Key Points
Describe human MK populations representing distinct developmental stages within a heterogeneous culture.
FV uptake identifies cultured MKs ready to release platelets upon infusion into mice.
Abstract
Stem cell–derived platelets have the potential to replace donor platelets for transfusion. Defining the platelet-producing megakaryocytes (MKs) within the heterogeneous MK culture may help to optimize the in vitro generation of platelets. Using 2 human stem cell models of megakaryopoiesis, we identified novel MK populations corresponding to distinct maturation stages. An immature, low granular (LG) MK pool (defined by side scatter on flow cytometry) gives rise to a mature high granular (HG) pool, which then becomes damaged by apoptosis and glycoprotein Ib α chain (CD42b) shedding. We define an undamaged HG/CD42b+ MK subpopulation, which endocytoses fluorescently labeled coagulation factor V (FV) from the media into α-granules and releases functional FV+CD42b+ human platelet-like particles in vitro and when infused into immunodeficient mice. Importantly, these FV+ particles have the same size distribution as infused human donor platelets and are preferentially incorporated into clots after laser injury. Using drugs to protect HG MKs from apoptosis and CD42b shedding, we also demonstrate that apoptosis precedes CD42b shedding and that apoptosis inhibition enriches the FV+ HG/CD42b+ MKs, leading to increased platelet yield in vivo, but not in vitro. These studies identify a transition between distinct MK populations in vitro, including one that is primed for platelet release. Technologies to optimize and select these platelet-ready MKs may be important to efficiently generate functional platelets from in vitro–grown MKs.
Introduction
The first report of in vitro–generated platelets1 raises the possibility that stem cell–derived platelets may be produced to complement or displace donor-derived platelets clinically.2-4 Although considerable progress has been made in the generation of platelets from hematopoietic stem cells,5-7 embryonic stem cells, and induced pluripotent stem cells (iPSCs),8-12 there remain important challenges to overcome before we can realize the goal of transfusing stem cell–derived platelets into humans. Low platelet yield and poor functionality have been important challenges in the field.13-16 In part, these unresolved challenges stem from gaps in a detailed understanding of megakaryocyte (MK) maturation in vitro, including defining the mature MKs that are optimally primed to release platelets and directing cultured MKs to this stage.
MK maturation is accompanied by an increase in cell size, ploidy, granular components, MK-specific surface receptors, and the formation of an invaginated membrane system.17-21 Whether these changes occur sequentially or as a continuum is unclear as is a definition of a mature MK that is primed for thrombopoiesis.
Human bone marrow MKs have been classified into 4 developmental stages based on size and ploidy: stage I MKs are 10 to 15 μm (2N/4N), stage II MKs are 14 to 20 μm (4N/8N), and stage III/IV MKs are 20 to 40 μm (8N-128N).22 The ultrastructural features at these stages have been described qualitatively using electron micrographs of primary human bone marrow MKs, but these MKs differ greatly from the in vitro–generated MKs, which are significantly smaller and with a ploidy that rarely exceeds 16N.23,24 The gene expression profiles of MKs of different ploidy have been compared,25,26 but gave little insights into what defines a mature MK primed for thrombopoiesis when infused into a bioreactor27-29 or into mice.30,31 We hypothesize that there may be a transient stage of MK development that is highly efficient in undergoing thrombopoiesis and that selective harvesting and/or optimizing the yield of these MKs may enhance functional platelet yield.
We examined cell size and granularity changes during human MK differentiation in vitro and identified discrete MK maturation stages with distinct granularity, which we termed the low granular (LG) and high granular (HG) MKs. We further demonstrated that the immature LG MKs give rise to mature HG MKs, which are subdivided into a functional CD42b+ population that produces platelets in vitro and after infusion into immunodeficient mice, and an apoptotic, nonfunctional CD42b− population. Moreover, using a labeled coagulation factor V (FV) variant, we showed that the HG/CD42b+ MKs endocytose FV into α-granules and release functional platelets with similar size distribution as human donor platelets. Additionally, treatment of MKs with a pan-caspase inhibitor, Q-VD-Oph, prevented both apoptosis and CD42b shedding, and enriched the functional FV+/CD42b+ population. These studies further our understanding of megakaryopoiesis and thrombopoiesis and may have implications for optimizing the production of stem cell–derived platelets for transfusion.
Methods
MK differentiation
Human CD34+ hematopoietic progenitor cells (HPCs), purchased from the Fred Hutchinson Cancer Research Center Co-operative Center for Excellence in Hematology (CCEH) Hematopoietic Cell Processing and Repository Core, were differentiated into MKs for 16 days in MK differentiation media described previously.6 The wild-type iPSC line CHOPWT6/WTBM1-832,33 was differentiated into primitive HPCs34 and then into MKs for 8 days in the same MK differentiation media.6
FV uptake into MKs
MKs were pulse-labeled with FV by incubating with 200 nM of a previously described FV variant35 tagged with Alexa 488 or Alexa 647 for 1 hour at 37°C. Excess FV was removed by washing. MKs were analyzed for FV content by flow cytometry and by immunofluorescence studies (described in supplemental Methods, available on the Blood Web site). The platelet-generating capability of FV-labeled MKs was evaluated by infusing MKs into immunodeficient mice and by collecting in vitro platelet-like particles (described in the supplemental Methods).
Infusion of MKs into immunodeficient NSG mice
Human MKs (2 × 106 to 7 × 106) were infused through the tail vein into 8- to 12-week-old NOD SCID interleukin receptor 2 γ (NSG) mice, produced at the Children’s Hospital of Philadelphia (CHOP) using breeders from The Jackson Laboratory. Fresh whole blood was drawn from the same mouse at specified time points postinfusion and stained with anti-human CD42a and CD42b antibodies prior to flow cytometric analysis.
To examine released human platelet incorporation into thrombi, laser injuries were induced in the cremaster arterioles of mice 30 minutes after the infusion of FV/calcein double-labeled MKs, and thrombus formation was recorded. Day 11 MKs were labeled with FV–Alexa 488 as described in the previous section and 2 μM calcein red-orange (ThermoFisher) for 20 minutes at 37°C before infusing into the jugular vein of NSG mice. To visualize the clot, anti-mouse CD41–Alexa 647 Fab fragments (BD Pharmingen) were injected IV prior to the induction of laser injuries. All animal studies were approved by CHOP’s institutional com-mittee for animal welfare.
Statistical analysis
All data are represented as mean ± standard error of the mean (SEM). Differences are analyzed using the Student t test and are considered significant if the P value is ≤.05.
Results
Identification of distinct MK populations during in vitro megakaryopoiesis
Identification of the mature, platelet-producing MKs within the heterogeneous, asynchronous MK culture would allow us to find novel ways of improving the yield and quality of stem cell–derived platelets. Thus, one goal is to determine whether we can identify distinct maturation intermediates within the MK culture and whether there is a specific maturation intermediate that is primed for thrombopoiesis. Key changes associated with MK maturation include: increase in cell size, ploidy, α-granule content, RNA, and the formation of an invaginated membrane system.17-21 These features lead to increased size and internal cellular complexity, which can be detected as increased forward scatter (FSC) and side scatter (SSC)/granularity, respectively, by flow cytometry. By examining these 2 parameters during the differentiation of human CD34+ HPCs into MKs, we identified 2 MK populations distinguishable by their granularity, LG and HG MKs (Figure 1A). We hypothesize that these novel MK populations represent distinct developmental stages.
Two MK populations with distinct granularity are present in the in vitro human stem cell–derived MK culture. (A) Representative size (forward-scatter area [FSC-A]) and granularity (side-scatter area [SSC-A]) profiles of CD42a+ MKs at specified time points during the differentiation of human CD34+ hematopoietic progenitors. LG and HG MKs are gated as shown. (B) Day 14 LG CD42a+ MKs were sorted and cultured for another 6 days (top). Size and granularity of MKs 3 days and 6 days postsort are shown. Day 14 HG CD42a+ MKs were similarly sorted and cultured (bottom). Representative studies of 3 independent studies.
Two MK populations with distinct granularity are present in the in vitro human stem cell–derived MK culture. (A) Representative size (forward-scatter area [FSC-A]) and granularity (side-scatter area [SSC-A]) profiles of CD42a+ MKs at specified time points during the differentiation of human CD34+ hematopoietic progenitors. LG and HG MKs are gated as shown. (B) Day 14 LG CD42a+ MKs were sorted and cultured for another 6 days (top). Size and granularity of MKs 3 days and 6 days postsort are shown. Day 14 HG CD42a+ MKs were similarly sorted and cultured (bottom). Representative studies of 3 independent studies.
The LG MKs appeared around day 7, followed by HG MKs around day 10, with their percentage changing over time (Figure 1A). Because HG MKs appeared later and had high granularity suggestive of cytoplasmic changes associated with maturation, we hypothesized that the LG MKs give rise to HG MKs. We tested this hypothesis by sorting LG and HG MKs and examined how the granularity of these sorted populations changed with further culture. We found that the LG MKs became HG MKs upon further culture (Figure 1B top), whereas the HG MKs remained HG (Figure 1B bottom), confirming that HG MKs came from LG MKs.
A subpopulation of HG MKs shows signs of injury
We further characterized these 2 MK populations by examining a panel of known MK markers. We found surface CD42b is selectively lost from the HG population, but not the LG population (Figure 2A). These data further divided the cells into 3 subpopulations: LG MKs, HG/CD42b+ MKs, and HG/CD42b− MKs. On platelets, the loss of surface CD42b is due to metalloproteinase cleavage of the extracellular glycocalicin domain of CD42b,36,37 and is associated with platelet activation, apoptosis, and clearance.36,38-41 We predict that CD42b shedding on HG MKs also signaled damage, thus we examined the MKs for signs of apoptosis. Indeed, 80% to 90% of the HG/CD42b− MKs were Annexin V+ and terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling–positive (TUNEL+) (Figure 2B), indicating that these cells were apoptotic. In contrast, <20% of the LG MKs and HG/CD42b+ MKs were apoptotic (Figure 2B). Over time, the percentage of LG MKs and HG/CD42b+ MKs decreased gradually with a concomitant accumulation of apoptotic HG/CD42b− MKs (Figure 2C), suggesting a progression of viable MKs to a nonphysiological terminal state that would likely decrease in vitro platelet yield.
HG/CD42b+MKs have characteristics of mature MKs. (A) Representative flow plots of CD42b expression on LG (green) and HG (red) MKs at specified time points of the differentiation. (B) Representative flow plots of Annexin V staining (left) and TUNEL staining (right) on day 14 MK cultures. (C) The percentage ± 1 SEM for each MK subpopulation at specified time points of the differentiation are shown of 4 independent studies. (D) Graph quantifying the percentages of MKs in each ploidy class ± 1 SEM of 5 independent studies. (E) Expression of α granule proteins (basic fibroblast growth factor [bFGF], vascular endothelial growth factor [VEGF], PF4, endostatin [Col18A]) and total RNA content of day 14 MKs was determined by intracellular staining and thiazole orange staining, respectively. Graphs quantifying the fold changes in mean fluorescence intensity over isotype/background ± 1 SEM are shown. (F) Day 14 MKs were stimulated with convulxin (500 ng/mL) or PAR-1–activating peptide (50 μM) for 20 minutes at room temperature. Representative flow plots showing percentages of activated (PAC-1+) MKs following convulxin stimulation (top). Black dotted line histogram indicates background staining in the absence of stimulation. Graph quantifying the percentages of PAC-1+ MKs following convulxin or PAR-1–activating peptide stimulation (bottom) (n = 5). *P < .05, **P < .01, ***P < .005, ****P < .001 for all statistical analyses shown.
HG/CD42b+MKs have characteristics of mature MKs. (A) Representative flow plots of CD42b expression on LG (green) and HG (red) MKs at specified time points of the differentiation. (B) Representative flow plots of Annexin V staining (left) and TUNEL staining (right) on day 14 MK cultures. (C) The percentage ± 1 SEM for each MK subpopulation at specified time points of the differentiation are shown of 4 independent studies. (D) Graph quantifying the percentages of MKs in each ploidy class ± 1 SEM of 5 independent studies. (E) Expression of α granule proteins (basic fibroblast growth factor [bFGF], vascular endothelial growth factor [VEGF], PF4, endostatin [Col18A]) and total RNA content of day 14 MKs was determined by intracellular staining and thiazole orange staining, respectively. Graphs quantifying the fold changes in mean fluorescence intensity over isotype/background ± 1 SEM are shown. (F) Day 14 MKs were stimulated with convulxin (500 ng/mL) or PAR-1–activating peptide (50 μM) for 20 minutes at room temperature. Representative flow plots showing percentages of activated (PAC-1+) MKs following convulxin stimulation (top). Black dotted line histogram indicates background staining in the absence of stimulation. Graph quantifying the percentages of PAC-1+ MKs following convulxin or PAR-1–activating peptide stimulation (bottom) (n = 5). *P < .05, **P < .01, ***P < .005, ****P < .001 for all statistical analyses shown.
As LG MKs progressed to HG/CD42b+ MKs, we observed an increase in ploidy (Figure 2D) and some α-granule proteins (Figure 2E), most notably platelet factor 4 (PF4), a known MK maturation marker.42 In contrast, HG/CD42b− MKs lost α-granule proteins and most of its RNA content (Figure 2E), consistent with their transition to a damaged state.
A feature of mature MKs is their ability to respond to platelet agonists.43 To assess the responsiveness of these MK subpopulations, we analyzed the percentage of activated MKs following stimulation with platelet agonists, convulxin, or protease-activated receptor 1 (PAR1)-activating peptide. Activation causes conformational changes in the surface glycoprotein IIb/IIIa, detectable by PAC-1 antibody.44,45 Although 70% to 80% of HG/CD42b+ MKs activated in response to stimulation, only 20% to 30% of LG MKs and virtually none of apoptotic HG/CD42b− MKs responded (Figure 2F). These data suggested that HG/CD42b+ MKs may be nearing their peak of maturation and that CD42b shedding indicated an undesirable decline of these mature MKs to an apoptotic, nonfunctional state.
To determine whether these distinct MK populations are present in the human bone marrow, we examined the size and granularity of primary MKs from marrow aspirates. Primary MKs form a continuum of maturing MKs with increasing cell size and granularity (supplemental Figure 1A). Because surface CD42a and CD42b are known to increase with maturation, we compared the size and granularity of CD42ahighCD42bhigh MKs and CD42alowCD42blow MKs. We observed that the mature CD42ahighCD42bhigh MKs in the bone marrow were both larger and more granular than CD42alowCD42blow MKs. In contrast, the cultured CD42ahighCD42bhigh MKs were more granular, but not bigger than the CD42alowCD42blow MKs (supplemental Figure 1B). We also did not detect the presence of CD42a+CD42b− MKs in bone marrow. These differences between marrow MKs and cultured MKs will be further discussed.
FV uptake labels mature, undamaged MKs
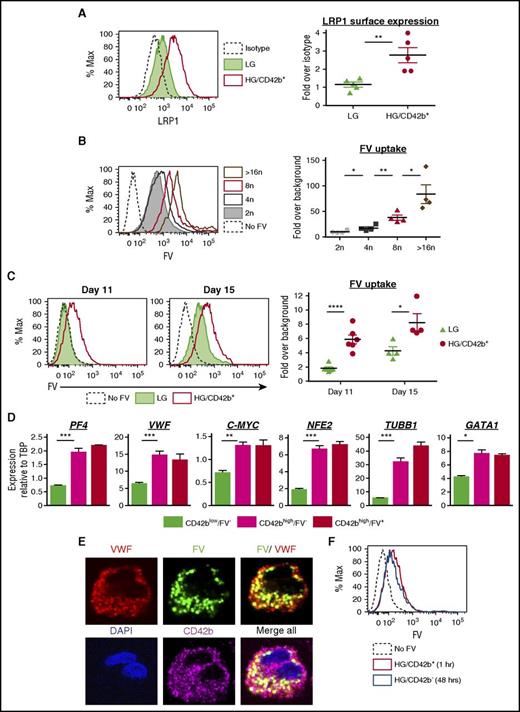
Human coagulation FV is synthesized by the liver and circulates in the plasma.46,47 Although mouse MKs produce FV endogenously,48 human MKs endocytose FV from their surroundings and package it into α-granules.49-52 However, the specific stage of maturation when human MKs endocytose FV is unknown.53 The HG/CD42b+ MKs have many characteristics of mature MKs and may be the population that is primed to release platelets. We hypothesized that the HG/CD42b+ MKs may be able to endocytose FV and that FV uptake may be a marker of fully mature MKs.
A putative receptor for FV endocytosis is the low-density lipoprotein receptor–related protein 1 (LRP1),54 which is upregulated on the surface of mature MKs.55 HG/CD42b+ MKs showed higher surface LRP1 expression as compared with LG MKs (Figure 3A). FV uptake by MKs, as assayed by pulse-labeling MKs with a fluorescently labeled FV variant,35 correlated with ploidy, suggesting that FV endocytosis increased with maturation (Figure 3B). Correspondingly, the HG/CD42b+ MKs endocytose more FV than the LG MKs (Figure 3C). To determine whether the kinetics of FV endocytosis change over time, we pulse-labeled MKs with fluorescently labeled FV on days 11 and 15 of the differentiation. On day 11, LG MKs showed negligible FV uptake whereas HG/CD42b+ exhibited greater FV uptake (Figure 3C). By day 15, both MK populations matured over time and took up FV, but the HG/CD42b+ MKs still endocytosed FV more efficiently than the LG MKs.
Mature MKs take up FV into their α granules. (A) Surface expression of LRP1 receptor on day 14 MKs. Left: representative flow plot of LRP1 surface expression on LG and HG/CD42b+ MKs. Right: graph quantifying median fluorescence intensity of LRP1 expression over isotype for LG and HG/CD42b+ MKs. Mean ± 1 SEM is shown (n = 5). (B) Day 14 MKs were pulse-labeled with 200 nM FV–Alexa 488 or Alexa 647 for 1 hour at 37°C. Left: representative flow plot of FV uptake by CD42b+ MKs of different ploidy classes. Right: graph quantifying median FV fluorescence over background associated with MKs of different ploidy classes (n = 4). (C) Day 11 or day 15 MKs pulse-labeled with FV as in panel B. Left: representative flow plot of FV uptake by LG and HG/CD42b+ MK populations is shown. Right: graph quantifying median FV fluorescence over background associated with LG and HG/CD42b+ MKs on days 11 and 15. (D) Day 11 or 14 MKs labeled with FV and CD42b were sorted for gene expression analysis by quantitative polymerase chain reaction (qPCR). Messenger RNA (mRNA) expression of late MK markers relative to housekeeping gene TATA box binding protein (TBP) in sorted CD42blow, CD42bhigh FV−, and CD42bhigh FV+ populations is shown (n = 4). (E) Immunostaining of CD42b and VWF was performed on day 14 FV-labeled MKs that were adhered to fibronectin-coated glass coverslips. Confocal images of the MKs show FV colocalizing with VWF in α-granules. Images were acquired using a DMi8 microscope (Leica Biosystems) equipped with a 63× Plan Apochromat objective (1.4 NA) and a Hamamatsu Photonics ORCA-Flash4.0 sCMOS digital camera. (F) Day 11 MKs were pulse-labeled with FV as in panel B. Excess FV was removed by washing, and MKs were followed for 48 hours. Representative flow plot showing that the intensity of FV fluorescence associated with HG/CD42b+ MKs 1 hour postlabeling is the same as that associated with HG/CD42b− MKs 48 hours later, indicating that HG/CD42b+ MKs give rise to HG/CD42b− MKs. *P < .05, **P < .01, ***P < .005, ****P < .001 for all statistical analyses. DAPI, 4′,6-diamidino-2-phenylindole; Max, maximum.
Mature MKs take up FV into their α granules. (A) Surface expression of LRP1 receptor on day 14 MKs. Left: representative flow plot of LRP1 surface expression on LG and HG/CD42b+ MKs. Right: graph quantifying median fluorescence intensity of LRP1 expression over isotype for LG and HG/CD42b+ MKs. Mean ± 1 SEM is shown (n = 5). (B) Day 14 MKs were pulse-labeled with 200 nM FV–Alexa 488 or Alexa 647 for 1 hour at 37°C. Left: representative flow plot of FV uptake by CD42b+ MKs of different ploidy classes. Right: graph quantifying median FV fluorescence over background associated with MKs of different ploidy classes (n = 4). (C) Day 11 or day 15 MKs pulse-labeled with FV as in panel B. Left: representative flow plot of FV uptake by LG and HG/CD42b+ MK populations is shown. Right: graph quantifying median FV fluorescence over background associated with LG and HG/CD42b+ MKs on days 11 and 15. (D) Day 11 or 14 MKs labeled with FV and CD42b were sorted for gene expression analysis by quantitative polymerase chain reaction (qPCR). Messenger RNA (mRNA) expression of late MK markers relative to housekeeping gene TATA box binding protein (TBP) in sorted CD42blow, CD42bhigh FV−, and CD42bhigh FV+ populations is shown (n = 4). (E) Immunostaining of CD42b and VWF was performed on day 14 FV-labeled MKs that were adhered to fibronectin-coated glass coverslips. Confocal images of the MKs show FV colocalizing with VWF in α-granules. Images were acquired using a DMi8 microscope (Leica Biosystems) equipped with a 63× Plan Apochromat objective (1.4 NA) and a Hamamatsu Photonics ORCA-Flash4.0 sCMOS digital camera. (F) Day 11 MKs were pulse-labeled with FV as in panel B. Excess FV was removed by washing, and MKs were followed for 48 hours. Representative flow plot showing that the intensity of FV fluorescence associated with HG/CD42b+ MKs 1 hour postlabeling is the same as that associated with HG/CD42b− MKs 48 hours later, indicating that HG/CD42b+ MKs give rise to HG/CD42b− MKs. *P < .05, **P < .01, ***P < .005, ****P < .001 for all statistical analyses. DAPI, 4′,6-diamidino-2-phenylindole; Max, maximum.
To correlate FV uptake with gene expression changes during MK maturation, we sorted CD42blow/FV− MKs, CD42bhigh/FV− MKs, and CD42bhigh/FV+ MKs (supplemental Figure 2A-B) and compared the expression of MK maturation genes in these populations. CD42blow/FV− MKs correspond to LG MKs, whereas CD42bhigh/FV− MKs correspond to the smaller HG/CD42b+ MKs and CD42bhigh/FV+ MKs correspond to the larger HG/CD42b+ MKs (supplemental Figure 2C), consistent with FV uptake being a marker of mature MKs. We found that the expression of MK maturation genes peaks as MKs gain CD42b, with little or no increase as MKs acquire the ability to endocytose FV (Figure 3D). These data suggest that the ability to endocytose FV is only acquired in the most mature MKs with peak expression of known MK maturation markers, indicating that FV uptake could be one of the final markers of a fully mature MK primed for thrombopoiesis.
To determine whether FV localized to the α-granules following endocytosis, we performed immunofluorescence studies on FV-labeled MKs, costaining for the MK surface marker (CD41 or CD42b) and an α-granule marker (von Willebrand factor [VWF]). CD42b+ MKs showed a punctate distribution of intracellular FV, which partially colocalized with VWF in granules (Figure 3E). In contrast, we observed that CD42b− MKs were associated with a very high level of FV fluorescence distinctly localized to the cell surface (supplemental Figure 3). We believe that this was due to FV binding to phosphatidylserine (PS) on the surface of apoptotic MKs.35
To confirm that the damaged HG/CD42b− MKs came from the HG/CD42b+ population, we pulse-labeled MKs on day 11, when all of the HG MKs are CD42b+. Forty-eight hours later, the newly generated HG/CD42b− MKs had the same level of FV fluorescence as the HG/CD42b+ MKs postlabeling (Figure 3F), indicating that the HG/CD42b− MKs came from the HG/CD42b+ population and that prevention of this damage may enhance platelet yield.
FV-labeled MKs release platelets when infused into NSG mice
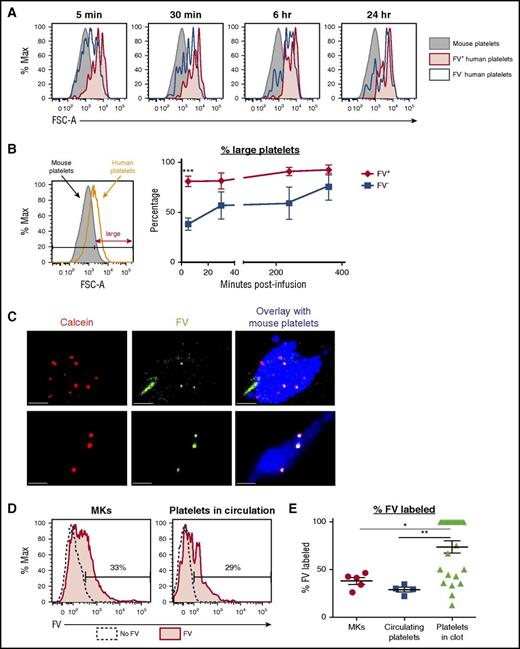
To determine whether FV-labeled HG/CD42b+ MKs preferentially generated functional platelets in vivo, we infused FV-labeled MKs into NSG mice. We previously demonstrated that human donor platelets infused into NSG mice could be detected in the blood and were ∼10-fold larger than mouse platelets.31 When cultured human MKs were infused into NSG mice, we observed 2 distinct populations of human platelet events: (1) platelet-like particles (PLPs) with a broad size-range, limited half-life, and functionality, and (2) platelets released from pulmonary-entrapped MKs that have similar size distribution, half-life, and functionality as donor platelets.31 These data suggest that size can be used as a discriminatory factor to examine whether the human platelet events detected in the circulation are more like nonfunctional PLPs or functional platelets.
For these studies, we used day 11 MKs to avoid the HG/CD42b− subpopulation that accumulates over time and binds FV nonspecifically (Figure 2C; supplemental Figure 3). Following the infusion of FV-labeled MKs, we detected both FV+ and FV− human platelet events in the circulation postinfusion (supplemental Figure 4A-B). We observed that all FV+ platelet events were MK-derived platelets, with sizes similar or larger than donor platelets, as early as 5 minutes postinfusion and at all time points following. In contrast, the FV− platelet events had a broad range of sizes initially, indicating that they were a mixture of PLPs and MK-derived platelets, but by 1 to 4 hours, the short-lived PLPs were cleared, leaving mostly the MK-derived platelets in circulation (Figure 4A-B; supplemental Figure 4C). These data suggest that although both FV+ and FV− MKs release platelets, FV+ MKs appear primed to release platelets as soon as they encounter the right in vivo environment.
FV+MKs release functional FV+platelets in vivo. (A) Day 11/12 MKs were incubated with 200 nM FV–Alexa 488 for 1 hour at 37°C prior to infusion into NSG mice. Representative flow plots showing the sizes of CD42a+CD42b+ FV+ (red) and FV− (black) human platelet events detected in the circulation of mice at the specified time points after the infusion of FV-labeled MKs. The sizes of mouse platelets are shown in gray. (B) Left: relative sizes of endogenous mouse platelets and infused human donor platelets. Platelets were considered large if their FSC-As were larger than 90% of the mouse platelets’ FSC-As. Right: percentage of FV+ (red) and FV− (black) platelets that were large at the various time points postinfusion. Mean ± 1 SEM is shown. (n = 4) (C) Day 11 MKs double-labeled with calcein red-orange (red) and FV–Alexa 488 (green) were infused into NSG mice 30 minutes prior to the induction of the first cremaster arteriole laser injury. Representative confocal images of clots formed after laser injury are shown. Mouse platelets are labeled with CD41–Alexa 647 (blue). All human platelets derived from infused MKs are calcein-labeled (red). FV-labeled (green) human platelets appear yellow in the overlay. Scale bars shown are 10 µm. (D) Representative flow plots showing the percentage of FV-labeled MKs that were infused (left) and FV-labeled human platelets circulating in mice postinfusion (right). (E) Quantification of the percentages of FV-labeled MKs that were infused with 5 independent studies, FV-labeled human platelets detected in the circulation postinfusion (n = 4), and FV-labeled platelets detected in the clot (n = 25). *P < .05, **P < .01, ***P < .005 for all statistical analyses.
FV+MKs release functional FV+platelets in vivo. (A) Day 11/12 MKs were incubated with 200 nM FV–Alexa 488 for 1 hour at 37°C prior to infusion into NSG mice. Representative flow plots showing the sizes of CD42a+CD42b+ FV+ (red) and FV− (black) human platelet events detected in the circulation of mice at the specified time points after the infusion of FV-labeled MKs. The sizes of mouse platelets are shown in gray. (B) Left: relative sizes of endogenous mouse platelets and infused human donor platelets. Platelets were considered large if their FSC-As were larger than 90% of the mouse platelets’ FSC-As. Right: percentage of FV+ (red) and FV− (black) platelets that were large at the various time points postinfusion. Mean ± 1 SEM is shown. (n = 4) (C) Day 11 MKs double-labeled with calcein red-orange (red) and FV–Alexa 488 (green) were infused into NSG mice 30 minutes prior to the induction of the first cremaster arteriole laser injury. Representative confocal images of clots formed after laser injury are shown. Mouse platelets are labeled with CD41–Alexa 647 (blue). All human platelets derived from infused MKs are calcein-labeled (red). FV-labeled (green) human platelets appear yellow in the overlay. Scale bars shown are 10 µm. (D) Representative flow plots showing the percentage of FV-labeled MKs that were infused (left) and FV-labeled human platelets circulating in mice postinfusion (right). (E) Quantification of the percentages of FV-labeled MKs that were infused with 5 independent studies, FV-labeled human platelets detected in the circulation postinfusion (n = 4), and FV-labeled platelets detected in the clot (n = 25). *P < .05, **P < .01, ***P < .005 for all statistical analyses.
FV+ platelets are incorporated into thrombi following laser injury
To examine the functionality of FV+ platelets, we performed laser injury studies following the infusion of MKs double-labeled with FV–Alexa 488 and calcein red-orange. To visualize the clot, we labeled mouse platelets with murine CD41–Alexa 647 (blue). We detected both FV+ (yellow) and FV− (red) human platelets/PLPs in the clots (Figure 4C). Given that the MKs infused were 38% ± 3% FV+ and the circulating platelets/PLPs were 29% ± 2% FV+, the observation that FV+ particles were present at 74% ± 6% in the clot (Figure 4D-E) supports that FV+ platelets were preferentially incorporated over FV− particles, suggesting that FV+ platelets had better functionality than FV− platelets/PLPs.
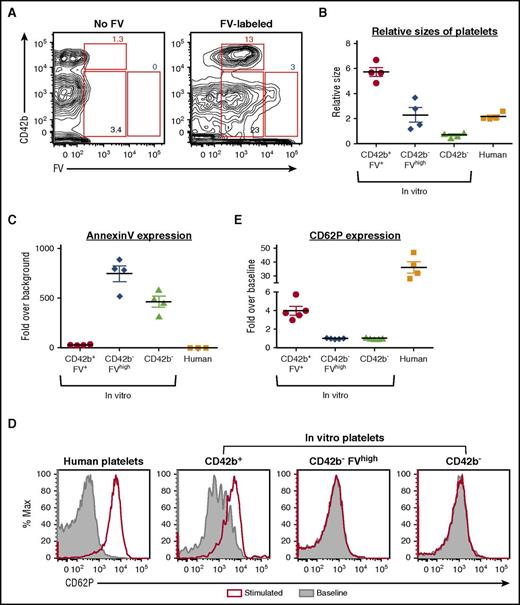
FV-labeled MKs generate FV-labeled PLPs in vitro that are partially functional
To determine whether FV-labeled MKs generate FV-labeled PLPs in vitro, we analyzed in vitro PLPs from day 11 FV-labeled MKs 24 hours postlabeling. All CD42b+ PLPs in vitro were FV-labeled (Figure 5A), indicating that they came from FV-labeled HG/CD42b+ MKs. There was a small but distinct subpopulation of CD42b− PLPs that were FVhigh (Figure 5A), which likely came from apoptotic HG/CD42b− MKs that bind FV through their surface PS. Consistent with published data,12,28 the FV-labeled CD42b+ PLPs were slightly larger than human donor platelets (Figure 5B). The FV-labeled CD42b+ platelets were Annexin Vlow and activated in response to agonist stimulation, although not as well as human donor platelets (Figure 5C-E), suggesting that they are partially functional. In contrast, the CD42b− PLPs were smaller than donor platelets, Annexin Vhigh, and did not respond to agonist stimulation (Figure 5B-E), indicating that they are likely nonfunctional cellular debris.
FV-labeled MKs give rise to functional CD42b+platelets in vitro. Day 11 MKs were pulse-labeled with FV, washed, and resuspended in fresh medium. In vitro platelets were harvested 24 hours later for analysis. (A) CD42b and FV expression of CD42a+ platelet-sized particles harvested from nonlabeled (left) and FV-labeled (right) MKs. CD42b+FV+, CD42b−FVhigh, and CD42b− particles were gated as shown. (B) Relative sizes of platelet-sized particles in vitro compared with human donor platelets. (C) Quantification of Annexin V staining on platelet-sized particles in vitro compared with human donor platelets. (D) In vitro platelets and human donor platelets are stimulated with PAR1-activating peptide (25 μM) for 20 minutes at 37°C. Platelet activation is indicated by the increase in surface CD62P expression. (E) Graph quantifying the fold change in CD62P expression over baseline when in vitro platelet-sized particles and human donor platelets are stimulated.
FV-labeled MKs give rise to functional CD42b+platelets in vitro. Day 11 MKs were pulse-labeled with FV, washed, and resuspended in fresh medium. In vitro platelets were harvested 24 hours later for analysis. (A) CD42b and FV expression of CD42a+ platelet-sized particles harvested from nonlabeled (left) and FV-labeled (right) MKs. CD42b+FV+, CD42b−FVhigh, and CD42b− particles were gated as shown. (B) Relative sizes of platelet-sized particles in vitro compared with human donor platelets. (C) Quantification of Annexin V staining on platelet-sized particles in vitro compared with human donor platelets. (D) In vitro platelets and human donor platelets are stimulated with PAR1-activating peptide (25 μM) for 20 minutes at 37°C. Platelet activation is indicated by the increase in surface CD62P expression. (E) Graph quantifying the fold change in CD62P expression over baseline when in vitro platelet-sized particles and human donor platelets are stimulated.
Metalloproteinase inhibition protects MKs from CD42b shedding, but not apoptosis
Metalloproteinase cleavage of CD42b on platelets occurs following activation, apoptosis, or improper storage,38-41,56 thus CD42b surface expression is a key parameter for assessing platelet quality. Metalloproteinase inhibitors like GM6001 prevent CD42b shedding on platelets and improve the hemostatic function and recovery of platelets postinfusion.36 These drugs have also been used to protect in vitro–generated platelets from damage.10,57 Because CD42b shedding and apoptosis are intricately linked events in platelets and the regulation of apoptosis in MKs is crucial for platelet formation, we examined whether inhibiting CD42b shedding can enrich the HG/CD42b+ subpopulation.
Treatment of MKs with GM6001 preserved CD42b expression, but did not affect apoptosis (supplemental Figure 5A-B). Importantly, although the percentage of CD42b+ MKs increased following treatment, many of these MKs remained apoptotic and fewer CD42b+ MKs responded to agonist stimulation (supplemental Figure 5C), indicating that the preservation of CD42b on MKs is insufficient to preserve MK viability.
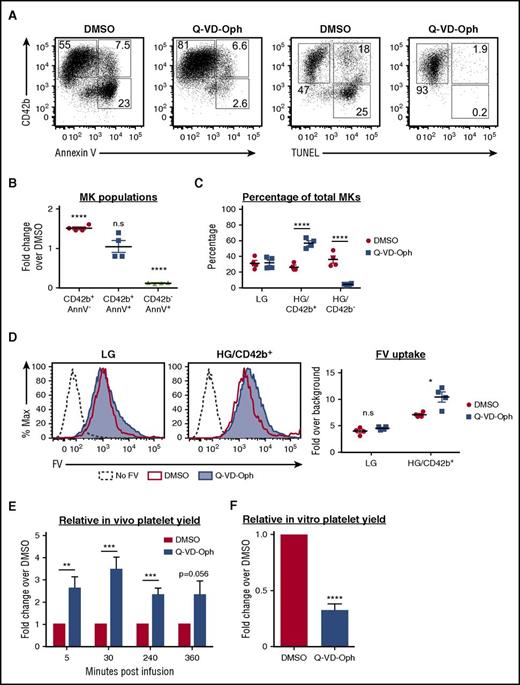
Apoptosis inhibition protects MKs from both apoptosis and CD42b shedding
To examine whether apoptosis inhibition can enrich the HG/CD42b+ subpopulation, we treated MKs with a pan-caspase inhibitor, Q-VD-Oph.58,59 Q-VD-Oph inhibited both apoptosis and CD42b shedding (Figure 6A), resulting in a 90% reduction in the percentage of apoptotic CD42b− MKs and a 50% increase in the percentage of CD42b+ MKs (Figure 6B). More specifically, Q-VD-Oph enriched the HG/CD42b+ MKs (Figure 6C). These data suggested that CD42b shedding occurred downstream of apoptosis. To determine whether Q-VD-Oph affected the localization of ADAM17, the metalloproteinase responsible for CD42b shedding,37 we analyzed ADAM17 surface expression on MKs. We found that ADAM17 surface expression was upregulated with maturation and was unaffected by Q-VD-Oph (supplemental Figure 6A). However, ADAM17’s activity requires PS exposure,60 thus ADAM17 is poised to cleave CD42b on mature MKs, but is unable to do so unless PS is exposed following apoptosis. This also explains why CD42b shedding occurs only on HG MKs but not on LG MKs. Therefore, we posit that by inhibiting apoptosis and PS exposure, Q-VD-Oph also prevents CD42b shedding.
Effect of apoptosis inhibition on MKs and platelet production. MKs were treated with Q-VD-Oph (25 µM) or dimethyl sulfoxide (DMSO; control) from day 8 to day 15 and analyzed on day 15. (A) Representative flow plots showing CD42b expression vs Annexin V (AnnV) binding or TUNEL positivity in MKs treated with DMSO or Q-VD-Oph. (B) Graph quantifying fold changes in the percentages of MK subpopulations with Q-VD-Oph treatment relative to DMSO. Mean ± 1 SEM is shown for 4 independent studies. (C) Quantification of the percentages of MK subpopulations with DMSO or Q-VD-Oph treatment. Mean ± 1 SEM is shown for 4 independent studies. (D) DMSO and Q-VD-Oph treated MKs were pulse-labeled with FV. Left: representative plot showing FV uptake by LG and HG/CD42b+ MKs treated with DMSO or Q-VD-Oph. Right: quantification of FV uptake by DMSO and Q-VD-Oph treated MKs. Mean ± 1 SEM is shown for 4 independent studies. (E) Graph quantifying the relative platelet production at specified time points following the infusion of Q-VD-Oph or DMSO-treated MKs into immunodeficient mice. Platelet production is normalized to DMSO control at each time point. Mean ± 1 SEM is shown for 6 independent studies. (F) Graph quantifying the relative in vitro platelet yield from DMSO or Q-VD-Oph treated MKs for 5 independent studies. *P < .05, **P < .01, ***P < .005, ****P < .001 for all statistical analyses. n.s., not significantly different between treatments for all statistical analyses.
Effect of apoptosis inhibition on MKs and platelet production. MKs were treated with Q-VD-Oph (25 µM) or dimethyl sulfoxide (DMSO; control) from day 8 to day 15 and analyzed on day 15. (A) Representative flow plots showing CD42b expression vs Annexin V (AnnV) binding or TUNEL positivity in MKs treated with DMSO or Q-VD-Oph. (B) Graph quantifying fold changes in the percentages of MK subpopulations with Q-VD-Oph treatment relative to DMSO. Mean ± 1 SEM is shown for 4 independent studies. (C) Quantification of the percentages of MK subpopulations with DMSO or Q-VD-Oph treatment. Mean ± 1 SEM is shown for 4 independent studies. (D) DMSO and Q-VD-Oph treated MKs were pulse-labeled with FV. Left: representative plot showing FV uptake by LG and HG/CD42b+ MKs treated with DMSO or Q-VD-Oph. Right: quantification of FV uptake by DMSO and Q-VD-Oph treated MKs. Mean ± 1 SEM is shown for 4 independent studies. (E) Graph quantifying the relative platelet production at specified time points following the infusion of Q-VD-Oph or DMSO-treated MKs into immunodeficient mice. Platelet production is normalized to DMSO control at each time point. Mean ± 1 SEM is shown for 6 independent studies. (F) Graph quantifying the relative in vitro platelet yield from DMSO or Q-VD-Oph treated MKs for 5 independent studies. *P < .05, **P < .01, ***P < .005, ****P < .001 for all statistical analyses. n.s., not significantly different between treatments for all statistical analyses.
Importantly, apoptosis inhibition by Q-VD-Oph did not affect ploidy or responsiveness to agonists (supplemental Figure 6B-C) and led to a small increase in FV uptake by the HG/CD42b+ MKs (Figure 6D). When Q-VD-Oph–treated MKs were infused into NSG mice, we detected increased numbers of human platelets postinfusion (Figure 6E). However, Q-VD-Oph treatment led to a decrease in the yield of CD42b+ PLPs in vitro (Figure 6F).
Distinct MK populations can also be found in iPSC-derived MK cultures
To determine whether these MK subpopulations were present in MK cultures derived from a different stem cell source, studies were repeated with human iPSC-derived MKs. LG, HG/CD42b+, and HG/CD42b− MK populations were present within iPSC-derived MK cultures (supplemental Figure 7A-B). Similarly, HG/CD42b+ MKs endocytosed more FV than LG MKs (supplemental Figure 7C). When we inhibited apoptosis with Q-VD-Oph, iPSC-derived MKs showed decreased apoptosis and CD42b shedding (supplemental Figure 7D). Thus, these distinct MK maturation stages are present in MK cultures derived from 2 different stem cell sources.
Discussion
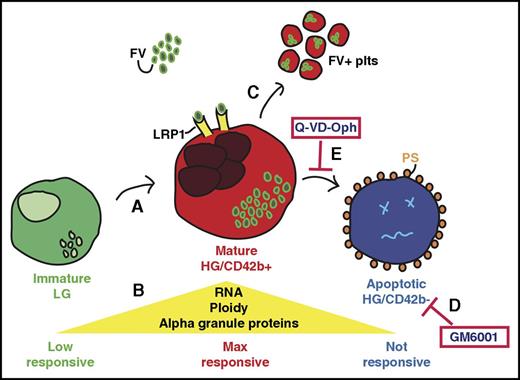
Despite advances in the understanding of megakaryopoiesis and thrombopoiesis, it remains unclear what defines a MK that is primed to release platelets. Our studies addressed this important question through developmental staging of human stem cell–derived MKs beginning with the idea that MKs increase in size and granularity with maturation.17 We identified distinct stages of MK differentiation with an initial maturation phase leading to a transient, functional stage, followed by a declining phase characterized by apoptosis, CD42b shedding, and a loss of RNA and α-granule proteins (Figure 7). These stages are not clearly distinguishable in primary marrow MKs as mature MKs exit the marrow to shed platelets in the circulation,61 thus, normally few or no MKs undergo apoptosis and/or CD42b shedding in the marrow. These insults may be more apparent in clinical settings like myelodysplastic syndromes and immune thrombocytopenia purpura, where intramedullary MKs fail to exit the marrow and/or undergo apoptosis.62-65 The loss of RNA and α-granule proteins may be due to degranulation, typically observed in idiopathic myelofibrosis, where growth factors released from MK α-granules promote marrow fibrosis.66-68 Collectively, apoptosis, CD42b shedding, and degranulation are associated with pathological clinical outcomes, suggesting that protecting cultured MKs from such insults may be important for producing high-quality stem cell–derived platelets. Additionally, although primary marrow MKs increase in size and granularity as they mature, in vitro–derived MKs increase in granularity but not in size as they mature. This result correlates with published observations that cultured MKs do not achieve high ploidy like those in vivo,17,23,24 reiterating the idea that the size and ploidy of in vitro–grown MKs may limit platelet yields.
Developmental staging of in vitro–generated MKs. Schematic representation of the stages as immature human MKs mature and then undergo injury. (A) The immature LG MK population has low ploidy and few internal granules and show limited response to agonist stimulation. These LG MKs mature to become the HG/CD42b+ MK population. (B) The mature HG/CD42b+ MKs have increased ploidy, RNA, and α-granular content as compared with the immature LG MKs. They are also maximally responsive to stimulation by various agonists. (C) Mature HG/CD42b+ MKs take up fluorescently labeled FV into their α granules and release FV-labeled platelets in vitro and in the circulation of mice when infused. The FV-labeled platelets are similar in size or larger than human donor platelets. FV-labeled platelets are incorporated into clots and activate in response to agonist stimulation, suggesting that they are functional. (D) GM6001 inhibits CD42b shedding from MKs, but does not prevent apoptosis or improve MK functionality. (E) On the other hand, blocking apoptosis also prevents CD42b shedding, and redirects MKs to increase the number of CD42b+ MKs that take up FV and release functional platelets.
Developmental staging of in vitro–generated MKs. Schematic representation of the stages as immature human MKs mature and then undergo injury. (A) The immature LG MK population has low ploidy and few internal granules and show limited response to agonist stimulation. These LG MKs mature to become the HG/CD42b+ MK population. (B) The mature HG/CD42b+ MKs have increased ploidy, RNA, and α-granular content as compared with the immature LG MKs. They are also maximally responsive to stimulation by various agonists. (C) Mature HG/CD42b+ MKs take up fluorescently labeled FV into their α granules and release FV-labeled platelets in vitro and in the circulation of mice when infused. The FV-labeled platelets are similar in size or larger than human donor platelets. FV-labeled platelets are incorporated into clots and activate in response to agonist stimulation, suggesting that they are functional. (D) GM6001 inhibits CD42b shedding from MKs, but does not prevent apoptosis or improve MK functionality. (E) On the other hand, blocking apoptosis also prevents CD42b shedding, and redirects MKs to increase the number of CD42b+ MKs that take up FV and release functional platelets.
Unlike murine MKs, which express FV endogenously,48 human MKs endocytose FV from the surroundings. We examined whether the FV uptake into α-granules would be a useful marker of mature, functional human MKs. When we pulse-labeled the heterogeneous MK culture with fluorescently labeled FV, the mature HG/CD42b+ MKs preferentially internalized the FV. The FV+ MKs released functional platelets with a size range similar or larger than human donor platelets almost immediately postinfusion. Because newly released young platelets and preplatelets are larger than circulating old platelets,69-71 FV+ platelets are likely young platelets released in vivo by infused FV-labeled MKs. Moreover, FV+ platelets are overrepresented in clots following laser injury, an indication of better functionality over FV− platelets. Additionally, all functional, CD42b+ in vitro PLPs are derived from the FV+ MKs, reaffirming that FV+ MKs are primed for platelet formation. With these new insights into the specific stage of MK maturation when FV endocytosis occurs, we can use this model to address the mechanisms of FV endocytosis and trafficking in human MKs, which is critical as mouse models do not accurately reflect human physiology in regard to FV uptake.
The heterogeneous and asynchronous nature of MK cultures makes it difficult to purify the mature MKs that are primed for platelet production. To improve the efficiency of in vitro platelet generation, we propose that FV labeling could be a tool to harvest the transient, mature MKs for platelet bioreactor use,27-29 particularly because MK’s ability to endocytose FV is acquired after peak expression of many maturation genes. Genome-wide studies to compare CD42bhigh/FV− MKs and CD42bhigh/FV+ MKs may lead to the identification of novel MK maturation markers, potentially cell surface receptors that could be useful alternatives to FV for the purification of mature MKs. Alternatively, FV+ MKs may be infused into patients with the anticipation that they would release a large number of platelets rapidly after infusion. Apart from FV, MKs also endocytose other proteins like fibrinogen72-74 and immunoglobulins.75 Whether endocytosis of these proteins by MKs occurs at the same stage of maturation and whether they could substitute for FV uptake is presently unclear.
We also found that CD42b shedding occurs downstream of apoptosis, likely due to ADAM17 activation by exposed PS on the MK following apoptosis, thus apoptosis inhibition also prevented CD42b shedding and enriched the mature FV-labeled MKs. The role of apoptosis in platelet formation is controversial.76 Initial studies supporting the requirement of apoptosis in platelet formation showed that caspase inhibition blocked proplatelet formation in culture and that compartmentalized activation of caspases is necessary for cytoskeletal rearrangements during platelet shedding.77,78 However, later studies showed that mice deficient in various components of the intrinsic and extrinsic apoptotic pathways shed platelets normally.79,80 In fact, apoptosis regulation is crucial for keeping MKs alive during platelet formation.81 We observed increased in vivo platelet yield following infusion of Q-VD-Oph–treated MKs but decreased in vitro yield of PLPs from the same MKs. This may be due to differences in platelet formation in vitro and in vivo. It is tempting to speculate that in vitro PLPs may arise through an apoptotic-related process while platelet formation in vivo occurs through a different mechanism. Future studies examining in vitro and in vivo platelet formation from human MKs deficient in various components of the apoptotic pathways may shed more light on the relationship between survival, apoptosis, and platelet formation.
In conclusion, we have identified distinct maturation stages within in vitro MK culture. Within these stages, a transient, mature, functional HG/CD42b+ MK population becomes damaged and apoptotic over time. MKs at peak maturation endocytose FV, and this ability to endocytose FV correlates with readiness to release functional platelets when infused into immunodeficient mice. Additionally, we found that apoptosis inhibition protects the mature, platelet-producing MKs and results in increased platelet yield in vivo, but not in vitro. These studies provide new insights into megakaryopoiesis and thrombopoiesis, and offers new tools that may be beneficial for improving platelet production from cultured MKs and to study human-specific MK physiology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chintan Jobaliya and Rebecca Kathryn Meyer at the Stem Cell Core at the Children’s Hospital of Philadelphia for cell sorting.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL130698 (P.G., M.P., D.L.F.) and U01 HL099656 (P.G., M.P., D.L.F.).
Authorship
Contribution: X.S. carried out the majority of the described studies, interpreted data, designed studies, and prepared the manuscript and figures; D.J. helped with the mice infusion studies; V.H. performed the cremaster laser injury studies; H.A.H. and M.S.M. performed the immunofluorescence studies; R.M.C. provided the fluorescently labeled FV; M.P. assisted in the overall concept, data interpretation, and manuscript editing; and D.L.F. and P.G. provided overall scientific guidance and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Gadue, Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, CTRB 5012, Philadelphia, PA 19104; e-mail: gaduep@e-mail.chop.edu; or Mortimer Poncz, Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, ARC 317, Philadelphia, PA 19104; e-mail: poncz@e-mail.chop.edu; or Deborah L. French, Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, CTRB 5014, Philadelphia, PA 19104; e-mail: frenchd@e-mail.chop.edu.

![Figure 1. Two MK populations with distinct granularity are present in the in vitro human stem cell–derived MK culture. (A) Representative size (forward-scatter area [FSC-A]) and granularity (side-scatter area [SSC-A]) profiles of CD42a+ MKs at specified time points during the differentiation of human CD34+ hematopoietic progenitors. LG and HG MKs are gated as shown. (B) Day 14 LG CD42a+ MKs were sorted and cultured for another 6 days (top). Size and granularity of MKs 3 days and 6 days postsort are shown. Day 14 HG CD42a+ MKs were similarly sorted and cultured (bottom). Representative studies of 3 independent studies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/2/10.1182_blood-2017-01-761049/4/m_blood761049f1.jpeg?Expires=1769167966&Signature=MZzM2IKAdFeDIzNXjV2XTceG9UXotk8JXjong4pb9LJJfWvSMoIhxPyVWc6-su4jVyGmsK10ZtnNn-qMoyW~KZX-KgLWUR-PnFYMEDBuM2z20VpuQIXjLSe5ILZtod4AXk9k2PxPgMv1u3Z7rFpp1R01N-ejVleY3EDoKLG~58j~tilr4bwHNIATpMKuNXLXSEmrSx8JYGWv3RixBbpKtc5aUFxsaqjLeuN-deu-Y2DV3tvooDSdcqJN-4k6qW8TpcPilo3oH7~TUMyVCLmLNzvm3kCDUwYh4OMc~qJSoZbqe3EklTrx88HbDp~P1FCGz1lpwM2OdJtdGszcqP7~SQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. HG/CD42b+ MKs have characteristics of mature MKs. (A) Representative flow plots of CD42b expression on LG (green) and HG (red) MKs at specified time points of the differentiation. (B) Representative flow plots of Annexin V staining (left) and TUNEL staining (right) on day 14 MK cultures. (C) The percentage ± 1 SEM for each MK subpopulation at specified time points of the differentiation are shown of 4 independent studies. (D) Graph quantifying the percentages of MKs in each ploidy class ± 1 SEM of 5 independent studies. (E) Expression of α granule proteins (basic fibroblast growth factor [bFGF], vascular endothelial growth factor [VEGF], PF4, endostatin [Col18A]) and total RNA content of day 14 MKs was determined by intracellular staining and thiazole orange staining, respectively. Graphs quantifying the fold changes in mean fluorescence intensity over isotype/background ± 1 SEM are shown. (F) Day 14 MKs were stimulated with convulxin (500 ng/mL) or PAR-1–activating peptide (50 μM) for 20 minutes at room temperature. Representative flow plots showing percentages of activated (PAC-1+) MKs following convulxin stimulation (top). Black dotted line histogram indicates background staining in the absence of stimulation. Graph quantifying the percentages of PAC-1+ MKs following convulxin or PAR-1–activating peptide stimulation (bottom) (n = 5). *P < .05, **P < .01, ***P < .005, ****P < .001 for all statistical analyses shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/2/10.1182_blood-2017-01-761049/4/m_blood761049f2.jpeg?Expires=1769167966&Signature=zJr2~lKMvAFJmW3a1N~Wym~MRKo21z2BCXzxBHWMv~ieHuc3pijVsM8XefZOzBz3Wxepep~nNcesFEURble7WKtor5tm5H7CNOUPPYwIP0Vhx5wmAyhJVy0Chgx05yesiDlhPGT2N7PRbHbhYy2nJLKRemDKL9xGWnnwkOTZlNmzpRAbM0nbUFiCd0OCpD61QO70xi3l6ZXprXKBgySrfALmGRT2lY6jrsKyB54hmYHHpLiFW5psXLpYke7uonq0Dj9oXow-lkr31M4FqJ1T858hfVjFQn71FuFwt5sl55D8ovZTIjsd0q-vgOFAtptXFL1MImZP06sdswJQvmpP0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)