Key Points
First characterization of neomorphic H3K27 mutations in AML.
H3K27 mutations are associated with and collaborate with RUNX1 mutations and translocations.
Abstract
Neomorphic missense mutations affecting crucial lysine residues in histone H3 genes significantly contribute to a variety of solid cancers. Despite the high prevalence of H3K27M mutations in pediatric glioblastoma and their well-established impact on global histone H3 lysine 27 di- and trimethylation (H3K27me2/3), the relevance of these mutations has not been studied in acute myeloid leukemia (AML). Here, we report the first identification of H3K27M and H3K27I mutations in patients with AML. We find that these lesions are major determinants of reduced H3K27me2/3 in these patients and that they are associated with common aberrations in the RUNX1 gene. We demonstrate that H3K27I/M mutations are strong disease accelerators in a RUNX1-RUNX1T1 AML mouse model, suggesting that H3K27me2/3 has an important and selective leukemia-suppressive activity in this genetic context.
Introduction
Methylation of histone H3 at lysine 27 (H3K27me) is crucial for transcriptional regulation during cellular development. Furthermore, abnormalities in the level or genomic distribution of H3K27 methylation resulting from mutations affecting polycomb repressive complex 2 (PRC2) are frequent in cancer.1-3
In hematological cancers, a considerable proportion of recurrent mutations are connected to PRC2 activity. Interestingly, these mutations are largely restricted to certain cellular and mutational contexts. For example, gain-of-function mutations of EZH2 at Y6414 resulting in increased H3K27me3 are associated with B-cell lymphoma,5 whereas deleterious mutations in EZH2 and SUZ12 are often observed in T-cell acute lymphoblastic leukemia with wild-type (WT) NOTCH1.6,7
In myeloid malignancies, loss-of-function EZH2 mutations are relatively frequent in myelodysplastic syndromes and myeloproliferative neoplasms8-10 but are generally rarer in de novo acute myeloid leukemia (AML). Relative enrichment for PRC2 mutations has been observed in t(8;21),11 RUNX1-mutant,12 and 7q AMLs,13 whereas they are virtually absent in inv(16) AML11 and AML with MLL rearrangements.14 Mutations in ASXL1, an accessory PRC2 member,15 are predominantly found in RUNX1-mutant AML12 and rarely in MLL-rearranged AML,14 whereas ASXL2 mutations are primarily associated with t(8;21) AML.11,16,17 Despite a large body of evidence supporting selective involvement of PRC2-associated mutations in AML, a majority of these mutations are thought to have pleiotropic effects and mostly subtle impacts on global H3K27me2/3 levels because of remaining functional WT alleles or compensatory mechanisms.
More recently, lysine-to-methionine mutations affecting H3.1K27 or H3.3K27 have been reported in pediatric brain cancers, specifically in diffuse intrinsic pontine glioma and supratentorial glioblastoma multiforme.18-20 Interestingly, K27M mutations are usually only present in 1 allele of several S-phase–regulated H3.1 or replication-independent histone H3.3 genes. Despite their low stoichiometric ratios, these mutations were shown to have a potent inhibitory effect on EZH1/2 enzymes, thereby causing profound and specific reductions in H3K27me2/3,21,22 in contrast to most heterozygous loss-of-function PRC2 mutations in cancer. Although H3K27M mutations are extremely frequent in pediatric gliomas18-20 and occur sporadically in pediatric T-cell acute lymphoblastic leukemia,2 their presence has not been documented in myeloid cancers to date.
Methods
Primary AML specimens
The Leucegene project is an initiative approved by the research ethics boards of Université de Montréal and Maisonneuve-Rosemont Hospital. As part of this project, RNA sequencing of 415 primary AML specimens from various cytogenetic groups was performed as previously described.14 All leukemia samples were collected and characterized by the Leukemia Cell Bank of Québec.
Patients
Patient 04H138 was a 54-year-old man who had undergone allogeneic stem-cell transplantation from a related female donor 7 years before the development of AML with intermediate-risk karyotype (47, XX, +8; female hematopoietic stem-cell transplant derived). The patient did not receive additional intensive therapy and died 146 days after the second diagnosis. No additional samples were available.
Patient 12H183 was an 18-year-old woman diagnosed with AML with t(8;21)(q22;q22) RUNX1-RUNX1T1. She underwent induction therapy (7+3) and received 4 cycles of consolidation therapy with high-dose cytarabine. She relapsed 12 months later and was treated with 7+3 induction followed by allogeneic stem-cell transplantation in second complete remission. No relapse samples were available.
Patient TCGA-AB-2927 was an 88-year-old woman who died 2 months after diagnosis.
Next-generation RNA sequencing and mutation validation
Sequenced data were mapped to the reference genome hg19 according to RefSeq annotations (University of California Santa Cruz; 16 April 2014). Variants were identified using CASAVA version 1.8.2 or km software (https://bitbucket.org/iric-soft/km) and validated by polymerase chain reaction (PCR) amplification of genomic regions and Sanger sequencing.
Flow cytometry analysis of H3K27me2/3
Cells were washed in phosphate-buffered saline, pelleted, and fixed by dropwise addition of ice-cold 95% ethanol under vortexing. Cells were immediately placed at 4°C for 1 hour followed by −20°C for long-term storage. For staining, cells were washed twice and blocked with staining buffer (phosphate-buffered saline with 1% bovine serum albumin and 0.25% Triton). Cells were then stained with mouse monoclonal anti-H3K27me2/3 (Active Motif #39536) and rabbit anti-H3 (Abcam #1791) in staining buffer for 20 minutes at room temperature. After a wash with staining buffer, cells were incubated with secondary antibodies anti-mouse Alexa-647 (Invitrogen #A-21235) and anti-rabbit Alexa-350 (Invitrogen #A-11046). Cells were washed again, and fluorescence-activated cell sorting (FACS) analysis was performed on a BD Biosciences LSR II (Institute for Research in Immunology and Cancer FACS core facility). FACS was performed on a BD FACSAria of cells that were costained with rabbit anti–H3K27me3-Alexa647 (Cell Signaling #12158) and rabbit anti–H3-BV421 (Cell Signaling #12167S).
Sanger sequencing of somatic mutations in AML subpopulations
After the cell sorts, genomic DNA was isolated using a standard Proteinase K digestion protocol. In patient 04H138, the mutated region of HIST1H3H was amplified using the primers 5′AGACTGCTCGCAAGTCCAC3′ (forward [fw]) and 5′GCTGAAAAGGCAGCTTTCTG3′ (reverse [rv]); the TET2 mutation by 5′AAAGGCACATTGGACATGCT3′ (fw) and 5′GCCCTGGGCTTCACTTACTC3′ (rv); BCOR, 5′TCCAAAGCTGACCACATGAA3′ (fw) and 5′GTTTTGGTGCCATCTGCATT3′ (rv); RUNX1, 5′CAAGCTAGGAAGACCGACCC3′ (fw) and 5′AAGGCCCCTGAACGTGTATG3′ (rv); USP7, 5′TGTGTTCAGTGTGGACTCATTTT3′ (fw) and 5′TACGCACCATGGTCTGAGAG3′ (rv); and CBL, 5′ACCATATCACTGGACACAAGC3′ (fw) and 5′CCTGCCAGGATGTAAGACAGG3′ (rv). In patient 12H183, the mutated region of HIST1H3F was amplified by 5′CACGAAGCAAACAGCTCGTA3′ (fw) and 5′GTACCAGACGCTGGAATGGT3′ (rv) and the ASXL2 mutation by 5′TACATCGCCAAGCAAACCCA3′ (fw) and GAGCACAGGGGTGGTTTACA (rv). All PCR products were sequenced with fw and rv PCR primers using standard protocols.
Bone marrow cell transduction and generation of mouse AML models
Bone marrow cells were extracted from adult C57/B6-congenic Pep3B mice and depleted from lineage-positive cells using biotin-labeled anti-B220, anti-Gr1, and anti-Ter119 (Biomedical Research Centre, UBC Vancouver) and streptavidin-coupled MicroBeads (Miltenyi) on an AutoMACS apparatus (Miltenyi). Lineage-negative cells (<10% of total bone marrow) and transformed mouse cells were cultured in mouse bone marrow medium (Iscove modified Dulbecco medium, 10% heat-inactivated fetal bovine serum, 100 ng/mL recombinant mouse stem cell factor [Shenandoah; #200-09], 10 ng/mL recombinant mouse interleukin-3 [Shenandoah; #200-01], 10 ng/mL recombinant human interleukin-6 [Shenandoah; #100-10], and 10−4 M 2-mercaptoethanol). Retroviral producer cells (GPE+86) were infected with VSV-G–pseudotyped MSCV AML1-ETO9a IRES GFP (addgene #12433) or with MSCV MLL-AF9 IRES Puro (subcloned from a construct by Frédéric Barabé, Université Laval, Québec City, QC, Canada) and used for ecotropic transduction of 48-hour prestimulated mouse bone marrow cells by coculture in the presence of 6 µg/mL polybrene. Infected cells were either FACS sorted based on GFP expression or puromycin selected (1.5 µg/mL, 3 days). Secondary infections with H3.3 IRES tdTomato–expressing MSCV vectors were produced by GPE+86 coculture under the same conditions or by spinoculation (1000g, 32°C, 2 hours) with VSV-G–pseudotyped Plat-A virus–containing supernatant in the presence of polybrene. Infected cells were transplanted into 8- to 10-week-old C57/B6 sublethally irradiated (60 cGy) recipients. Mice were monitored for enlarged spleens and euthanized at disease end stage. All procedures conformed with institutional guidelines.
Additional methods
All additional methods are provided in the supplemental Data (available on the Blood Web site).
Results
H3K27M and H3K27I mutations occur at low frequencies in AML with RUNX1 alterations
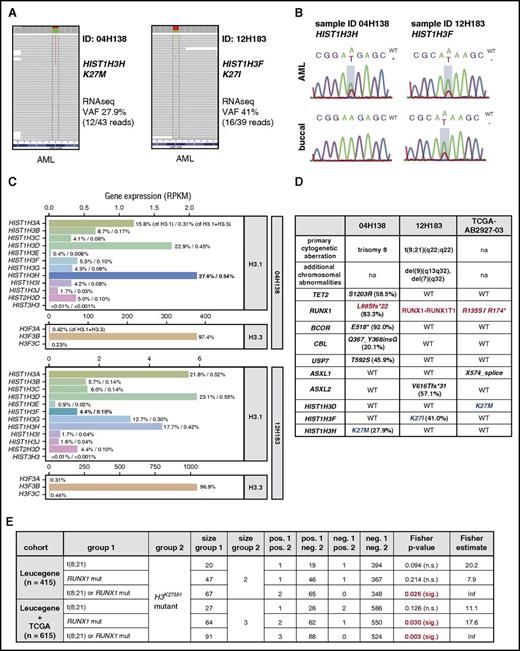
As part of an initiative to identify disease-associated mutations and gene expression signatures in adult AML, we discovered single-nucleotide variations on canonical histone H3 genes affecting lysine 27 resulting in either lysine-to-methionine or lysine-to-isoleucine substitutions based on the mapped RNA sequencing information (Figure 1A). Of note, no mutations of histone H3.3 genes H3F3A, H3F3B, or H3F3C were detected. Although H3.1K27 mutations were observed at low frequencies (2 of 415 patient samples investigated), the previous description of histone H3.1K27M and H3.3K27M mutations in pediatric gliomas and their documented impact on global levels of H3K27me2/3 merited further investigation. We confirmed both mutations by Sanger sequencing and determined that the HIST1H3HK27M mutation was absent in normal tissue from the same patient and was thus acquired somatically (Figure 1B, top panels). The HIST1H3FK27I mutation in patient 12H183 was also detected in normal reference DNA (Figure 1B, bottom panels), along with other AML-associated mutations in this patient, because of AML cell contamination in the buccal swap sample. Variant allele frequencies calculated based on RNA sequencing data were <50% in both cases (Figure 1B). Given that all sequenced AML specimens were enriched to contain blast populations of >90% before banking and sequencing, this suggested that either variant was likely present in a major heterozygous mutant subclone. Furthermore, we found that the mutated H3 genes contributed to 27.6% of canonical H3.1 expression and 0.54% of total histone H3 (H3.1 and H3.3) gene expression in patient 04H138 (HIST1H3HK27M) and to 4.4% of canonical H3.1 expression and 0.1% of total H3 gene expression in patient 12H183 (HIST1H3FK27M; Figure 1C). Considering that H3.1 but not H3.3 transcripts lack polyadenylation23 and therefore are likely underrepresented in our transcriptome data, where RNA was purified using polyA enrichment, it is important to note that the ratio between H3.1 and H3.3 chromatin content likely deviates from these values. In addition to the 2 H3K27-mutant AML samples in our cohort, 1 of 200 adult AML cases cataloged in TCGA cohort24 carried a heterozygous K27M mutation in HIST1H3D. Interestingly, all 3 H3K27-mutant AML samples additionally carried either RUNX1 mutations or a RUNX1-to-RUNX1T1 translocation [t(8;21), AML1-ETO; Figure 1D, in red). Within our AML cohort (Leucegene) or in combined Leucegene and TCGA cohorts, Fisher’s exact test indicated a significant enrichment of H3K27 mutations in RUNX1-altered vs all other AMLs (Figure 1E). In the combined Leucegene and TCGA cohorts, a significant enrichment of H3K27 mutations in RUNX1-mutant AML was also identified (Figure 1E). Despite the small number of patients with H3K27-mutant AML, these statistics raised the possibility that a selective cooperation between impaired or altered RUNX1 function and H3K27M/I-mediated inhibition of H3K27 methylation may exist. In support of this, all 3 AML specimens exhibited additional lesions with previously suggested deleterious effects on H3K27 methylation affecting either ASXL1 (through a T592S point mutation of its deubiquitinase USP725 ) or ASXL2 [an out-of-frame V616Tfs*31 mutation similar to those described before in the t(8;21) context16 ] or through heterozygous loss of the EZH2 gene located on chromosome 7q32 (patient 12H183; Figure 1D, additional chromosomal abnormalities).
H3K27Mand H3K27Imutations occur in human AML with RUNX1 alterations. (A) Discovery of histone H3 mutations in RNA sequencing (RNAseq) of primary AML. Variant allele frequencies (VAFs) and RNAseq coverage (variant/total reads) are provided for sequenced AML samples with H3K27 mutations (2 of 415 investigated specimens). (B) Validation of H3 mutation status. Genomic DNA from AML cells (top) and buccal swaps (bottom) was amplified using gene-specific primers and subjected to Sanger sequencing. Mutated nucleotides are boxed in red. (C) H3 variant expression varies based on the mutated H3 gene family member. Gene expression values of H3.1 and H3.3 genes in the 2 H3K27-mutant AML specimens are plotted. In patient 04H138 (top panels), the HIST1H3HK27M variant is present on the highest expressed H3 (27.6% of all H3.1 genes, 0.31% of all H3.1 and H3.3 genes; highlighted in bold). In patient 12H183 (bottom panels), expression of the HIST1H3F gene constitutes 4.4% of total canonical H3 and 0.10% of total H3 gene expression. (D) Summary of mutational contexts of H3K27-mutant AML samples. Note that all specimens with H3K27 mutations (in blue) have aberrations in the RUNX1 gene (highlighted in red). (E) Statistical analysis (Fisher’s exact test) of coassociations between H3K27 mutations and RUNX1 anomalies in the Leucegene or combined Leucegene and The Cancer Genome Atlas (TCGA) cohorts. Inf, infinite; na, not applicable; n.s., not significant; RPKM, reads per kilobase million.
H3K27Mand H3K27Imutations occur in human AML with RUNX1 alterations. (A) Discovery of histone H3 mutations in RNA sequencing (RNAseq) of primary AML. Variant allele frequencies (VAFs) and RNAseq coverage (variant/total reads) are provided for sequenced AML samples with H3K27 mutations (2 of 415 investigated specimens). (B) Validation of H3 mutation status. Genomic DNA from AML cells (top) and buccal swaps (bottom) was amplified using gene-specific primers and subjected to Sanger sequencing. Mutated nucleotides are boxed in red. (C) H3 variant expression varies based on the mutated H3 gene family member. Gene expression values of H3.1 and H3.3 genes in the 2 H3K27-mutant AML specimens are plotted. In patient 04H138 (top panels), the HIST1H3HK27M variant is present on the highest expressed H3 (27.6% of all H3.1 genes, 0.31% of all H3.1 and H3.3 genes; highlighted in bold). In patient 12H183 (bottom panels), expression of the HIST1H3F gene constitutes 4.4% of total canonical H3 and 0.10% of total H3 gene expression. (D) Summary of mutational contexts of H3K27-mutant AML samples. Note that all specimens with H3K27 mutations (in blue) have aberrations in the RUNX1 gene (highlighted in red). (E) Statistical analysis (Fisher’s exact test) of coassociations between H3K27 mutations and RUNX1 anomalies in the Leucegene or combined Leucegene and The Cancer Genome Atlas (TCGA) cohorts. Inf, infinite; na, not applicable; n.s., not significant; RPKM, reads per kilobase million.
In summary, lysine-to-methionine or -isoleucine substitutions of H3K27 represent sporadic (<1%) but recurrent mutations in adult AML and may co-occur with altered RUNX1 gene function.
H3K27 mutations are subclonal and accompanied by globally reduced H3K27me2/3
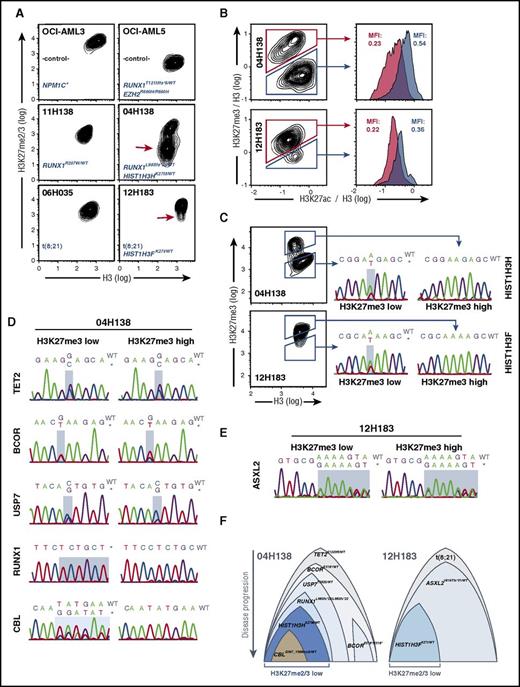
Because K27M mutations of histones H3.1 and H3.3 cause largely reduced H3K27me2/3 in patients with glioma,21,22,26 we sought to examine global H3K27me2/3 levels in the identified H3K27-mutant AML samples. To this end, we first established a FACS-based method to reliably quantify global H3K27me2/3. We used OCI-AML3 and EZH2R690H-mutant27 OCI-AML5 cell lines that were either untreated or treated with EZH1/2 inhibitors to set up FACS-staining conditions. This allowed us to detect changes in H3K27me2/3 levels with high sensitivity and at the single-cell level (supplemental Figure 1). Using this protocol, we identified an AML cell population with substantially reduced H3K27me2/3 in patient 04H138 (Figure 2A, middle panels, red arrow). This reduction was on average >10-fold and thus comparable to levels in OCI-AML5 cells (Figure 2A, top right panel). Furthermore, ∼50% of cells were affected, consistent with the HISTH3HK27M allele frequency of 27.9% in this patient. The analysis of patient sample 12H183, bearing an HISTH3FK27I mutation, revealed a less severe change in H3K27me2/3 in a small subset of cells, compared with a control t(8;21) patient (Figure 2A, bottom panels, red arrow). This was likely a result of the smaller inhibitory potency of the H3K27I mutant22 and its presence on a less expressed H3 gene (Figure 1C). Both specimens exhibited increased H3K27 acetylation (H3K27ac) levels in their respective H3K27me3-low subfractions (Figure 2B), as was reported in H3.3K27M-mutant diffuse intrinsic pontine glioma specimens.22 To confirm whether the observed reductions in H3K27me3 could be ascribed to the presence of H3K27 mutations, we sorted H3K27me3-high and -low subpopulations and performed Sanger sequencing of PCR-amplified H3 gene regions. With this strategy, we efficiently separated H3 WT from H3K27-mutant cells, based on the presence of the mutated H3K27 alleles in the H3K27me3-low and their complete absence in the respective H3K27me3-high populations (Figure 2C). These results provided a first indication that reduced H3K27me3 levels were indeed attributable to H3K27 mutations. For additional confirmation and to assess the potential contribution of other mutations to the hypo-H3K27me3 phenotype (ie, those in USP7, ASXL2, and BCOR, linked to PRC1/2 activity), we sequenced the remaining identified mutated regions in the same respective subpopulations (Figure 2D-E). In patient 04H138, this revealed that both subpopulations carried equal frequencies of the likely heterozygous TET2 mutation (Figure 2D, top panels), consistent with the early appearance of this lesion. Interestingly, although the H3K27me3-low subfraction contained a balanced allele frequency of WT and mutant BCOR, the H3K27me3-high subfraction carried the mutant allele at a noticeably higher frequency (Figure 2D, second row), suggesting the presence of a homozygous mutant subclone in addition to otherwise heterozygous cells. Moreover, the mutant USP7T592S allele was detectable at levels compatible with heterozygosity in the H3K27me3-low subfraction and at a noticeably reduced level in the H3K27me3-high fraction, respectively (Figure 2D, third row). Most strikingly, the RUNX1L98Sfs*22 allele was essentially confined to the sorted H3K27me3-low fraction and appeared at a homozygous level, whereas it was virtually absent in the sorted H3K27me3-high fraction (Figure 2D, fourth row). Finally, the mutant CBL allele was only detectable at a subclonal level in the H3K27me3-low fraction and was excluded from the H3K27me3-high portion (Figure 2D, fifth row), suggesting its appearance at a late stage in the disease. In patient 12H183, the ASXL2V616Tfs*31 mutation was equally detectable in both H3K27me3-defined subfractions and appeared at near-identical frequencies as the ASXL2 WT allele, suggesting heterozygosity in both subfractions (Figure 2E). Thus, the ASXL2V616Tfs*31 mutation by itself has a smaller impact on global H3K27me3 levels compared with its combination with the H3K27I mutation. This is in line with a study demonstrating that ASXL2-deficient hematopoietic cells exhibit a smaller reduction in H3K27me3 levels compared with their ASXL1-deficient counterparts.28
H3K27mutations are associated with globally reduced H3K27me2/3 and are subclonal. (A) H3K27me2/3 levels in AML cell lines and patient specimens. Cell lines were ethanol fixed and stained with H3K27me2/3 and pan-H3–specific primary antibodies and anti-mouse Alexa-647/anti-rabbit Alexa-350 secondary antibodies. Cell line and patient identifier in addition to the relevant genotype information are provided in each panel. Approximately 10-fold less H3K27me2/3 is detected in a subclonal population in patient 04H138 (red arrow), and a population with reduced H3K27me2/3 is detected in a subclonal population in patient 12H183 (red arrow). (B) Increased H3K27ac in H3K27me3-low AML subpopulations. Cells were treated as in panel A and costained for pan-H3 (BV421), H3K27me3 (Alexa647), and H3K27ac (PE). H3K27me3 and H3K27ac signals were normalized against H3 signals (derived parameters; Flowjo) and plotted and gated as indicated. (C) H3K27 mutations are restricted to H3K27me2/3-low subpopulations in primary AML specimens. Cells were treated and stained as in panel B. H3K27me3-high and -low populations were FACS sorted as indicated and subjected to Sanger sequencing of PCR-amplified mutated genomic DNA regions. Detected mutation sites are shaded in light blue. In both cases, the H3K27-mutant alleles were exclusively present in the H3K27me3-low fractions. (D) Distribution of the remaining mutations in the H3K27me3-high and -low subfractions of patient 04H138. Genomic DNA from panel C was used to determine the relative distribution of mutations in the H3K27me3-defined subpopulations. Mutated regions are highlighted in light blue. (E) Distribution of the ASXL2 mutation in the H3K27me3-high and -low subfractions of patient 12H183. (F) Depictions of proposed subclonal compositions and their possible evolutionary trajectories. Suggested initiating lesions are positioned at the top, followed by mutations in their suspected order of appearance. Relative sizes of subclones are assigned based on the detected frequencies of H3K27me3-low and -high subpopulations shown in panels D and E. MFI, mean fluorescence intensity.
H3K27mutations are associated with globally reduced H3K27me2/3 and are subclonal. (A) H3K27me2/3 levels in AML cell lines and patient specimens. Cell lines were ethanol fixed and stained with H3K27me2/3 and pan-H3–specific primary antibodies and anti-mouse Alexa-647/anti-rabbit Alexa-350 secondary antibodies. Cell line and patient identifier in addition to the relevant genotype information are provided in each panel. Approximately 10-fold less H3K27me2/3 is detected in a subclonal population in patient 04H138 (red arrow), and a population with reduced H3K27me2/3 is detected in a subclonal population in patient 12H183 (red arrow). (B) Increased H3K27ac in H3K27me3-low AML subpopulations. Cells were treated as in panel A and costained for pan-H3 (BV421), H3K27me3 (Alexa647), and H3K27ac (PE). H3K27me3 and H3K27ac signals were normalized against H3 signals (derived parameters; Flowjo) and plotted and gated as indicated. (C) H3K27 mutations are restricted to H3K27me2/3-low subpopulations in primary AML specimens. Cells were treated and stained as in panel B. H3K27me3-high and -low populations were FACS sorted as indicated and subjected to Sanger sequencing of PCR-amplified mutated genomic DNA regions. Detected mutation sites are shaded in light blue. In both cases, the H3K27-mutant alleles were exclusively present in the H3K27me3-low fractions. (D) Distribution of the remaining mutations in the H3K27me3-high and -low subfractions of patient 04H138. Genomic DNA from panel C was used to determine the relative distribution of mutations in the H3K27me3-defined subpopulations. Mutated regions are highlighted in light blue. (E) Distribution of the ASXL2 mutation in the H3K27me3-high and -low subfractions of patient 12H183. (F) Depictions of proposed subclonal compositions and their possible evolutionary trajectories. Suggested initiating lesions are positioned at the top, followed by mutations in their suspected order of appearance. Relative sizes of subclones are assigned based on the detected frequencies of H3K27me3-low and -high subpopulations shown in panels D and E. MFI, mean fluorescence intensity.
In summary, we conclude that the mutated H3K27 alleles are the most significant genetic determinants of global H3K27me3 in the 2 analyzed specimens. It is important to note, however, that in both AML patient cases, additional genetic lesions exist that likely cooperate with mutant H3K27 to reduce deposition of the H3K27me2/3 marks.
Possible mutational hierarchies can be extrapolated from these data (Figure 2F). For the 04H138 specimen, the RUNX1 mutation may have occurred after TET2, BCOR, and USP7 mutations. Irrespective of the scenario that resulted in this apparent hierarchy, homozygous RUNX1L98Sfs*22 is clearly associated with the H3K27M mutation and with the H3K27me3-low phenotype, strengthening the possibility of a functional collaboration between these 2 lesions.
Strong genetic interaction between H3K27 mutants and AML1-ETO in a mouse leukemia model
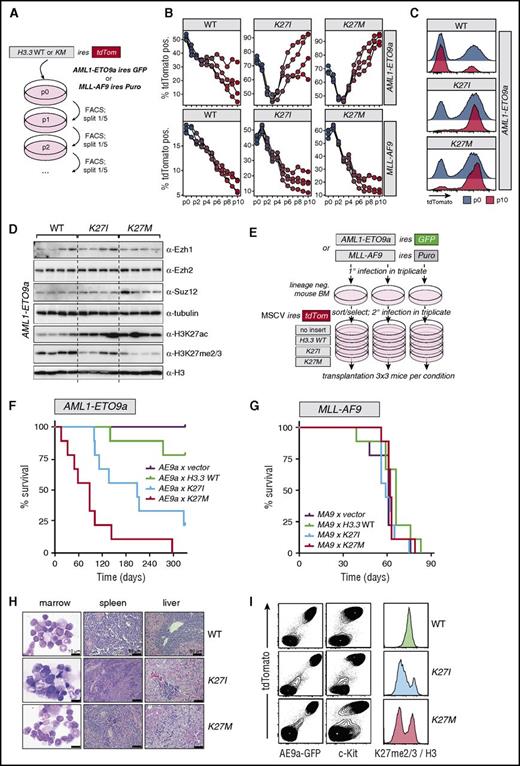
We next tested the possibility that H3.3K27 mutants may specifically collaborate with the product of the t(8;21)(q22;q22) translocation (RUNX1-RUNX1T1; AML1-ETO transcript 9a, AE9a) in vitro and in vivo. Selectivity of this collaboration was assessed by comparison with the combination of the H3.3K27-mutant genes with the MLL-AF9 fusion product. We first investigated whether expression of any of the H3.3 mutants resulted in altered proliferation kinetics in vitro. To this end, we tracked tdTomato-marked cells infected with H3.3WT, H3.3K27I, or H3.3K27M over several passages in competitive proliferation assays (Figure 3A). Expression of H3.3WT conferred a mild competitive proliferation disadvantage in both AE9a and MLL-AF9 cells (Figure 3B, left panels). In contrast, expression of H3.3K27I/M initially resulted in competitive loss in AE9a cells, but tdTomato+ fractions recovered and surpassed their initial postinfection frequencies in 3 of 4 H3.3K27I-infected and in all H3.3K27M-infected cultures (Figure 3B, top panels). At the end of the culture period, H3.3K27I- or H3.3K27M-infected cultures contained mostly cells with strong transgene expression based on high tdTomato fluorescence (Figure 3C). Most importantly, this was characteristic of AE9a-immortalized cells, because H3.3K27I- and H3.3K27M-infected MLL-AF9 cells were progressively outcompeted in all replicate cultures (Figure 3B, bottom panels). H3.3K27I/M-infected AE9a cells exhibited significant reductions in H3K27me2/3 compared with H3.3WT-infected controls despite undiminished Ezh1, Ezh2, and Suz12 protein levels (Figure 3D). In accordance with previous reports,22,29 H3.3K27I/M expression and loss of H3K27me2/3 were generally accompanied by elevated levels of H3K27ac (Figure 3D), albeit with a significant level of heterogeneity. In addition, 1 of 4 H3.3K27M-infected cultures exhibited elevated Suz12 protein levels (Figure 3D). Although exact causes of this heterogeneity are difficult to reconstruct after extended culture, this suggests that H3K27 mutations cause reprogramming of H3K27 modification profiles with some plasticity, driving clonal selection under constraints of additional epigenetic factors and compensatory mechanisms.
H3.3K27Iand H3.3K27Mselectively accelerate in vitro proliferation and disease latency in an AML1-ETO mouse model. (A) Experimental strategy to assess in vitro competitiveness of H3.3KM mutant–expressing cells in AML1-ETO9a (AE9a) and MLL-AF9 cells. Mouse hematopoietic stem and progenitor cells (HSPCs) transformed by either MSCV AE9a IRES GFP or MSCV MLL-AF9 IRES Puro were infected with MSCV IRES tdTomato retroviruses expressing either WT or mutant H3.3. Cells were passaged, and percentages of tdTomato+ cells were determined at each passage. (B) A proliferative advantage of H3.3K27I/M-infected cells was observed in AE9a but not in MLL-AF9 cells. Trajectories of 4 independent cultures per condition are represented by connected dots; passage numbers are indicated below. (C) Representative tdTomato FACS profiles at passages 0 and 10 indicate selection for high expression of H3.3K27I/M at the end of the culture period in AE9a cells. (D) Western analysis of AE9a cells infected with the indicated H3.3 variants. AE9a cells from the 4 replicate cultures in panel B were further expanded, lysed in Laemmli buffer, and subjected to western analysis. Reduced H3K27me2/3 and increased H3K27ac levels, but no reductions in PRC2 protein levels (Ezh1/2, Suz12), were observed. (E) Experimental strategy to assess AML progression upon H3.3KM expression in vivo. (F) Increased AML penetrance and progression upon expression of H3.3K27I/M in AE9a cells (n = 9 mice per condition). (G) Expression of H3.3K27I/M in MLL-AF9 cells leads to no change in AML progression (n = 9 mice per condition). (H) Pathological analysis of diseased AE9a recipients indicates typical AML end-stage characteristics. (I) Phenotypic analysis of bone marrow (BM) cells at disease end stage in AE9a model. Selection for cells coexpressing high levels of AE9a (GFP), H3.3 variants (tdTomato; left panels), and c-kit (middle panels). Reduced H3K27me2/3 in H3.3K27I/M-infected cells at disease end stage (right panels). H3K27me2/3 signal was normalized to H3 signal intensity and plotted.
H3.3K27Iand H3.3K27Mselectively accelerate in vitro proliferation and disease latency in an AML1-ETO mouse model. (A) Experimental strategy to assess in vitro competitiveness of H3.3KM mutant–expressing cells in AML1-ETO9a (AE9a) and MLL-AF9 cells. Mouse hematopoietic stem and progenitor cells (HSPCs) transformed by either MSCV AE9a IRES GFP or MSCV MLL-AF9 IRES Puro were infected with MSCV IRES tdTomato retroviruses expressing either WT or mutant H3.3. Cells were passaged, and percentages of tdTomato+ cells were determined at each passage. (B) A proliferative advantage of H3.3K27I/M-infected cells was observed in AE9a but not in MLL-AF9 cells. Trajectories of 4 independent cultures per condition are represented by connected dots; passage numbers are indicated below. (C) Representative tdTomato FACS profiles at passages 0 and 10 indicate selection for high expression of H3.3K27I/M at the end of the culture period in AE9a cells. (D) Western analysis of AE9a cells infected with the indicated H3.3 variants. AE9a cells from the 4 replicate cultures in panel B were further expanded, lysed in Laemmli buffer, and subjected to western analysis. Reduced H3K27me2/3 and increased H3K27ac levels, but no reductions in PRC2 protein levels (Ezh1/2, Suz12), were observed. (E) Experimental strategy to assess AML progression upon H3.3KM expression in vivo. (F) Increased AML penetrance and progression upon expression of H3.3K27I/M in AE9a cells (n = 9 mice per condition). (G) Expression of H3.3K27I/M in MLL-AF9 cells leads to no change in AML progression (n = 9 mice per condition). (H) Pathological analysis of diseased AE9a recipients indicates typical AML end-stage characteristics. (I) Phenotypic analysis of bone marrow (BM) cells at disease end stage in AE9a model. Selection for cells coexpressing high levels of AE9a (GFP), H3.3 variants (tdTomato; left panels), and c-kit (middle panels). Reduced H3K27me2/3 in H3.3K27I/M-infected cells at disease end stage (right panels). H3K27me2/3 signal was normalized to H3 signal intensity and plotted.
Together, these results demonstrate that expression of H3.3K27I and H3.3K27M variants confer a selective proliferation advantage to AE9a but not MLL-AF9 AML cells in vitro and that this is likely mediated by global changes in H3K27me2/3 and H3K27ac.
Next, we engineered mouse AML models to further investigate these observations in vivo. To this end, AE9a- or MLL-AF9–infected mouse HSPCs were transduced with either empty MSCV IRES tdTomato or MSCV IRES tdTomato–expressing H3.3WT, H3.3K27I, or H3.3K27M, resulting in gene transfer rates between 38% to 73% (Figure 3E; supplemental Figure 2). In the context of AE9a-driven AML, expression of H3.3K27M caused a striking disease acceleration, leading to all mice reaching end-stage disease within a median of 88 days (Figure 3F, red line). This contrasted with recipients of AE9a/empty vector or AE9a/H3.3WT cells, of which none or only 2 of 9 mice receiving transplants succumbed to AML within 330 days, respectively. Seven of 9 mice receiving AE9a/H3.3K27I transplants developed AML with a median latency of 208 days (Figure 3F, blue line). Importantly, combined expression of H3.3K27I or H3.3K27M with MLL-AF9 did not change disease latencies compared with control groups (Figure 3G). Of note, diseased AE9a mice were characterized by the accumulation of characteristic AML blasts in the bone marrow, and histological analyses showed high infiltration of leukemic cells in livers and spleens (Figure 3H). In addition, leukemic AE9a-expressing cells (marked by GFP) invariably expressed high levels of the H3.3K27I/M mutants indicated by high tdTomato fluorescence (Figure 3I left panels). Moreover, all diseased recipients contained high fractions of c-Kit+ cells characteristic of AML blasts in this model,30,31 and leukemic bone marrow cells contained distinct cell populations with largely decreased H3K27me2/3 levels specific to the H3.3K27I and H3.3K27M groups (Figure 3I middle and right panels).
Taken together, these data demonstrate that H3K27me2/3 restricts the progression of AML1-ETO9a–driven leukemia formation and that loss of H3K27me2/3 through the expression of H3.3K27I or H3.3K27M strongly cooperates with this model. This collaboration is selective given that expression of H3.3K27I or H3.3K27M results in no accelerated disease in the context of MLL-AF9.
Notably, the more potent inhibitory effect of the H3.3K27M mutant on PRC2 compared with H3.3K27I22 resulted in a stronger cooperation with AE9a, demonstrating that varying degrees of PRC2 inhibition have quantitatively different effects on leukemia progression in t(8;21) AML. In addition, we observed a more pronounced H3K27me2/3 reduction in leukemic AE9a/H3.3K27I mice compared with patient 12H183, bearing an HIST1H3FK27I mutation. This is likely caused by higher expression of the transgene in our AML model, strongly suggesting that the abundance of K27-mutant H3 and the loss of H3K27me2/3 stand in direct correlation with each other.
Impact of H3.3K27M expression on a subset of immature human cord blood cells
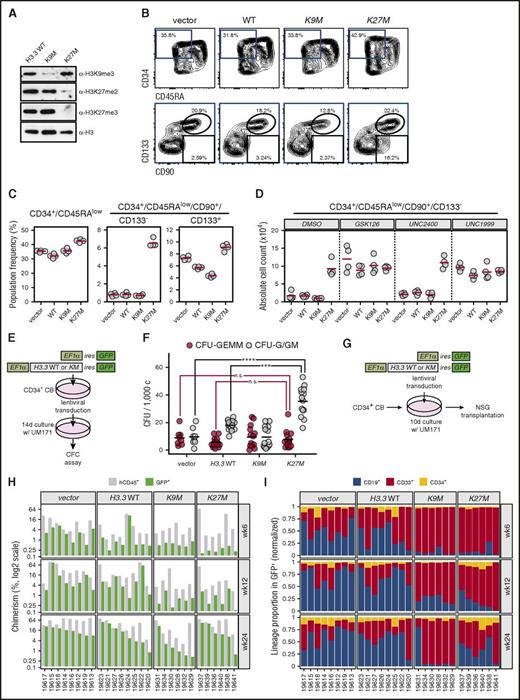
Finally, we investigated the influence of the H3K27M mutation on the differentiation and proliferation of human cord blood CD34+ HSPCs. As seen with mouse cells, ectopic expression of the H3.3K27M mutant driven by a strong promoter (full-length EF1-α) markedly reduced H3K27me2/3 levels in these cells (Figure 4A). As an additional control, CD34+ cells were infected with H3.3K9M, resulting in the expected reduction of H3K9me3 (Figure 4A). Interestingly, H3.3K27M overexpression led to an eightfold net expansion of a phenotypically primitive CD34+/CD45RA−/CD90+ cell population, which surprisingly lacked expression of the CD133 stem-cell marker (Figure 4B-C). Furthermore, the EZH1/2 inhibitors GSK12632 and UNC1999,33 but not the inactive control compound UNC2400, reproduced this phenotype in a dose-dependent manner (supplemental Figure 3). GSK126- or UNC1999-treated H3.3K27M-expressing cells displayed no additional increase in absolute expansion of the CD34+/CD45RA−/CD90+/CD133− population compared with control-treated H3.3K27M-expressing cultures (Figure 4D), confirming that the activity of the H3.3K27M mutant is indeed mediated through inhibition of PRC2 as previously suggested.22
H3.3K27Mexpression in human HSPCs leads to expansion of phenotypically immature cells through inhibition of H3K27me2/3. (A) Lentiviral expression of H3.3KM mutants induces specific reductions in H3 methylation in cultured human HSPCs. After 48 hours in culture, human umbilical cord blood (UCB)–derived CD34+ cells were infected with lentiviruses expressing the indicated H3.3 variants and IRES GFP under control of the EF1a promoter. After 10 days of total culture, GFP+ cells were FACS sorted, lysed in Laemmli buffer, and subjected to western analysis at 105 cells per lane. Decreased H3K27me2, H3K27me3, or H3K9me3 was detected upon expression of H3K27M or H3K9M, respectively. (B) Phenotypic characterization of H3.3KM mutant–expressing human HSPCs. UCB-derived CD34+ cells were infected and cultured for 10 days as in panel A, stained with the indicated antibodies, and FACS analyzed. Differences in the CD34+/CD45RA− population (top panels) and CD34+/CD45RA−/CD90+/CD133-− or CD34+/CD45RA−/CD90+/CD133+ populations (bottom) within infected (GFP+) cells were assessed. Percentages of each gate are indicated. (C) Summary of phenotypic changes in HSPCs expressing H3.3K27M. Population frequencies were calculated based on analysis in panel B. Quadruplicate infections/cultures of CD34+ cells expressing H3.3K27M revealed a prominent expansion of CD34+CD45RA−CD90+CD133− cells in H3.3K27M-infected HSPCs. (D) Addition of EZH1/2 inhibitors (GSK126 and UNC1999; 1 µM) leads to no increased expansion of the CD34+/CD45RA−/CD90+/CD133− population in H3.3KM-expressing cells compared with controls (DMSO; 10−4 and UNC2400; 1 µM). Cells were analyzed at day 10 postinfection and EZH1/2 inhibitor treatment. All analyses were restricted to the transduced (GFP+) subsets. (E-F) Assessment of myeloid progenitor activity in response to H3.3K27M expression in HSPCs. The experimental strategy is depicted in panel E. GFP+ cells were FACS isolated and plated in methylcellulose media supporting erythromyeloid differentiation. Colonies were morphologically assessed and scored after 14 days. Granulo/erythoid/monocytic/megakaryocytic colony-forming units (CFU-GEMM) and granulocytic/granulomonocytic colony-forming units (CFU-G/GM) were scored. Combined results from 2 independent experiments are presented in panel F. ***P < .001, ****P < .0001; 2-way analysis of variance statistics with Tukey adjustment. (G) Experimental strategy for transplantation of HSPCs expressing H3.3KM mutants. Cells were infected with lentiviruses as in panel A and cultured for 10 days in the presence of UM171. Progenies of 104 CD34+ UCB cells were transplanted into NSG recipients (8 mice per condition). (H) Summary of human hematopoietic stem-cell engraftment from the experiment depicted in panel G. Bone marrow chimerism of NSG mice receiving transplants was determined by FACS analysis based on GFP and human CD45 surface expression at the specified posttransplantation time points. Individual mouse identifiers are indicated at the bottom of the graph. (I) Lineage contribution of H3.3KM-expressing HSPCs in transplant-recipient NSG mice. Presented data refer to the distribution of myeloid (CD33), B-lymphoid (CD19), and immature (CD34) surface phenotypes within the GFP+ compartments. CFC, colony-forming cell; n.s., not significant.
H3.3K27Mexpression in human HSPCs leads to expansion of phenotypically immature cells through inhibition of H3K27me2/3. (A) Lentiviral expression of H3.3KM mutants induces specific reductions in H3 methylation in cultured human HSPCs. After 48 hours in culture, human umbilical cord blood (UCB)–derived CD34+ cells were infected with lentiviruses expressing the indicated H3.3 variants and IRES GFP under control of the EF1a promoter. After 10 days of total culture, GFP+ cells were FACS sorted, lysed in Laemmli buffer, and subjected to western analysis at 105 cells per lane. Decreased H3K27me2, H3K27me3, or H3K9me3 was detected upon expression of H3K27M or H3K9M, respectively. (B) Phenotypic characterization of H3.3KM mutant–expressing human HSPCs. UCB-derived CD34+ cells were infected and cultured for 10 days as in panel A, stained with the indicated antibodies, and FACS analyzed. Differences in the CD34+/CD45RA− population (top panels) and CD34+/CD45RA−/CD90+/CD133-− or CD34+/CD45RA−/CD90+/CD133+ populations (bottom) within infected (GFP+) cells were assessed. Percentages of each gate are indicated. (C) Summary of phenotypic changes in HSPCs expressing H3.3K27M. Population frequencies were calculated based on analysis in panel B. Quadruplicate infections/cultures of CD34+ cells expressing H3.3K27M revealed a prominent expansion of CD34+CD45RA−CD90+CD133− cells in H3.3K27M-infected HSPCs. (D) Addition of EZH1/2 inhibitors (GSK126 and UNC1999; 1 µM) leads to no increased expansion of the CD34+/CD45RA−/CD90+/CD133− population in H3.3KM-expressing cells compared with controls (DMSO; 10−4 and UNC2400; 1 µM). Cells were analyzed at day 10 postinfection and EZH1/2 inhibitor treatment. All analyses were restricted to the transduced (GFP+) subsets. (E-F) Assessment of myeloid progenitor activity in response to H3.3K27M expression in HSPCs. The experimental strategy is depicted in panel E. GFP+ cells were FACS isolated and plated in methylcellulose media supporting erythromyeloid differentiation. Colonies were morphologically assessed and scored after 14 days. Granulo/erythoid/monocytic/megakaryocytic colony-forming units (CFU-GEMM) and granulocytic/granulomonocytic colony-forming units (CFU-G/GM) were scored. Combined results from 2 independent experiments are presented in panel F. ***P < .001, ****P < .0001; 2-way analysis of variance statistics with Tukey adjustment. (G) Experimental strategy for transplantation of HSPCs expressing H3.3KM mutants. Cells were infected with lentiviruses as in panel A and cultured for 10 days in the presence of UM171. Progenies of 104 CD34+ UCB cells were transplanted into NSG recipients (8 mice per condition). (H) Summary of human hematopoietic stem-cell engraftment from the experiment depicted in panel G. Bone marrow chimerism of NSG mice receiving transplants was determined by FACS analysis based on GFP and human CD45 surface expression at the specified posttransplantation time points. Individual mouse identifiers are indicated at the bottom of the graph. (I) Lineage contribution of H3.3KM-expressing HSPCs in transplant-recipient NSG mice. Presented data refer to the distribution of myeloid (CD33), B-lymphoid (CD19), and immature (CD34) surface phenotypes within the GFP+ compartments. CFC, colony-forming cell; n.s., not significant.
Interestingly, granulocytic/granulomonocytic colony-forming cells, but not multipotent colony-forming cells (granulo/erythroid/monocytic/megakaryocytic), were expanded in 14-day cultures initiated with H3.3K27M-transduced CD34+ umbilical cord blood cells when compared with controls (Figure 4E-F). Using the protocol depicted in Figure 4G, we assessed the repopulation activity of these cells in NSG mice at 6, 12, and 24 weeks posttransplantation. These results indicated a marginal loss in in vivo reconstitution activity by H3.3K27M-transduced cells (Figure 4H), mostly at 6- and 12-week time points and some skewing toward myeloid reconstitution (Figure 4I). Of note, expression of H3.3K9M reduced overall long-term engraftment and resulted in a noticeable myeloid bias, suggesting impaired long-term hematopoietic stem-cell function (Figure 4I). Within the scope of these experiments, H3.3K27M expression led to the selective expansion of a phenotypically defined immature population and to increased self-renewal of committed myeloid progenitors (granulocytic/granulomonocytic colony-forming cells) in vitro, but to no obvious advantage in vivo. These results consolidate the idea that the K27M mutation is a collaborating lesion but is insufficient to initiate AML on its own.
Discussion
Our study represents the first characterization of histone H3K27 missense mutations in the context of AML. We show that AML cells with heterozygous K27 mutations in 1 of 12 canonical H3 genes, HIST1H3H or HISTH3F, exhibit readily detectable global reductions in H3K27me2/3. The extent of this reduction depends on the specific amino acid conversion, that is K27M or K27I, as well as the expression level of the affected H3 gene and its relative contribution to the H3 pool. In the most severe demonstrated case, K27M mutation of HIST1H3H led to approximately 10-fold reduction in H3K27me2/3, reminiscent of cells lacking enzymatic activity of EZH2, the major H3K27me2/3 methyltransferase.34
Three observations support the central notion that H3K27I/M mutations may act as opportunistic lesions that exacerbate AML progression after RUNX1 gene alterations: their subclonal stoichiometry in RUNX1-mutant and t(8;21) AML patient samples, the significant and selective role in disease acceleration in an AML1-ETO9a–driven t(8;21) mouse model, and the promotion of committed myeloid progenitor self-renewal but failure to cause leukemic transformation in human cord blood–derived HSPCs.
Interestingly, H3K27M mutations in AML occur in different genetic contexts compared with in patients with glioma, where collaborating events predominantly involve loss of p53 activity and activated receptor tyrosine kinase signaling.19,35 In line with the high frequency of mutations in PRC2-associated genes in AML with RUNX1 gene alterations,11,12,17 our results suggest that genetic events causing impaired H3K27me2/3 deposition, either locally or globally, are under strong positive selective pressure in t(8;21) and RUNX1-mutant AMLs. On this basis, it is tempting to speculate that the propensity to accumulate secondary lesions resulting in reduced H3K27me2/3 may be a common hallmark of RUNX1-altered AML.
Given the profound perturbations in H3K27 modification states that are present in H3K27-mutant gliomas, several studies have suggested that these cancers are selectively vulnerable to epigenetic therapies including inhibition of EZH2,36 the KDM6 H3K27 demethylase family,37 the H3K27ac reader BRD4,38 or histone deacetylases.39 On the basis of our study, it is conceivable that demethylase inhibitor–mediated modulation of H3K27me2/3 may have selective pharmacological potency in AML with reduced H3K27me2/3 and/or RUNX1 gene alterations. In addition, disruption of aberrant transcriptional activation downstream of increased H3K27ac using BET family inhibitors may exhibit selectivity in these cases. To test this, we propose that chemogenomic studies using sufficiently large cohorts of genetically characterized AML specimens be conducted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Allis (Rockefeller University) for H3.3KM expression constructs, M. Draoui for project coordination, S. Corneau for sample coordination, and M. Arteau and R. Lambert at the Institute for Research in Immunology and Cancer (IRIC) genomics platform for sequencing. The authors acknowledge the dedicated work of the Quebec Leukemia Cell Bank staff, namely G. d’Angelo for morphological diagnoses and H. Chaker for fluorescence in situ hybridization analyses of acute myeloid leukemia samples. The authors also thank D. Gagné and G. Dulude at IRIC for technical assistance with flow cytometry sorts, M. Fréchette and V. Blouin-Chagnon for assistance with mouse experiments, and Sauvageau laboratory members for helpful discussions and proofreading of the manuscript.
This work was supported by the Government of Canada through Genome Canada and the Ministère de l’Économie and de l’Innovation et des Exportations du Québec through Génome Québec, with supplementary funds from Amorchem and the Canadian Cancer Society Research Institute. G.S. and J.H. are recipients of research chairs from Industrielle-Alliance (Université de Montréal) and the Canada Research Chair program, respectively. The Leukemia Cell Bank of Québec is supported by grants from the Cancer Research Network of the Fonds de Recherche du Québec–Santé (FRQS). Sequencing read mapping and transcript quantification were performed on the supercomputer Briaree from the Université de Montréal, managed by Calcul Québec and Compute Canada. The operation of this supercomputer is funded by the Canada Foundation for Innovation, NanoQuébec, Réseau de Médecine Génétique Appliquée, and Fonds de Recherche du Québec–Nature et Technologies. Y.W.Z is supported by a scholarship from FRQS. E.T. and V.-P.L. are supported by fellowships from the Cole Foundation Montreal.
Authorship
Contribution: B.L. conceived and supervised the study, performed experiments and data analysis, and wrote the manuscript; Y.W.Z. performed experiments and data analysis and contributed to the writing of the manuscript; I.B. validated somatic mutations in patient samples and H3K27me2/3 subpopulations; N.M. assisted in analysis of mice receiving human hematopoietic stem-cell transplants; E.T. and J.C. provided expertise in the analysis of phenotypic changes in human hematopoietic cells; V.-P.L. provided mutational profiling of human acute myeloid leukemia (AML) specimens; J.H. analyzed AML cytogenetics and provided all AML samples; and G.S. contributed to project conception, coordinated and supervised the study, and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guy Sauvageau, Institute for Research in Immunology and Cancer (IRIC), P.O. Box 6128, Downtown Station, Montreal, QC, Canada, H3C 3J7; e-mail: guy.sauvageau@umontreal.ca; and Bernhard Lehnertz, Institute for Research in Immunology and Cancer (IRIC), P.O. Box 6128, Downtown Station, Montreal, QC, Canada, H3C 3J7; e-mail: bernhard.j.lehnertz@umontreal.ca.
References
Author notes
B.L. and Y.W.Z. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal