRS.17,18
A comprehensive description of the time course of presentation and biomarkers of CRS in a large cohort of patients has not been reported. Here, we report the clinical and laboratory findings from 133 adult patients with CD19+ relapsed/refractory B-ALL, CLL, or NHL who received lymphodepletion chemotherapy followed by infusion of CD19 CAR T cells. We identify risk factors before and after CAR T-cell infusion that are associated with the incidence and severity of subsequent CRS, which allows identification of patients at high risk of severe toxicity who might be candidates for early intervention studies. The data will facilitate recognition, diagnosis, and treatment of CRS.
Methods
Study design
We enrolled patients with relapsed/refractory CD19+ B-cell malignancies in a phase 1/2 clinical trial that evaluated lymphodepletion chemotherapy followed by CD19 CAR T cells.1,2 The study titled “Phase I/II Study of Immunotherapy for Advanced CD19+ Chronic Lymphocytic Leukemia, Acute Lymphoblastic Leukemia/Lymphoma and Non-Hodgkin Lymphoma with Defined Subsets of Autologous T Cells Engineered to Express a CD19-Specific Chimeric Antigen Receptor” (NCT01865617) was conducted according to the principles of the Declaration of Helsinki with approval by the Fred Hutchinson Cancer Research Center Institutional Review Board. This article reports clinical and laboratory data from 133 consecutively treated patients in the study who received their first cycle of lymphodepletion and CAR T-cell infusion.
Lymphodepletion chemotherapy and CD19 CAR T-cell infusion
The design of the CAR transgene and CAR T-cell manufacturing from CD4+ T cells and either bulk or central memory–enriched CD8+ T cells have been previously described (supplemental Data available on the Blood Web site).1,2 A truncated human epidermal growth factor receptor (EGFRt) was encoded in the lentiviral vector to allow precise enumeration of transduced CAR T cells by flow cytometry.19 Patients received lymphodepletion chemotherapy with a cyclophosphamide-based regimen with or without fludarabine (supplemental Table 1), followed 2 to 4 days later by infusion with CD19 CAR T cells formulated in a 1:1 ratio of CD4+:CD8+ and infused at 1 of 3 dose levels (DLs; DL1, 2 × 105 EGFRt+ cells per kg; DL2, 2 × 106 EGFRt+ cells per kg; DL3, 2 × 107 EGFRt+ cells per kg).
CRS grading and neurotoxicity
The severity of CRS was graded according to consensus criteria.20 Neurologic adverse events (AEs) were graded according to Common Terminology Criteria for Adverse Events v4.0.3 and did not contribute to organ toxicity criteria for CRS grading.
Evaluation of clinical laboratory parameters, CAR T-cell counts, and serum biomarkers
Blood was collected before lymphodepletion, on day 0 before CAR T-cell infusion, and at intervals after CAR T-cell infusion for analyses of complete blood counts, renal function, hepatic function, coagulation, and serum cytokine concentrations. In a subset of patients, serum angiopoietin 1 (Ang-1), Ang-2, and von Willebrand factor (VWF) concentrations were measured. CD4+ and CD8+ CAR T cells were identified by flow cytometry as viable CD45+CD3+CD4+CD8–EGFRt+ and CD45+CD3+CD4–CD8+EGFRt+ events, respectively, in a lymphocyte forward scatter/side scatter gate. Additional details are provided in supplemental Data.
Statistical analyses
Statistical methods are reported in supplemental Data.
Results
Patient and treatment characteristics
One hundred thirty-three patients with relapsed/refractory B-cell malignancies were included in the analyses (B-ALL, n = 47; CLL, n = 24; NHL, n = 62). The median age was 54 years (range, 20-73 years), and the median number of prior therapies was 4 (range, 1-11 prior therapies; Table 1). Twenty-five patients (19%) had previously undergone allogeneic hematopoietic stem cell transplantation (HCT), 22 (17%) had undergone autologous HCT, and 3 (2%) had undergone both allogeneic and autologous HCT. The lymphodepletion regimens given before CAR T-cell infusion are shown in supplemental Table 1. A majority of patients (78%) received a regimen containing both cyclophosphamide and fludarabine. Thirty-five patients (26%) received CAR T cells at DL1, 86 (65%) at DL2, and 12 (9%) at DL3.
Univariable and multivariable analysis of baseline and therapy-related characteristics by severity of CRS
| Characteristic . | CRS grade . | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1-3 . | 4-5 . | Total . | . | . | |||||||||||||||||
| No. . | % . | Median . | IQR . | Range . | No. . | % . | Median . | IQR . | Range . | No. . | % . | Median . | IQR . | Range . | No. . | % . | Median . | IQR . | Range . | Univariable P* . | Multivariable P† . | |
| No. of patients | 40 | 83 | 10 | 133 | ||||||||||||||||||
| Age, years | 56 | 44-65 | 27-70 | 54 | 43-61 | 20-73 | 53.5 | 43-62 | 20-70 | 54 | 43-62 | 20-73 | .55 | — | ||||||||
| Sex | .79 | — | ||||||||||||||||||||
| Male | 28 | 30 | 59 | 63 | 6 | 7 | 93 | 70 | ||||||||||||||
| Female | 12 | 30 | 24 | 60 | 4 | 10 | 40 | 30 | ||||||||||||||
| Karnofsky performance score | .30 | — | ||||||||||||||||||||
| 60-70 | 2 | 14 | 10 | 71 | 2 | 14 | 14 | 10 | ||||||||||||||
| 80-90 | 32 | 30 | 67 | 63 | 7 | 7 | 106 | 80 | ||||||||||||||
| 100 | 6 | 46 | 6 | 46 | 1 | 8 | 13 | 10 | ||||||||||||||
| Disease type | .30 | — | ||||||||||||||||||||
| ALL | 12 | 25 | 31 | 66 | 4 | 9 | 47 | 35 | ||||||||||||||
| CLL | 4 | 17 | 18 | 75 | 2 | 8 | 24 | 18 | ||||||||||||||
| NHL | 24 | 39 | 34 | 55 | 4 | 6 | 62 | 47 | ||||||||||||||
| Prior lines of therapy | 3 | 2-5 | 1-11 | 4 | 3-5 | 1-11 | 5 | 3-7 | 2-9 | 4 | 3-5 | 1-11 | .13 | — | ||||||||
| Prior transplant | .38‡ | — | ||||||||||||||||||||
| Allogeneic only | 3 | 12 | 21 | 84 | 1 | 4 | 25 | 19 | ||||||||||||||
| Autologous only | 9 | 41 | 11 | 50 | 2 | 9 | 22 | 17 | ||||||||||||||
| Both | 0 | 0 | 3 | 100 | 0 | 0 | 3 | 2 | ||||||||||||||
| Marrow disease burden by flow cytometry (%) | 0 | 0-1.3 | 0-79 | 20 | 0-65 | 0-97 | 21 | 3.6-40 | 0-89.8 | 1.3 | 0-42 | 0-97 | <.0001 | <.0001 | ||||||||
| Not involved | 23 | 47 | 25 | 51 | 1 | 2 | 49 | 37 | ||||||||||||||
| CD19+ cells in marrow by flow cytometry | 3.6 | 1.3-6.6 | 0-79 | 22 | 3.0-66 | 0-99 | 22 | 11-40 | 0.3-90 | 8.8 | 2.2-48 | 0-99 | .0001§ | - | ||||||||
| Platelet count (1000/μL) | 98 | 58-159 | 11-265 | 69 | 38-119 | 1-553 | 32 | 19-85 | 5-162 | 77 | 40-133 | 1-553 | .002 | .05 | ||||||||
| CD8+ selection method | .001 | .03 | ||||||||||||||||||||
| Bulk CD8+ | 9 | 15 | 47 | 77 | 5 | 8 | 61 | 46 | ||||||||||||||
| Central memory enriched | 31 | 43 | 36 | 50 | 5 | 7 | 72 | 54 | ||||||||||||||
| Lymphodepletion | .67 | .02 | ||||||||||||||||||||
| Cyclophosphamide/fludarabine based | 30 | 29 | 65 | 62 | 9 | 9 | 104 | 78 | ||||||||||||||
| Not cyclophosphamide/fludarabine based | 10 | 35 | 18 | 62 | 1 | 3 | 29 | 22 | ||||||||||||||
| CAR T-cell dose | .002 | .003 | ||||||||||||||||||||
| 2 × 105 EGFRt+ cells per kg | 10 | 29 | 25 | 71 | 0 | 0 | 35 | 26 | ||||||||||||||
| 2 × 106 EGFRt+ cells per kg | 27 | 31 | 54 | 63 | 5 | 6 | 86 | 65 | ||||||||||||||
| 2 × 107 EGFRt+ cells per kg | 3 | 25 | 4 | 33 | 5 | 42 | 12 | 9 | ||||||||||||||
| Lymphodepletion/CAR T-cell dose interaction effect|| | .03 | .009 | ||||||||||||||||||||
| Characteristic . | CRS grade . | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 1-3 . | 4-5 . | Total . | . | . | |||||||||||||||||
| No. . | % . | Median . | IQR . | Range . | No. . | % . | Median . | IQR . | Range . | No. . | % . | Median . | IQR . | Range . | No. . | % . | Median . | IQR . | Range . | Univariable P* . | Multivariable P† . | |
| No. of patients | 40 | 83 | 10 | 133 | ||||||||||||||||||
| Age, years | 56 | 44-65 | 27-70 | 54 | 43-61 | 20-73 | 53.5 | 43-62 | 20-70 | 54 | 43-62 | 20-73 | .55 | — | ||||||||
| Sex | .79 | — | ||||||||||||||||||||
| Male | 28 | 30 | 59 | 63 | 6 | 7 | 93 | 70 | ||||||||||||||
| Female | 12 | 30 | 24 | 60 | 4 | 10 | 40 | 30 | ||||||||||||||
| Karnofsky performance score | .30 | — | ||||||||||||||||||||
| 60-70 | 2 | 14 | 10 | 71 | 2 | 14 | 14 | 10 | ||||||||||||||
| 80-90 | 32 | 30 | 67 | 63 | 7 | 7 | 106 | 80 | ||||||||||||||
| 100 | 6 | 46 | 6 | 46 | 1 | 8 | 13 | 10 | ||||||||||||||
| Disease type | .30 | — | ||||||||||||||||||||
| ALL | 12 | 25 | 31 | 66 | 4 | 9 | 47 | 35 | ||||||||||||||
| CLL | 4 | 17 | 18 | 75 | 2 | 8 | 24 | 18 | ||||||||||||||
| NHL | 24 | 39 | 34 | 55 | 4 | 6 | 62 | 47 | ||||||||||||||
| Prior lines of therapy | 3 | 2-5 | 1-11 | 4 | 3-5 | 1-11 | 5 | 3-7 | 2-9 | 4 | 3-5 | 1-11 | .13 | — | ||||||||
| Prior transplant | .38‡ | — | ||||||||||||||||||||
| Allogeneic only | 3 | 12 | 21 | 84 | 1 | 4 | 25 | 19 | ||||||||||||||
| Autologous only | 9 | 41 | 11 | 50 | 2 | 9 | 22 | 17 | ||||||||||||||
| Both | 0 | 0 | 3 | 100 | 0 | 0 | 3 | 2 | ||||||||||||||
| Marrow disease burden by flow cytometry (%) | 0 | 0-1.3 | 0-79 | 20 | 0-65 | 0-97 | 21 | 3.6-40 | 0-89.8 | 1.3 | 0-42 | 0-97 | <.0001 | <.0001 | ||||||||
| Not involved | 23 | 47 | 25 | 51 | 1 | 2 | 49 | 37 | ||||||||||||||
| CD19+ cells in marrow by flow cytometry | 3.6 | 1.3-6.6 | 0-79 | 22 | 3.0-66 | 0-99 | 22 | 11-40 | 0.3-90 | 8.8 | 2.2-48 | 0-99 | .0001§ | - | ||||||||
| Platelet count (1000/μL) | 98 | 58-159 | 11-265 | 69 | 38-119 | 1-553 | 32 | 19-85 | 5-162 | 77 | 40-133 | 1-553 | .002 | .05 | ||||||||
| CD8+ selection method | .001 | .03 | ||||||||||||||||||||
| Bulk CD8+ | 9 | 15 | 47 | 77 | 5 | 8 | 61 | 46 | ||||||||||||||
| Central memory enriched | 31 | 43 | 36 | 50 | 5 | 7 | 72 | 54 | ||||||||||||||
| Lymphodepletion | .67 | .02 | ||||||||||||||||||||
| Cyclophosphamide/fludarabine based | 30 | 29 | 65 | 62 | 9 | 9 | 104 | 78 | ||||||||||||||
| Not cyclophosphamide/fludarabine based | 10 | 35 | 18 | 62 | 1 | 3 | 29 | 22 | ||||||||||||||
| CAR T-cell dose | .002 | .003 | ||||||||||||||||||||
| 2 × 105 EGFRt+ cells per kg | 10 | 29 | 25 | 71 | 0 | 0 | 35 | 26 | ||||||||||||||
| 2 × 106 EGFRt+ cells per kg | 27 | 31 | 54 | 63 | 5 | 6 | 86 | 65 | ||||||||||||||
| 2 × 107 EGFRt+ cells per kg | 3 | 25 | 4 | 33 | 5 | 42 | 12 | 9 | ||||||||||||||
| Lymphodepletion/CAR T-cell dose interaction effect|| | .03 | .009 | ||||||||||||||||||||
IQR, interquartile range.
Two-sided P values were calculated by using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables.
Step-wise multivariable proportional odds models were performed to assess the impact of baseline factors on the occurrence of CRS (grade 0 vs 1-3 vs 4-5), where log10 values were used to transform data as appropriate, with 0.001 substituting for values of 0.
Any transplant type vs no transplant.
Because marrow disease burden and total CD19+ cells in marrow have a strong correlation (r = 0.99; P < .0001), only marrow disease was included in the multivariable analysis.
The interaction effect demonstrates that at increasing CAR T-cell dose levels, the incorporation of fludarabine into the lymphodepleting regimen has a greater association with CRS.
The incidence and kinetics of CRS and neurotoxicity
CRS of any grade developed in 93 (70%) of 133 patients. The incidence, severity, and clinical presentation of CRS in B-ALL, CLL, and NHL patients were similar (Table 1; supplemental Figures 1-2). A majority of patients (123 [92.5%] of 133) had either no CRS (grade 0, 30%), or grade 1 to 3 CRS (grade 1, 26%; grade 2, 32%; grade 3, 4.5%). Ten patients (7.5%) developed grade ≥4 CRS (grade 4, 3.8%; grade 5, 3.8%) (Figure 1). Five of these 10 patients died within the first 30 days after CAR T-cell infusion as a result of complications associated with CRS and/or neurotoxicity. One additional patient died 4 months after CAR T-cell therapy as a result of irreversible neurotoxicity. Of the 10 patients (7.5%) with grade ≥4 CRS, 8 were enrolled and received CAR T cells during the dose-escalation phase of the study. At the maximum tolerated dose (MTD) of CAR T cells, grade ≥4 CRS was observed in 2 (2.5%) of 79 patients.
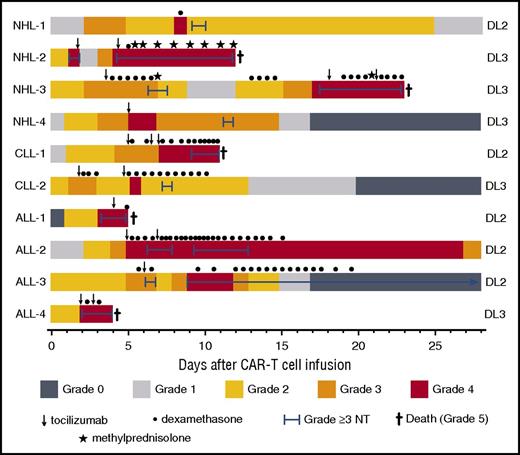
Presentation, management, and outcomes of patients with grade ≥4 CRS. Colors on the swimmer plot indicate the CRS grade on each day through 28 days after CAR T-cell infusion in all patients who developed grade ≥4 CRS. The duration of grade ≥3 neurotoxicity and interventions with tocilizumab and/or corticosteroids are indicated in the figure. ALL-2 developed dialysis-dependent acute kidney injury (AKI) through day 26 followed by resolution of CRS-associated organ toxicity (grade 0) on day 37. ALL-3 died 4 months after CAR T-cell infusion with irreversible neurotoxicity, despite resolution of fever and hypotension associated with CRS on day 13 after CAR T-cell infusion. NHL-1 had ongoing grade 1 AKI at last available laboratory value on day 83. Doses of medications: dexamethasone 10 mg intravenously or orally, methylprednisolone 1g intravenously, tocilizumab 4 to 8 mg/kg intravenously. NT, neurotoxicity.
Presentation, management, and outcomes of patients with grade ≥4 CRS. Colors on the swimmer plot indicate the CRS grade on each day through 28 days after CAR T-cell infusion in all patients who developed grade ≥4 CRS. The duration of grade ≥3 neurotoxicity and interventions with tocilizumab and/or corticosteroids are indicated in the figure. ALL-2 developed dialysis-dependent acute kidney injury (AKI) through day 26 followed by resolution of CRS-associated organ toxicity (grade 0) on day 37. ALL-3 died 4 months after CAR T-cell infusion with irreversible neurotoxicity, despite resolution of fever and hypotension associated with CRS on day 13 after CAR T-cell infusion. NHL-1 had ongoing grade 1 AKI at last available laboratory value on day 83. Doses of medications: dexamethasone 10 mg intravenously or orally, methylprednisolone 1g intravenously, tocilizumab 4 to 8 mg/kg intravenously. NT, neurotoxicity.
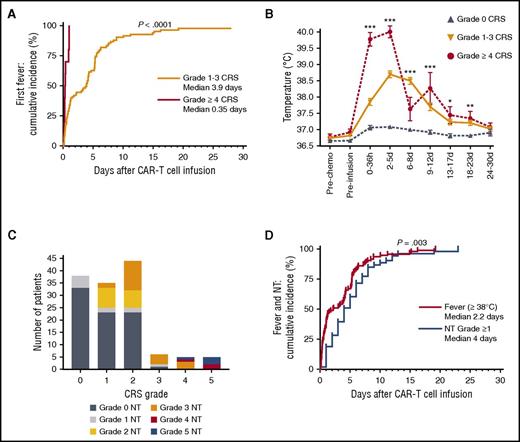
Fever ≥38°C was the first objective sign of CRS with the exception of 1 patient who presented with hypotension without fever. Fever occurred a median of 2.2 days (IQR, 0.9-5.6 days) after CAR T-cell infusion and lasted for a median of 3.0 days (IQR, 1.2-4.8 days) (Table 2). Compared with patients who had grade 1 to 3 CRS, patients who had grade ≥4 CRS experienced fever earlier after CAR T-cell infusion (P < .0001) and the fever peaked earlier (P = .001), reached a higher maximum temperature (P < .0001), and was of longer duration (P = .03; Table 2; Figure 2A-B). All patients who ultimately had grade ≥4 CRS were febrile within 25 hours after CAR T-cell infusion, and only 4 patients (all with grade ≤3 CRS) developed their first fever more than 12 days after CAR T-cell infusion (Figure 2A). Fifty-three (40%) of 133 patients had 1 or more grade ≥1 neurologic AEs (grade 1-2, 18%; grade ≥3, 21%), and the severity of neurotoxicity was associated with the severity of CRS (P < .0001; supplemental Table 2). All patients with grade ≥4 CRS also developed grade ≥3 neurotoxicity (Figure 2C). Neurotoxicity typically presented after CRS (P = .003), with the first neurologic AE of any grade presenting a median of 4 days (IQR, 2-7 days) after CAR T-cell infusion (Figure 2D). The first grade ≥3 neurologic AE presented 4.5 days (IQR, 3.2-6.2 days) after the first fever.
Characterization of fever in patients who develop CRS
| Fever . | CRS grade . | P* . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-3 . | 4-5 . | Total . | |||||||||||
| No. . | Median . | IQR . | Range . | No. . | Median . | IQR . | Range . | No. . | Median . | IQR . | Range . | ||
| No. of patients | 83 | 10 | 92 | ||||||||||
| Fever onset (days after CAR T-cell infusion) | 3.9 | 0.8-5.6 | 0.1-19 | 0.4 | 0.3-0.9 | 0.2-1.0 | 2.2 | 0.9-5.6 | 0.1-19 | <.0001 | |||
| Time to peak temperature (days after CAR T-cell infusion) | 5.7 | 4.3-7.6 | 0.2-30 | 2.8 | 1.3-3.2 | 0.4-11 | 5.3 | 3.4-7.3 | 0.2-30 | .001 | |||
| Maximum temperature (°C) | 39.4 | 39.2-39.6 | 37.7-41.3 | 40.4 | 40.1-40.6 | 39.9-40.9 | 39.5 | 39.2-39.8 | 37.7-41.3 | <.0001 | |||
| Fever duration (days after first fever) | 2.5 | 1.2-4.7 | 0.02-15 | 4.4 | 3.6-5.4 | 3.1-6.8 | 3.0 | 1.2-4.8 | 0.02-15 | .03 | |||
| Fever . | CRS grade . | P* . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-3 . | 4-5 . | Total . | |||||||||||
| No. . | Median . | IQR . | Range . | No. . | Median . | IQR . | Range . | No. . | Median . | IQR . | Range . | ||
| No. of patients | 83 | 10 | 92 | ||||||||||
| Fever onset (days after CAR T-cell infusion) | 3.9 | 0.8-5.6 | 0.1-19 | 0.4 | 0.3-0.9 | 0.2-1.0 | 2.2 | 0.9-5.6 | 0.1-19 | <.0001 | |||
| Time to peak temperature (days after CAR T-cell infusion) | 5.7 | 4.3-7.6 | 0.2-30 | 2.8 | 1.3-3.2 | 0.4-11 | 5.3 | 3.4-7.3 | 0.2-30 | .001 | |||
| Maximum temperature (°C) | 39.4 | 39.2-39.6 | 37.7-41.3 | 40.4 | 40.1-40.6 | 39.9-40.9 | 39.5 | 39.2-39.8 | 37.7-41.3 | <.0001 | |||
| Fever duration (days after first fever) | 2.5 | 1.2-4.7 | 0.02-15 | 4.4 | 3.6-5.4 | 3.1-6.8 | 3.0 | 1.2-4.8 | 0.02-15 | .03 | |||
Two-sided P values were calculated by using the Wilcoxon test.
Kinetics of presentation of CRS and neurotoxicity. (A) Cumulative incidence curve for first fever ≥38°C in patients with grade 1 to 3 (n = 82) or grade ≥4 CRS (n = 10). (B) Mean ± standard error of the mean (SEM) of the maximum temperature after CAR T-cell infusion. (C) Incidence and grading of neurotoxicity within each CRS grade. (D) The median time of onset of fever ≥38°C (red, n = 92) or neurotoxicity (blue, n = 53) after CAR T-cell infusion. One patient with grade 2 CRS who developed hypotension without fever is not included. Kruskal-Wallis test ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005. d, days after CAR T-cell infusion; h, hours after CAR T-cell infusion; pre-chemo, before the start of lymphodepletion chemotherapy; pre-infusion, before CAR T-cell infusion.
Kinetics of presentation of CRS and neurotoxicity. (A) Cumulative incidence curve for first fever ≥38°C in patients with grade 1 to 3 (n = 82) or grade ≥4 CRS (n = 10). (B) Mean ± standard error of the mean (SEM) of the maximum temperature after CAR T-cell infusion. (C) Incidence and grading of neurotoxicity within each CRS grade. (D) The median time of onset of fever ≥38°C (red, n = 92) or neurotoxicity (blue, n = 53) after CAR T-cell infusion. One patient with grade 2 CRS who developed hypotension without fever is not included. Kruskal-Wallis test ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005. d, days after CAR T-cell infusion; h, hours after CAR T-cell infusion; pre-chemo, before the start of lymphodepletion chemotherapy; pre-infusion, before CAR T-cell infusion.
One hundred nine patients (82%) received both the lymphodepletion chemotherapy and CAR T-cell infusion in the outpatient setting. Outpatients were admitted at the first fever ≥38°C. Because the severity of CRS did not reach grade ≥3 until a median of 3.4 days after onset of fever (range, 1.4-4.7 days), there was sufficient time for hospital admission and therapeutic interventions to mitigate the progression of CRS. The median duration of hospitalization for all patients was 7 days (IQR, 3-14 days), and it was associated with the maximum severity of CRS (grade 0, median 0 days; grade 1-3, 9 days; grade ≥4, 18 days; P < .0001; supplemental Table 2). Twenty-six patients (20%) with CRS and/or neurotoxicity received tocilizumab and/or dexamethasone to treat CRS and/or neurotoxicity. Twenty patients received dexamethasone and tocilizumab, 5 received dexamethasone alone, and one received tocilizumab alone. All patients who received tocilizumab had grade ≥3 CRS and/or neurotoxicity, except 2 patients who had progressive grade 2 CRS. Fever resolved at a median of 0.4 days (IQR, 0.2-2.0 days) following the first dose of tocilizumab or dexamethasone.
Severe CRS is associated with vascular instability and organ dysfunction
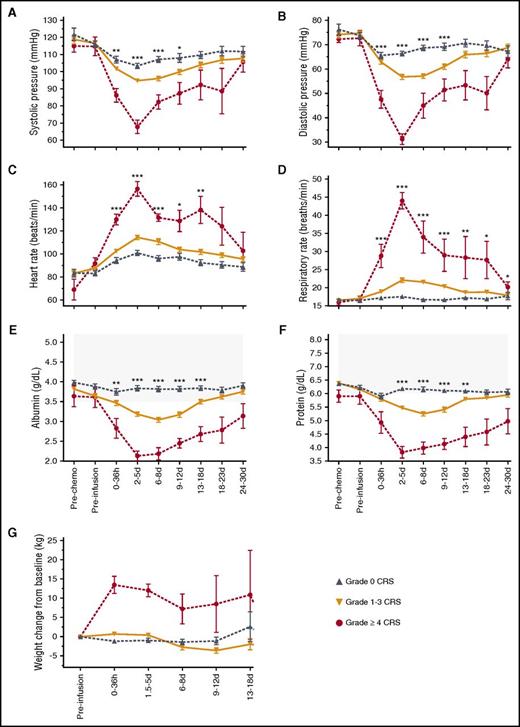
After CAR T-cell infusion, patients with severe CRS exhibited hemodynamic instability and capillary leak with hypotension, tachycardia, tachypnea, hypoalbuminemia, hypoproteinemia, and weight gain (Figure 3). Seventeen of 133 patients (13%) required admission to the intensive care unit (ICU) for management of CRS and/or neurologic AEs, and the median duration of their stay in the ICU was 3 days (IQR, 2-7 days). Eleven (8%) of 133 patients received vasopressor support. Only 2 patients with grade ≤3 CRS required vasopressor support. Ten patients (7.5%) required intubation to manage respiratory failure associated with severe neurotoxicity (n = 5), management of pulmonary dysfunction (n = 3), or disease progression (n = 2).
Hemodynamic instability and clinical capillary leak in grade ≥4 CRS. (A-G) Mean ± SEM of (A) minimum systolic blood pressure, (B) minimum diastolic blood pressure, (C) maximum heart rate, (D) maximum respiratory rate, (E) minimum serum protein concentration, (F) minimum albumin concentration, and (G) weight gain from the start of lymphodepletion are shown at the indicated times after CAR T-cell infusion. Gray shading indicates the normal range. Kruskal-Wallis test: ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005.
Hemodynamic instability and clinical capillary leak in grade ≥4 CRS. (A-G) Mean ± SEM of (A) minimum systolic blood pressure, (B) minimum diastolic blood pressure, (C) maximum heart rate, (D) maximum respiratory rate, (E) minimum serum protein concentration, (F) minimum albumin concentration, and (G) weight gain from the start of lymphodepletion are shown at the indicated times after CAR T-cell infusion. Gray shading indicates the normal range. Kruskal-Wallis test: ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005.
All patients with grade ≥4 CRS also developed grade ≥3 nonneurologic organ toxicity, which resolved a median of 24 days (range, 12-32 days) after resolution of fever. Nine of the 10 patients with grade ≥4 CRS developed hepatic dysfunction, manifested by elevated aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin, with 5 patients having grade ≥3 transaminase elevation (supplemental Figure 3A-D). The aspartate aminotransferase peaked between days 2 and 5, whereas the alanine aminotransferase, alkaline phosphatase, and total bilirubin peaked later at day 6 to 8. One patient developed late hepatic dysfunction on day 20 associated with severe hypotension as a result of gastrointestinal hemorrhage. Three of the 10 patients with grade ≥4 CRS developed grade ≥3 acute kidney injury, with 1 patient requiring hemodialysis for 15 days until recovery of renal function (supplemental Figure 3E-F). Only 3 patients with grade ≤3 CRS developed grade 3 nonneurologic organ toxicity (2 hepatic, 1 cardiac), and these events resolved in 1 to 2 days.
Delayed hematopoietic recovery in patients with grade ≥4 CRS
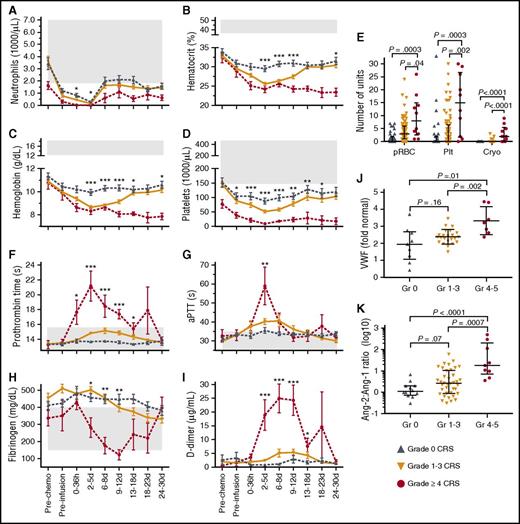
We evaluated recovery of blood counts in all patients who had received lymphodepletion chemotherapy and CAR T-cell infusion. To ensure that observed differences in hematopoietic toxicity in patients with distinct grades of CRS were not the result of differences in intensity of the lymphodepletion regimen, only patients who received cyclophosphamide/fludarabine lymphodepletion (n = 104) were included in this analysis. The absolute neutrophil count, hematocrit, hemoglobin concentration, and platelet count declined after cyclophosphamide/fludarabine chemotherapy, reaching nadirs between days 2 and 5 after CAR T-cell infusion (Figure 4A-D). The absolute neutrophil count, hematocrit, and platelet nadirs were lower in patients with more severe CRS, and patients with grade ≥4 CRS received more platelet (P = .002) and red cell (P = .04) transfusions than those with grade ≤3 CRS (Figure 4E). Five of 10 patients with grade ≥4 CRS became refractory to platelet transfusion. Marrow tumor burden (P < .0001), the number of prior therapies (P = .02), and the occurrence of CRS (P = .0002) were associated with longer hematologic recovery. The time to hematologic recovery was longer than expected in most patients with grade 4 CRS (supplemental Table 3) and was delayed in patients with grade 1 to 3 CRS (median, 13.5 days [IQR, 6.5-18.1 days]) compared with those without CRS (median, 4.1 days [IQR, 2.9-7.5 days]; P = .0002).
Hematopoietic toxicity, laboratory coagulopathy, and endothelial injury in grade ≥4 CRS. (A) Minimum absolute neutrophil count, (B) hematocrit, (C) hemoglobin, and (D) platelet count are shown for patients receiving cyclophosphamide/fludarabine lymphodepletion at the indicated times after CAR T-cell infusion (n = 104). (E) Total transfused units of packed red blood cells (pRBC), platelets (Plt), and cryoprecipitate (Cryo) in the first 28 days after CAR T-cell infusion. (F) Maximum PT, (G) maximum aPTT, (H) minimum fibrinogen, and (I) maximum D-dimer concentrations are shown at the indicated times after CAR T-cell infusion. (J) The fold change in VWF concentration in serum from a subset of patients at the peak of CAR T-cell expansion (n = 60; grade 0, n = 12; grade 1-3, n = 39; grade ≥4 CRS, n = 9) compared with the VWF concentration in pooled normal plasma (12.2 μg/mL; CRYOcheck, Precision Biologic, Dartmouth, NS, Canada). (K) The Ang-2:Ang-1 ratio at the peak of CAR T-cell expansion (n = 60; grade 0, n = 12; grade 1-3, n = 39; grade ≥4 CRS, n = 9). For (A-D) and (F-I), data represent the mean ± SEM. P values were determined using the Kruskal-Wallis test. For (E,J,K), each point represents data from 1 patient. The median and IQR are shown. P values were determined using the Wilcoxon test. Gray shading indicates the normal range. ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005. Gr, grade.
Hematopoietic toxicity, laboratory coagulopathy, and endothelial injury in grade ≥4 CRS. (A) Minimum absolute neutrophil count, (B) hematocrit, (C) hemoglobin, and (D) platelet count are shown for patients receiving cyclophosphamide/fludarabine lymphodepletion at the indicated times after CAR T-cell infusion (n = 104). (E) Total transfused units of packed red blood cells (pRBC), platelets (Plt), and cryoprecipitate (Cryo) in the first 28 days after CAR T-cell infusion. (F) Maximum PT, (G) maximum aPTT, (H) minimum fibrinogen, and (I) maximum D-dimer concentrations are shown at the indicated times after CAR T-cell infusion. (J) The fold change in VWF concentration in serum from a subset of patients at the peak of CAR T-cell expansion (n = 60; grade 0, n = 12; grade 1-3, n = 39; grade ≥4 CRS, n = 9) compared with the VWF concentration in pooled normal plasma (12.2 μg/mL; CRYOcheck, Precision Biologic, Dartmouth, NS, Canada). (K) The Ang-2:Ang-1 ratio at the peak of CAR T-cell expansion (n = 60; grade 0, n = 12; grade 1-3, n = 39; grade ≥4 CRS, n = 9). For (A-D) and (F-I), data represent the mean ± SEM. P values were determined using the Kruskal-Wallis test. For (E,J,K), each point represents data from 1 patient. The median and IQR are shown. P values were determined using the Wilcoxon test. Gray shading indicates the normal range. ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005. Gr, grade.
CRS has been associated with macrophage activation syndrome.20,21 Consistent with this, we observed higher ferritin and C-reactive protein (CRP) levels, and more prolonged monocytopenia in the blood of patients with grade ≥4 CRS compared with those who had grade ≤3 CRS (supplemental Figure 3G-I). However, examination of bone marrow biopsies from patients with grade ≥4 CRS showed no evidence of increased hemophagocytosis that might contribute to delayed hematopoietic recovery. Rather, in 5 of 7 patients with grade ≥4 CRS and available marrow pathologic examination, the bone marrow was hypocellular without morphologic evidence of residual tumor, suggesting that there may be alternative mechanisms for delayed recovery (supplemental Table 3).
Consumptive coagulopathy in grade ≥4 CRS
We examined the prothrombin time (PT), activated partial thromboplastin time (aPTT), D-dimer, and fibrinogen in patients at intervals after CAR T-cell infusion. Patients receiving therapeutic anticoagulation were excluded from the analyses (n = 9). In the first week after CAR T-cell infusion, patients with grade ≤3 CRS had normal or mildly elevated PT, aPTT, D-dimer, and fibrinogen. In contrast, those with grade ≥4 CRS developed early prolongation of the PT and aPTT, which peaked approximately 2 to 5 days after CAR T-cell infusion (Figure 4F-G). Increasing D-dimer and falling fibrinogen concentrations started on days 2 to 5, with hypofibrinogenemia occurring from days 9 to 12, consistent with disseminated intravascular coagulation (Figure 4H-I). Compared with patients with grade 1 to 3 CRS, those with grade ≥4 CRS received more cryoprecipitate transfusions to correct coagulopathy (P < .0001; Figure 4E) and had more severe and prolonged thrombocytopenia (Figure 4D). Grade ≥3 bleeding occurred in only 3 patients (2%), all of whom had grade ≥4 CRS. Red cell fragmentation was not a prominent feature on analysis of blood film morphology. The findings were consistent with development of a consumptive coagulopathy in patients with severe CRS.
Biomarkers of endothelial activation are elevated in severe CRS
The presence of hemodynamic instability, capillary leak, and a consumptive coagulopathy raised the possibility that endothelial activation might contribute to the clinical findings of severe CRS. VWF is released from Weibel-Palade bodies upon endothelial activation22 and plays a key role in the initiation of coagulation. To determine whether in vivo endothelial activation was present in patients with severe CRS, we evaluated serum concentrations of VWF at the peak of CAR T-cell expansion in blood in a subset of 60 patients with different severities of CRS (grade 0, n = 12; grade 1-3, n = 39; grade ≥4, n = 9). We found that patients with grade ≥4 CRS had higher VWF concentrations compared with those who had grade ≤3 CRS (Figure 4J). Serum concentrations of Ang-2, which is also released from Weibel-Palade bodies on endothelial activation and promotes capillary leak,23,24 were also higher in patients with grade ≥4 CRS (supplemental Figure 4A). Ang-1 promotes endothelial stability, and an increase in the Ang-2:Ang-1 ratio has been associated with morbidity and mortality in sepsis and cerebral malaria.25-30 At the peak of CAR T-cell expansion in blood, increasing severity of CRS was associated with lower Ang-1, higher Ang-2, and an increased Ang-2:Ang-1 ratio (Figure 4K; supplemental Figure 4A). Of note, before both lymphodepletion and CAR T-cell infusion, and on day 1 after CAR T-cell infusion, increasing serum VWF concentration was associated with increased severity of subsequent CRS (supplemental Figure 4B). Furthermore, before lymphodepletion and on day 1 after CAR T-cell infusion, there was an association between increased Ang-2:Ang-1 and severity of CRS (supplemental Figure 4C). Together, these data indicate that biomarkers of endothelial activation are elevated during severe CRS and that endothelial activation even prior to commencing lymphodepletion and CAR T-cell therapy may increase the risk of subsequent development of severe CRS.
Patient and treatment characteristics associated with the development and severity of CRS
To identify patients at risk of developing CRS, we performed univariable analyses of the impact of baseline clinical and laboratory characteristics on the development of any grade of CRS. Patients with higher marrow tumor burden (P < .0001), a higher percentage of CD19+ cells in the marrow (P = .0001), and more severe thrombocytopenia (P = .002) were at higher risk of developing CRS (Table 1). Manufacturing of CAR T cells using bulk CD8+ T cells without selection of the central memory subset (P = .001) and the infused CAR T-cell dose (P = .002) were associated with increased risk of CRS. Despite our previous observation that addition of fludarabine to cyclophosphamide in lymphodepletion enhanced in vivo CAR T-cell expansion,1,2 this was not associated with increased occurrence of CRS in univariable analysis. However, analysis of the interaction between CAR T-cell dose and cyclophosphamide/fludarabine lymphodepletion showed that addition of fludarabine at any given CAR T-cell dose increased the risk of CRS (P = .03). Stepwise multivariable analysis showed that higher bone marrow CD19+ tumor burden (P < .0001), more severe thrombocytopenia (P = .05), bulk CD8+ T-cell selection (P = .03), cyclophosphamide/fludarabine lymphodepletion (P = .02), higher CAR T-cell dose (P = .003), and the interaction effect of CAR T-cell dose and cyclophosphamide/fludarabine lymphodepletion (P = .009) were independently associated with development of CRS (Table 1). Risk factors for CRS within each disease cohort are presented in supplemental Tables 4A-C.
We then examined risk factors for the occurrence of any grade of CRS that were identified in the multivariable model to see if these factors also impacted the severity of CRS (Table 3). Univariable pairwise analysis showed that only higher CAR T-cell dose (P = .0003) and cyclophosphamide/fludarabine lymphodepletion (P = .03) were associated with the development of grade ≥4 compared with grade 1 to 3 CRS.
Univariable pairwise analysis of factors significant in the multivariable proportional odds model
| . | Univariable pairwise P values for CRS grade . | ||
|---|---|---|---|
| 0 vs 1-3 . | 0 vs 4-5 . | 1-3 vs 4-5 . | |
| Marrow burden of disease (%) | <.0001 | .0001 | .8 |
| Platelet count | .01 | .005 | .06 |
| CAR T-cell dose level | .7 | .005 | .0003 |
| Bulk CD8+ T-cell selection | .0005 | .12 | .7 |
| Fludarabine/cyclophosphamide stratified by dose level | .8 | .4 | .03 |
| . | Univariable pairwise P values for CRS grade . | ||
|---|---|---|---|
| 0 vs 1-3 . | 0 vs 4-5 . | 1-3 vs 4-5 . | |
| Marrow burden of disease (%) | <.0001 | .0001 | .8 |
| Platelet count | .01 | .005 | .06 |
| CAR T-cell dose level | .7 | .005 | .0003 |
| Bulk CD8+ T-cell selection | .0005 | .12 | .7 |
| Fludarabine/cyclophosphamide stratified by dose level | .8 | .4 | .03 |
Mitigation of toxicity by reduction in peak CAR T-cell counts in blood will be associated with reduced response rates
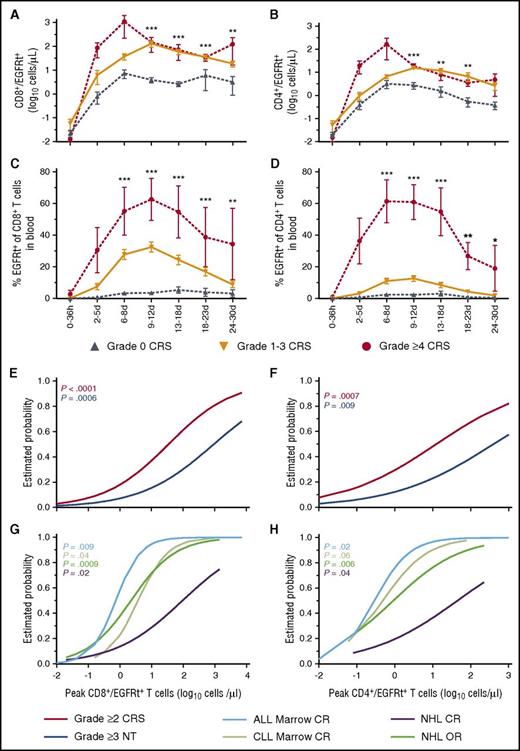
Consistent with the observation that a high CAR T-cell dose and cyclophosphamide/fludarabine lymphodepletion were associated with severity of CRS, our data found earlier and higher peaks in absolute CAR T-cell numbers in blood of patients with grade ≥4 CRS compared with grade 1 to 3 or no CRS (Figure 5A-D). To identify a therapeutic window of absolute CAR T-cell numbers that would minimize the risk of CRS and neurotoxicity while retaining a high probability of antitumor activity in each disease, we modeled the relationship between the peak CAR T-cell counts in blood and the occurrence of toxicity or disease response by using logistic regression (Figure 5E-H). B-ALL patients who achieved a peak of 10 CD8+ CAR T cells per μL had an estimated probability of minimal residual disease (MRD)–negative CR of 95%, and the estimated probabilities of grade ≥2 CRS and grade ≥3 neurotoxicity were 37% and 15%, respectively. Similar findings were noted for patients with 5 CD4+ CAR T cells per μL (MRD-negative CR, 94%; grade ≥2 CRS, 42%; grade ≥3 neurotoxicity, 19%). Reduction of the infused CAR T-cell dose in B-ALL patients with high marrow tumor burden resulted in consistent targeting of peak CAR T-cell counts in the ranges associated with high efficacy without undue toxicity2 (supplemental Table 5); however, the therapeutic window was narrow. The probabilities of marrow response and toxicity in CLL patients were similar to those in patients with B-ALL. In NHL patients, a therapeutic window with high efficacy and low toxicity could not be established. These data suggest that CAR T-cell dose reduction as a sole strategy to mitigate toxicity will reduce efficacy and that early intervention approaches that do not involve reduction in the peak CAR T-cell counts in blood should be investigated.
CAR T-cell counts in blood and estimated probabilities of response or toxicity. (A-B) The absolute number and (C-D) percentage of CD8+ (left) and CD4+ (right) CAR T-cells in blood. The mean ± SEM of the maximum values are shown. P values were determined by using the Kruskal-Wallis test. (E-F) Estimated probabilities by logistic regression of grade ≥2 CRS and grade ≥3 neurotoxicity at (E) peak CD8+ and (F) CD4+ CAR T-cell counts in blood. (G-H) Estimated probabilities by logistic regression of bone marrow CR in ALL and CLL patients by flow cytometry and CR or overall response (OR) in NHL patients according to Cheson imaging criteria (2014) at peak (G) CD8+ and (H) CD4+ CAR T-cell counts in blood. Lymph node CR in CLL patients is not depicted because of the limited cohort size available for analysis. P values are color-coded to indicate the association between the CAR T-cell peak counts and outcomes. ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005.
CAR T-cell counts in blood and estimated probabilities of response or toxicity. (A-B) The absolute number and (C-D) percentage of CD8+ (left) and CD4+ (right) CAR T-cells in blood. The mean ± SEM of the maximum values are shown. P values were determined by using the Kruskal-Wallis test. (E-F) Estimated probabilities by logistic regression of grade ≥2 CRS and grade ≥3 neurotoxicity at (E) peak CD8+ and (F) CD4+ CAR T-cell counts in blood. (G-H) Estimated probabilities by logistic regression of bone marrow CR in ALL and CLL patients by flow cytometry and CR or overall response (OR) in NHL patients according to Cheson imaging criteria (2014) at peak (G) CD8+ and (H) CD4+ CAR T-cell counts in blood. Lymph node CR in CLL patients is not depicted because of the limited cohort size available for analysis. P values are color-coded to indicate the association between the CAR T-cell peak counts and outcomes. ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005.
Early identification of patients at high risk of severe CRS
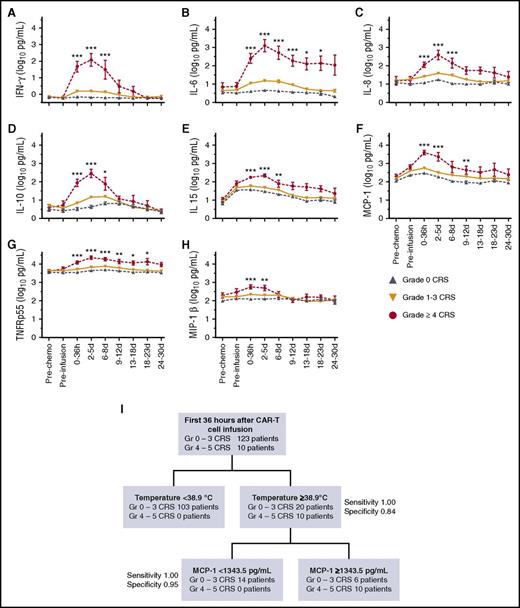
We investigated whether patients who would subsequently develop life-threatening CRS could be identified early after CAR T-cell infusion so that intervention strategies that might prevent progression of CRS could be started. All patients who developed grade ≥4 CRS had fever ≥38.9°C within the first 36 hours after CAR T-cell infusion; however, using fever ≥38.9°C within 36 hours as the only indication for intervention would have resulted in unnecessary treatment of 20 patients with grade ≤3 CRS (sensitivity, 1.00; specificity, 0.84). Patients who developed grade ≥4 CRS also exhibited higher concentrations of interferon-γ, interleukin-6 (IL-6), IL-8, IL-10, IL-15, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor receptor p55 (TNFRp55), and macrophage inflammatory protein-1β (MIP-1β) within 36 hours after CAR T-cell infusion compared with those with grade ≤3 CRS (P < .0001; Figure 6A-H). These cytokines were elevated before the onset of grade ≥3 CRS and demonstrated similar kinetics in B-ALL, CLL, and NHL patients (supplemental Figure 5), suggesting that 1 or more of these cytokines could be useful as predictive biomarkers for grade ≥4 CRS (Figure 6A-H). We performed classification-tree modeling and found that in patients with fever ≥38.9°C within 36 hours of CAR T-cell infusion, a serum MCP-1 concentration ≥1343.5 pg/mL performed better than CRP, ferritin, or other cytokines and enhanced identification of patients who developed grade ≥4 CRS (sensitivity, 1.00; specificity, 0.95) (Figure 6I). By using this approach, 6 (4.5%) of 133 patients were misclassified as being at high risk of grade ≥4 CRS, 4 of whom developed grade ≥2 CRS and/or neurotoxicity, indicating that unnecessary early intervention would uncommonly be used for patients who did not develop moderate or severe CRS and/or neurotoxicity.
Biomarkers for early prediction of grade ≥4 CRS. (A-H) Concentrations of listed cytokines in serum obtained from patients at the indicated time points. P values were determined by using the Kruskal-Wallis test. (I) An algorithm for early identification of patients at high risk of grade ≥4 CRS using classification-tree modeling. Early high fever (≥38.9°C) within the first 36 hours after CAR T-cell infusion triggers evaluation of serum MCP-1 concentration. Patients with fever ≥38.9°C and serum MCP-1 ≥1343.5 pg/mL are at high risk for subsequent development of grade ≥4 CRS. ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005.
Biomarkers for early prediction of grade ≥4 CRS. (A-H) Concentrations of listed cytokines in serum obtained from patients at the indicated time points. P values were determined by using the Kruskal-Wallis test. (I) An algorithm for early identification of patients at high risk of grade ≥4 CRS using classification-tree modeling. Early high fever (≥38.9°C) within the first 36 hours after CAR T-cell infusion triggers evaluation of serum MCP-1 concentration. Patients with fever ≥38.9°C and serum MCP-1 ≥1343.5 pg/mL are at high risk for subsequent development of grade ≥4 CRS. ***P < .0001; **P ranges from >.0001 to <.001; *P ranges from >.001 to <.005.
Discussion
The success of CD19-specific CAR T-cell immunotherapy for treating patients with relapsed/refractory B-cell malignances has resulted in an increase in the number of clinicians who are required to manage the novel toxicities associated with this therapy. We describe the clinical presentation, laboratory findings, and correlative and predictive biomarkers of CRS in a large cohort of patients who received lymphodepletion chemotherapy and CD19 CAR T cells to guide physicians caring for these patients. CRS was a frequent event after CAR T-cell immunotherapy, occurring in 70% of patients; however, in a majority of patients, it was mild to moderate and resolved within days without a requirement for tocilizumab or dexamethasone intervention. Life-threatening CRS was uncommon (7.5%) and mainly occurred during the CAR T-cell dose escalation phase of our study. At the CAR T-cell MTD, grade ≥4 CRS was rare (2.5%). Despite the potential for severe toxicity, a strategy of hospital admission at the first fever made outpatient lymphodepletion chemotherapy and CAR T-cell infusion a feasible approach, even in heavily pretreated patients with advanced B-cell malignancies.
The risk of CD19 CAR T-cell therapy could potentially be reduced by identifying patients who are at high risk of developing severe CRS before therapy and modifying the treatment regimen or recognizing them early after CAR T-cell infusion when preventative interventions could be instituted. Multivariable analysis identified baseline and treatment-related risk factors for CRS, including those associated with more robust CAR T-cell expansion, such as higher marrow tumor burden, cyclophosphamide/fludarabine lymphodepletion, and higher CAR T-cell dose. Other pretreatment factors that were associated with CRS, such as thrombocytopenia and manufacturing of CAR T cells from bulk CD8+ T cells, may be a reflection of the higher tumor burden in these patients; however, distinct mechanisms cannot be excluded.
Because in vivo CAR T-cell expansion is driven by recognition of cells expressing CD19, a logical approach to reducing the risk of severe CRS is to reduce the CAR T-cell dose in patients with a high tumor burden. This strategy was effective in mitigating toxicity in B-ALL patients without impairing efficacy.2 However, our analyses indicate that the therapeutic window is narrow and that a reduction in CAR T-cell dose that results in peak CD8+ CAR T-cells <10 cells per μL and CD4+ CAR T cells <5 per μL will likely result in reduced efficacy. This is particularly true in NHL, in which the probabilities of CR, grade ≥2 CRS, and grade ≥3 neurotoxicity were similar at any given peak CAR T-cell count.
The risk of impaired efficacy with CAR T-cell dose reduction suggests that the optimal strategy might enable delivery of an adequate CAR T-cell dose followed by early intervention in those patients who exhibit clinical or laboratory findings associated with a high risk of subsequent toxicity. We previously reported that increases in distinct serum cytokines within the first day after CAR T-cell infusion were associated with subsequent requirement for ICU care.1,2 An association of these cytokines with subsequent grade ≥4 CRS was confirmed in the current larger cohort of ALL, CLL, and NHL patients. A predictive algorithm based on evaluating multiple cytokine concentrations (as reported in pediatric ALL21 ) may be complex and expensive to implement. Therefore, we used classification-tree modeling to design a simple 2-step algorithm to predict grade ≥4 CRS in which a single serum cytokine concentration was measured only in the small subset of patients with fever ≥38.9°C within 36 hours of infusion. The model was designed to identify patients who would develop life-threatening CRS, despite appropriate intervention with tocilizumab and/or dexamethasone for grade 2 to 3 CRS. The best sensitivity and specificity was obtained by testing serum MCP-1 in patients with fever ≥38.9°C within 36 hours of infusion. Fever and CRP evaluation have previously been used to identify those at risk of severe toxicity9 ; however, in our study, MCP-1 evaluation was superior to CRP testing. The optimal preemptive therapy of high-risk patients is unknown. Although widely used approaches to treat severe CRS (eg, tocilizumab 8 mg/kg intravenously and dexamethasone 8 mg/kg intravenously twice per day) might be suitable, these and other strategies to modify cytokine signaling should be studied in suitably designed clinical trials.
The presentation of vascular instability, capillary leak, and consumptive coagulopathy suggested that endothelial activation or dysfunction coincides with severe CRS. This was confirmed by demonstrating that severe CRS was accompanied by high serum concentrations of VWF and Ang-2, which are released from Weibel-Palade bodies on endothelial activation. The mechanisms that lead to endothelial activation in CRS have not been characterized; however, the high serum concentrations of endothelium-activating cytokines, such as IL-6 and interferon-γ, observed in patients with severe CRS suggest that these cytokines may contribute. Additional studies will be required to determine whether regulation of endothelial activation, for example by modification of the Ang-1/2 axis, could be used to treat patients with severe CRS, as proposed for patients with infection-related microvascular dysfunction.31 We also found that serum VWF and the Ang-2:Ang-1 ratio were higher before starting CAR T-cell immunotherapy in patients who subsequently developed more severe CRS, suggesting that preexisting endothelial activation might be a previously unrecognized risk factor for severe CRS. It is noteworthy that thrombocytopenia before lymphodepletion chemotherapy was also associated with subsequent severe CRS. Platelets are one of the few sources of the endothelial stabilizing cytokine Ang-1, suggesting that patients with severe thrombocytopenia might be prone to endothelial activation.26
CRS is a common AE after CAR T-cell therapy, but it is well tolerated in most patients who receive an optimized lymphodepletion regimen and dose of CAR T cells. The safety profile supports delivery of this treatment in the outpatient setting, and effective therapies are available for most patients who develop severe CRS. Additional understanding of the risk factors and mechanisms that lead to severe CRS will facilitate testing of interventions to prevent or reverse toxicity and improve the safety of CD19 CAR T cells, and potentially of CAR T cells targeting other malignancies.32
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Fred Hutchinson Cancer Research Center Cell Processing Facility, Seattle Cancer Care Alliance (SCCA) Cell Therapy Laboratory, and the staff of the Program in Immunology and SCCA Immunotherapy Clinic.
This work was supported by National Institutes of Health (NIH) National Cancer Institute grants R01 CA136551 and P30 CA15704, NIH National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK56465, Life Science Discovery Fund, Bezos Family Foundation, University of British Columbia Clinical Investigator Program, and Juno Therapeutics, Inc.
Authorship
Contribution: K.A.H. and J.G. collected and analyzed research data; S.C. and X.C. collected research data; L.-A.H., W.C.L., M.M.W., J.A.L., J.C., D.C., and S.H.-B. designed and performed experiments; D.L. performed statistical analyses; K.A.H., S.R.R., D.G.M., and C.J.T. wrote and edited the manuscript; and all authors reviewed the final version of the manuscript.
Conflict-of-interest disclosure: C.J.T., S.R.R., and D.G.M. receive research funding from Juno Therapeutics, Inc. D.L. is an employee of and has equity interests in Juno Therapeutics, Inc. S.R.R. has equity interests in Juno Therapeutics, Inc. C.J.T., S.R.R., W.C.L., and D.L. are named as inventors on 1 or more patents or patent applications related to this work. The Fred Hutchinson Cancer Research Center receives research funding from Juno Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Kevin A. Hay, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: khay@fredhutch.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal