Abstract
New therapies for hemophilia A and hemophilia B will likely continue to change clinical practice. Ranging from extended half-life to nonfactor products and gene therapy, these innovative approaches have the potential to enhance the standard of care by decreasing infusion frequency to increase compliance, promoting prophylaxis, offering alternatives to inhibitor patients, and easing route of administration. Each category has intrinsic challenges that may limit the broader application of these promising therapies. To date, none specifically address the challenge of dispersing treatment to the developing world.
Introduction
The field of novel therapeutics for hemophilia has seen significant advances in recent years. Several extended half-life (EHL) products are available and nonfactor therapies (NFTs), such as a bispecific antibody mimicking activated factor VIII (FVIIIa) for hemophilia A (HA), small interfering RNA (siRNA) to antithrombin (AT), and antibodies to tissue factor pathway inhibitor (TFPI), are in clinical trials. These provide the benefits of prophylaxis and reduce the need for frequent injections. Gene therapy trials are reporting data that are fulfilling expectations with some studies ameliorating phenotype from severe to mild and even achieving curative factor levels after a single vector injection. The early success of these therapies warrants restrained optimism regarding their long-term safety, efficacy, and potential to reduce bleeding frequency, especially in patients with inhibitors.
EHL factor products
Recently, modifications to optimize the half-life of factor products have become the focus of product development. Several EHL products have emerged, affording patients alternatives with less frequent infusions compared with standard products for both prophylaxis and on-demand therapy. Several extensive comparative reviews of EHL recombinant (r)FVIII, rFIX, and rFVIIa products are available.1-3 Half-life (t1/2) prolongation techniques include the following: (1) site directed or non–site-specific pegylation,4-6 (2) fusion with prolonged half-life proteins, such as IgG-Fc or albumin,7-12 and (3) protein modifications.
EHL-rFIX products have successfully prolonged t1/2 2.5- to 5-fold, decreasing infusion frequency to 7 to 14 days depending on the product. The half-lives of rFIX-FP (albumin fused) and N9-GP (pegylated) are longer than rFIX-Fc, allowing every 10- to 14-day dosing; however, comparative studies are lacking.9,10,13 Preclinical studies are now investigating how prolonged t1/2 might translate to improved clinical outcomes. In hemophilia B (HB) mice, improved joint wound healing was observed with a single dose of glycopegylated rFIX compared with repeated rFIX infusions.14 However, rFIX and rFIX-Fc exhibited equivocal hemostatic efficacy in a murine saphenous vein bleeding model, thought to be secondary to similar extravascular rFIX pools.15
Regardless of strategy, EHL-rFVIII products all prolong t1/2 approximately only 1.5-fold rFVIII, allowing for a decrease in infusion frequency to twice instead of thrice weekly. Why EHL technologies prolong rFIX t1/2 more than rFVIII has been a subject of ongoing research. Most FVIII circulates with von Willebrand factor (VWF) via a noncovalent interaction between FVIII-C1, -C2, and -a3 and the D′D3 region of VWF.16 Clearance of VWF and FVIII occurs mostly as a complex through a variety of receptors in hepatocytes, macrophages, and sinusoidal endothelium and is influenced by many host, receptor, and VWF-related parameters.16 VWF t1/2 is ∼15 hours, which may impose a ceiling on EHL-rFVIII half-life prolongation. Hypothesizing that EHL-rFVIII products rely on endogenous VWF for circulation, the ability of VWF D′D3 molecules fused with half-life extending domains to decrease FVIII clearance is being investigated.17,18
The SIPPET trial demonstrates improved immunogenicity profile in VWF-enriched plasma-derived (pd) FVIII compared with rFVIII products19,20 ; whether EHL-rFVIII products will provide a tolerance advantage compared with other FVIII products remains unknown. Animal data suggest that Fc fusion protein may be more likely to induce tolerance by expansion of regulatory T cells.21 Although there are case reports of first-line and salvage immune tolerance induction (ITI) using rFVIIIFc,22,23 whether it offers a tolerance advantage remains to be addressed in ongoing clinical trials (#NCT03093480 and #NCT03103542).
Although EHL products are promising, the optimal strategy for treatment of bleeds between prophylactic doses and dosing regimens will likely need to be individualized to patient pharmacokinetics accounting for age and physical activity. Nevertheless, these are attractive options for young patients to potentially avoid central lines and may offer alternatives for ITI, but it remains to be seen if these products have better immunologic profiles than pdFVIII and/or rFVIII. Attempts at administering these products subcutaneously should be cautiously approached, because trials of subcutaneous pdFIX before rFIX were associated with inhibitor development.24 Post–licensure surveillance of these products will better inform the risk of inhibitors and long-term safety. Finally, caution must be applied in development and administration of these products with modified primary sequence as demonstrated by the development of cross-reactive antibodies to FVIIa with some EHL rFVIIa products.25-27
NFTs
Several strategies designed to provide a procoagulant prophylactic effect, without factor replacement, are rapidly progressing through clinical development, including FVIII-mimetics and inhibition of endogenous anticoagulants.28 Attributes of these approaches are that (1) they are not neutralized by antifactor inhibitors and (2) subcutaneous delivery with attractive pharmacokinetic parameters allows for weekly to monthly dosing.
Emicizumab (ACE910; Hoffman-La Roche Pharmaceutical) is a bispecific humanized monoclonal antibody with arms that recognize FIXa or FX that is designed to mimic FVIIIa function; simultaneous binding of emicizumab to FIXa and FX sufficiently orients these coagulation factors to allow for efficient FIXa-catalyzed proteolytic activation of FX, without FVIIIa cofactor activity.29 Emicizumab has demonstrated efficacy in preventing bleeding in HA patients with and without inhibitors.30,31 In the recent phase 3 study, inhibitor subjects that received emicizumab prophylaxis (n = 35) had an annualized bleeding rate (ABR) of 3 compared with 23 in untreated subjects (n = 18, P < .001), whereas subjects previously on standard bypassing regimens for prophylaxis (n = 24) decreased their ABR by 80% with emicizumab prophylaxis (from 16 to 3, P < .001).30 Plasma levels of emicizumab in this study are estimated to be hemostatically equivalent to 10% to 15% of normal FVIII activity with weekly subcutaneous injections.30,32 However, 47% of subjects that received emicizumab prophylaxis still required activated prothrombin complex concentrate (aPCC) and/or rFVIIa administration. Three subjects that received aPCC developed thrombotic microangiopathy, 1 of which continued to have serious bleeding and died of anemia after refusing red cell transfusion. In addition, 2 subjects that received aPCC had venous thrombotic complications. Preliminary ex vivo studies suggest that aPCC enhances the procoagulant effect of FVIII-mimetics much more than rFVIIa.33,34 This is likely due to the presence of FIXa,35 the catalytic efficiency of which is enhanced 20 000-fold by emicizumab.29 Thus, even small amounts of FIXa may become highly potent. These adverse events emphasize the potential problems with manipulating the coagulation system to promote hemostasis through unregulated mechanisms. How best to combine nonfactor and factor therapies will likely remain an important issue requiring additional studies.
Other NFTs target endogenous anticoagulants, including AT, TFPI, and activated protein C (APC), with the rationale that decreasing these pathways may offset the procoagulant deficiency. Fitusiran (Alnylam Pharmaceuticals) is an AT siRNA therapeutic36 that has entered early-phase clinical trials for patients with hemophilia with and without inhibitors.37,38 Subcutaneous injections of fitusiran resulted in dose-dependent decreases in AT levels.37 Notably, only reductions of AT levels to ≤20% normalized ex vivo thrombin generation of plasma from hemophilia subjects without inhibitors; this reduction appeared to be efficacious in preventing bleeding, with 10 out of the 12 subjects decreasing their ABR or maintaining no bleeds.37 However, the remaining 2 subjects increased their ABR.37 Bleeding was acutely managed mostly with 1 to 2 doses of factor replacement without reported complications.37 Several approaches are also being investigated to inhibit TFPI.39-45 Concizumab (Novo Nordisk), a humanized monoclonal antibody against TFPI, has advanced the furthest in clinical development. Results from the concizumab phase 1 study (n = 24) demonstrate a dose-dependent decrease in TFPI levels after subcutaneous delivery and no pathological bleeding when TFPI levels are ≤20% baseline,39 which was also associated with normalization of ex vivo thrombin generation.41 Alternative anti-TFPI antibodies are also in therapeutic development.42,43 However, the emerging complexity of the biology of TFPI46 as well as the experience of a TFPI inhibitory aptamer that increased bleeding likely secondary to paradoxically increasing the half-life of TFPI44,45 mandates careful clinical progression. Targeting the anticoagulant effect of APC has also restored hemostasis in hemophilia mouse models.47,48
NFTs appear to be able to improve hemostasis in hemophilia patients, likely including those with inhibitors; however, they do not currently appear to be able to prevent all bleeding. The experience with emicizumab should engender caution about the potential for thrombotic consequences especially when combining therapies. Treatment with fitusiran and concizumab were both associated with elevated D-dimer levels in some subjects,37,39 although the clinical relevance of this observation is unknown. However, a recent report of a fatal thrombotic event in an HA subject on fitusiran raises continued safety concerns.49 Global coagulation assays may assist with determining efficacious levels of these therapeutics. NFTs are also susceptible to antidrug antibodies, with ∼4% of emicizumab30,32 and fitusiran subjects37 demonstrating confirmed or suspected antidrug antibodies, which is consistent with other macromolecular therapeutics50 and may compromise efficacy. No antidote to these therapies exists, except AT protein, which should temporally reverse fitusiran.
Gene therapy
Over the last 2 decades, preclinical and clinical studies on adenoassociated viral (AAV) vector-based gene therapy for hemophilia have identified successful strategies.51-54 Because of the limited packing capacity of AAV vectors, most of the early studies were focused on HB because of the small size of the F9 gene, but early clinical data are now emerging for HA.
All current studies use a codon optimized transgene under the control of a liver-specific promoter to restrict expression to hepatocytes.55,56 There are several AAV vector serotypes being investigated, but all exhibit liver tropism that allows vector delivery by peripheral intravascular injection. Two ongoing trials are using AAV serotype 5 (AAV5), manufactured with baculovirus transduction systems and insect cell lines,57,58 whereas the others are using plasmid-based systems in mammalian cell lines for AAV8,56,59 AAVSpark100,60 and AAVrh10. Although these studies all use an AAV vector, from the product perspective, they are very different drugs.
The immunogenicity of AAV is the main limitation.61 The presence of neutralizing antibodies (NAb) to the AAV capsid, which occur in ∼30% to 40% of the general population depending on the serotype, precludes effective gene transfer62 ; subjects with NAbs are excluded from current trials. The second limitation is AAV capsid-mediated cellular immune response resulting in transient hepatotoxicity with an increase in liver enzyme levels (alanine aminotransferase [ALT]) and, if left untreated, loss of transgene expression.55 This complication is clearly vector dose–dependent, and the onset depends on the vector serotype ranging from 4 to 10 weeks.51 Currently, it is not possible to predict who will develop this immune response; thus, minimizing the vector dose is a feasible and safe strategy.
The first long-term expression of AAV liver gene therapy from a dose-escalation study on AAV8 using FIX-wild type resulted in FIX expression levels of 1.5% to 4% in the low- (n = 2) and mid- (n = 2) dose cohort with reduction of ABR.56,59 In the high-dose cohort, 2 × 1012 vg/kg (vector genomes per kilogram), FIX levels rose to ∼5% with normal specific activity and 90% reduction of ABR, confirming the biological activity of the transgene. However, in this cohort, ALT levels increased in 4/6 (60%) subjects from weeks 7 to 10, and oral steroids were successfully used to control the cellular immune response. Over 3.2 years, there was no evidence of adverse events; transgene expression levels and the improvement of the disease phenotype remained stable.
Because of the vector-dose dependency of AAV-mediated cellular immune response, the next generation of studies took advantage of a FIX variant with 8- to 10-fold enhanced protein-specific activity. Identified in a man with venous thrombosis with FIX activity >700%, FIX-Padua results from substitution of arginine to a leucine at position 338.63 The use of FIX-Padua in preclinical studies, including inhibitor-prone HB canine model, showed excellent safety profile without increased immunogenicity.64-67 Two clinical studies have used this variant. The study by Spark Therapeutics is using AAV-FIX-Padua at a single dose of 5 × 1011 vg/kg (n = 10) with FIX levels ∼30%; all subjects have stopped prophylaxis for ≥12 weeks (ongoing).60 Notably, no immune response to FIX-Padua was noted. Although 2 out of 10 subjects developed increased ALT levels after 4 weeks, initiation of oral steroids prevented total FIX expression loss. Thus, by reducing the vector dose fivefold from the initial AAV8 trial, FIX-Padua resulted in sixfold higher expression levels while limiting the risk of capsid-mediated immune response to only 20%. Previously, Shire carried out a study of AAV8-FIX-Padua (n = 8).68,69 Only 1 subject exhibited stable FIX levels of 20% over 2.5 years, whereas the remainder of subjects only exhibited transient expression for unclear reasons. The use of FIX-Padua provides a safe strategy to lower the therapeutic vector dose while modifying the disease phenotype to a mild range.
Other studies for FIX-wild type by Dimension and Uniqure used AAVrh10 and AAV5, respectively. Uniqure carried out a study of AAV5 at 2- to >10-fold higher than the therapeutic doses used in the studies described above to achieve levels of 5% to 7%, whereas ALT increased in 3 subjects (30%).57 Data from the use of AAVrh10 are not available. Zinc finger–mediated nucleases for in vivo gene editing delivered by AAV vectors to replace a normal copy of the F9 gene for HB is ongoing (#NCT02695160), but no public data have been disclosed by Sangamo Therapeutics.
The only HA gene therapy study with reported results is by Biomarin using AAV5-FVIII-B-domain deleted,58 with identical primary sequence as current factor products.70 A total of 15 patients were enrolled. In the low- (n = 1) and mid-dose (n = 1) cohort of 6 × 1012 and 2 × 1013 vg/kg, respectively, only 1 subject increased his FVIII levels, to 2%. In the high-dose cohort of 6 × 1013 vg/kg, FVIII levels, surprisingly, ranged from 40% to 150% over an 8-month period (ongoing); all subjects stopped prophylaxis, and ABR was reduced by 97%. ALT increased in 10/15 subjects, and prophylactic therapy with steroids was initiated at week 4. There is no reported immune response to FVIII.
Collectively, these emerging data are very encouraging. Long-term follow-up, however, is needed to better assess the impact on the disease phenotype and quality of life. A promising strategy has to consider both the prevalence of NAbs to the vector capsid and the vector therapeutic dose, because the latter will impact both the rates of capsid-mediated immune responses and the manufacturing limitations associated with large-scale production.71 Transient immunosuppression may not be efficacious in some situations as reported.61 A better understanding of why the capsid-mediated toxicity occurs at significantly different vector doses for a given AAV serotype is needed.
All of these studies enrolled adult subjects heavily exposed to FIX or FVIII without any evidence or history of inhibitors. The risk of the immune response to the transgene, however, will likely become a prominent concern in future studies that include pediatric patients with fewer exposure days. Encouragingly, preclinical studies support the concept that liver gene therapy may provide benefits of ITI by continuous endogenous expression of the transgene and inhibitor eradication with subsequent increased levels of the transgene and amelioration of the disease phenotype.64,72,73
Conclusion
In a relatively short period of time, providers will likely be contemplating diverse therapeutic options for the treatment of hemophilia with both distinct limitations and advantages (Figure 1; Table 1). Some therapies are likely to be adjuvants of the current treatments and may facilitate the benefits of prophylaxis for standard patients and those who currently have limited options, such as patients with refractory inhibitors. Further development of antidotes specific for nonfactor replacement will improve safety. Curative strategies with gene therapy are likely feasible, but patients with NAbs will not be eligible. New approaches to overcome this limitation are needed, because immunosuppression and plasmapheresis are not efficacious for high-titer NAbs.51 Lentiviral vectors could be an alternative for these patients; however, data from translational studies in large animal models using in vivo or ex vivo strategies are challenging.74,75 Last, the excitement over the potential of these novel therapies to improve the lives of people of with hemophilia in the developed world should not conceal the economic reality that, because of costs, >75% of the hemophilia worldwide population (∼350 000) is not regularly, if at all, treated. Closing this treatment gap between patients in the developed and the developing world remains an unmet ethical imperative. It would be remarkable if some of these technologies could be adapted to economically sensitive therapies to address this critical problem.
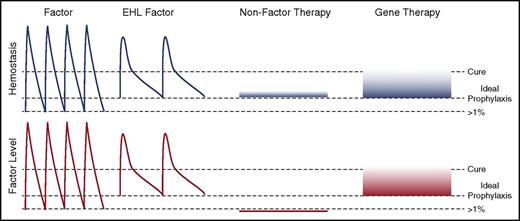
Novel approaches to hemophilia therapy. The peaks and troughs of factor levels (red) and provided hemostasis (blue) differ substantially between the classes of therapies. Current prophylaxis regimens with standard factor therapies aim for trough levels of >1% normal with dosing from every other day to twice weekly. EHL therapies decrease the frequency of administration and likely can provide higher trough levels. NFTs are ideally being dosed at an equivalent hemostatic level to prevent pathological bleeding. This may be achievable with weekly to monthly subcutaneously delivery that results in very stable hemostatic, but no factor level. Gene therapy is likely to be able to provide a sustained factor level that approaches a cure.
Novel approaches to hemophilia therapy. The peaks and troughs of factor levels (red) and provided hemostasis (blue) differ substantially between the classes of therapies. Current prophylaxis regimens with standard factor therapies aim for trough levels of >1% normal with dosing from every other day to twice weekly. EHL therapies decrease the frequency of administration and likely can provide higher trough levels. NFTs are ideally being dosed at an equivalent hemostatic level to prevent pathological bleeding. This may be achievable with weekly to monthly subcutaneously delivery that results in very stable hemostatic, but no factor level. Gene therapy is likely to be able to provide a sustained factor level that approaches a cure.
Novel therapeutics for hemophilia
| Product . | Mechanism . | Advantages . | Limitations . | Status . | ||||
|---|---|---|---|---|---|---|---|---|
| Dosing frequency . | Route . | Relative ease of compliance . | Immunogenicity . | Monitoring . | Study population . | |||
| EHL-rFVIII | ||||||||
| Efmoroctocog alfa (BDD-rFVIII-Fc, Eloctate)7 | IgG1-Fc fusion | Every 3-5 d | IV | Low | 3% NNA | Standard | PTP | Approved |
| Rurioctacog alfa pegol (BAX 855, Adynovate)8 | 20-kDa pegylation | Twice weekly | IV | Low | 4% NNA | Standard | PTP | Approved |
| Damoctocog alfa pegol (BAY 94-9027)4 | 60-kDa site-specific pegylation | Every 3-7 d | IV | Low | 3% NNA0.6% anti-PEG | Chromogenic* | PTP | Phase 3 |
| Turoctocog alfa pegol (N8-GP)5 | 40-kDa site-specific pegylation | Every 4 d | IV | Low | 0.6% NNA | TBD | PTP | Phase 3 |
| 0.6% NAb† | ||||||||
| EHL-rFIX | ||||||||
| Eftrenonacog alfa (FIX-Fc, Alprolix)9 | IgG1-Fc fusion | Every 7-10 d | IV | Medium | 0.8% NNA | Standard* | PTP | Approved |
| Albutrepenonacog alfa (FIX-FP, Idelivion)10 | Albumin fusion | Weekly to bimonthly | IV | Medium | 0% NNA | Standard* | PTP | Approved |
| Nonacog β pegol (N9-GP, Rebinyn)13 | 40-kDa site-specific pegylation | Weekly | IV | Medium | 4% NNA | Chromogenic* | On demand only in USA | Approved |
| NFTs | ||||||||
| Emicizumab30-32 | Bispecific antibody FVIIIa-mimetic | Weekly | SQ | Medium | 3% NNA | TBD | HA with inhibitor | Phase 3 |
| Fitusiran36,37 | AT siRNA | Weekly to monthly | SQ | Medium | 4% NNA | TBD | HA/HB without inhibitor | Phase 1/2 |
| Concizumab39 | TFPI monoclonal antibody | TBD | IV/SQ | TBD | 0% | TBD | HA/HB without inhibitor | Phase 1/2 |
| Gene therapy | ||||||||
| SPK-900160 | Endogenous expression with AAV vectors | Vector dependent | IV | High | 0% | Standard | HA/HB without inhibitor; no NAb to vector | Phase 1/2 |
| AMT-06057 | ||||||||
| BMN27058 | ||||||||
| Product . | Mechanism . | Advantages . | Limitations . | Status . | ||||
|---|---|---|---|---|---|---|---|---|
| Dosing frequency . | Route . | Relative ease of compliance . | Immunogenicity . | Monitoring . | Study population . | |||
| EHL-rFVIII | ||||||||
| Efmoroctocog alfa (BDD-rFVIII-Fc, Eloctate)7 | IgG1-Fc fusion | Every 3-5 d | IV | Low | 3% NNA | Standard | PTP | Approved |
| Rurioctacog alfa pegol (BAX 855, Adynovate)8 | 20-kDa pegylation | Twice weekly | IV | Low | 4% NNA | Standard | PTP | Approved |
| Damoctocog alfa pegol (BAY 94-9027)4 | 60-kDa site-specific pegylation | Every 3-7 d | IV | Low | 3% NNA0.6% anti-PEG | Chromogenic* | PTP | Phase 3 |
| Turoctocog alfa pegol (N8-GP)5 | 40-kDa site-specific pegylation | Every 4 d | IV | Low | 0.6% NNA | TBD | PTP | Phase 3 |
| 0.6% NAb† | ||||||||
| EHL-rFIX | ||||||||
| Eftrenonacog alfa (FIX-Fc, Alprolix)9 | IgG1-Fc fusion | Every 7-10 d | IV | Medium | 0.8% NNA | Standard* | PTP | Approved |
| Albutrepenonacog alfa (FIX-FP, Idelivion)10 | Albumin fusion | Weekly to bimonthly | IV | Medium | 0% NNA | Standard* | PTP | Approved |
| Nonacog β pegol (N9-GP, Rebinyn)13 | 40-kDa site-specific pegylation | Weekly | IV | Medium | 4% NNA | Chromogenic* | On demand only in USA | Approved |
| NFTs | ||||||||
| Emicizumab30-32 | Bispecific antibody FVIIIa-mimetic | Weekly | SQ | Medium | 3% NNA | TBD | HA with inhibitor | Phase 3 |
| Fitusiran36,37 | AT siRNA | Weekly to monthly | SQ | Medium | 4% NNA | TBD | HA/HB without inhibitor | Phase 1/2 |
| Concizumab39 | TFPI monoclonal antibody | TBD | IV/SQ | TBD | 0% | TBD | HA/HB without inhibitor | Phase 1/2 |
| Gene therapy | ||||||||
| SPK-900160 | Endogenous expression with AAV vectors | Vector dependent | IV | High | 0% | Standard | HA/HB without inhibitor; no NAb to vector | Phase 1/2 |
| AMT-06057 | ||||||||
| BMN27058 | ||||||||
NAb, neutralizing antibodies; NNA, nonneutralizing antibodies; PTP, previously treated patients; SQ, subcutaneously; TBD, to be determined.
Differing results may be obtained with specific aPTT reagents; for detailed information on performance of standard vs chromogenic assays with these products, see Kitchen et al.76
One patient developed inhibitor (initially low titer and then high at 13.5 BU) after 93 exposure days to N8-GP in phase 3 trial.
Authorship
Contribution: V.R.A., B.S.D., and B.J.S.-J. researched, wrote, and edited the manuscript.
Conflict-of-interest disclosure: B.J.S.-J. is an investigator on AAV gene therapy clinical trials for hemophilia sponsored by Spark Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Valder R. Arruda, The Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, 5056 Colket Translational Research Center, Philadelphia, PA 19104; e-mail: arruda@e-mail.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal