Key Points
Deficiency in twinfilin 2a causes macrothrombocytopenia and hyperreactivity of platelets in mice.
We provide the first in vivo evidence for an inhibitory function of twinfilin 2a in platelet actin dynamics.
Abstract
Regulated reorganization of the actin cytoskeleton is a prerequisite for proper platelet production and function. Consequently, defects in proteins controlling actin dynamics have been associated with platelet disorders in humans and mice. Twinfilin 2a (Twf2a) is a small actin-binding protein that inhibits actin filament assembly by sequestering actin monomers and capping filament barbed ends. Moreover, Twf2a binds heterodimeric capping proteins, but the role of this interaction in cytoskeletal dynamics has remained elusive. Even though Twf2a has pronounced effects on actin dynamics in vitro, only little is known about its function in vivo. Here, we report that constitutive Twf2a-deficient mice (Twf2a−/−) display mild macrothrombocytopenia due to a markedly accelerated platelet clearance in the spleen. Twf2a−/− platelets showed enhanced integrin activation and α-granule release in response to stimulation of (hem) immunoreceptor tyrosine-based activation motif (ITAM) and G-protein–coupled receptors, increased adhesion and aggregate formation on collagen I under flow, and accelerated clot retraction and spreading on fibrinogen. In vivo, Twf2a deficiency resulted in shortened tail bleeding times and faster occlusive arterial thrombus formation. The hyperreactivity of Twf2a−/− platelets was attributed to enhanced actin dynamics, characterized by an increased activity of n-cofilin and profilin 1, leading to a thickened cortical cytoskeleton and hence sustained integrin activation by limiting calpain-mediated integrin inactivation. In summary, our results reveal the first in vivo functions of mammalian Twf2a and demonstrate that Twf2a-controlled actin rearrangements dampen platelet activation responses in a n-cofilin– and profilin 1–dependent manner, thereby indirectly regulating platelet reactivity and half-life in mice.
Introduction
Platelets are small anucleate cell fragments that are continuously produced from megakaryocytes (MKs) in the bone marrow by a cytoskeleton-driven process. Proplatelet formation is characterized by the extension of long cytoplasmic protrusions into bone marrow sinusoids, where larger fragments, so-called preplatelets, are shed and further mature within the circulation. However, the molecular mechanisms orchestrating platelet biogenesis are incompletely understood.1 While microtubule sliding enables proplatelet elongation and transport of organelles, the actin cytoskeleton regulates branching of extending proplatelets, thereby increasing the number of available proplatelet tips.2,3 In line with this model, we previously demonstrated that the small actin-binding proteins ADF/cofilin and profilin 1 (Pfn1) are critical determinants of platelet formation and sizing.4,5
In circulating platelets, the actin cytoskeleton is essential to maintain cell morphology and exert key functions, such as the transduction of mechanical forces, granule release, and filopodia and lamellipodia formation.6 The critical role of the actin cytoskeleton for platelet production and function is further evidenced by the association of genetic variants in actin cytoskeleton-related genes such as DIAPH1,7 FLNA,8 WASP,9,10 ACTN1,9 MYH9,11 or TRPM712 with platelet disorders in humans and mice. In addition, orchestrated rearrangements of the actin cytoskeleton also control the affinity of αIIbβ3- and α2β1-integrins to their ligands, and deficiencies in actin-related proteins result in altered platelet and MK integrin function.4,13-18 Nonetheless, the precise molecular mechanisms linking actin dynamics and platelet integrin function have remained ill-defined.
Twinfilins (Twfs) are evolutionarily conserved (40 kDa) actin-binding proteins that are composed of 2 actin depolymerizing factor (ADF)-homology domains connected by a short linker region and followed by a C-terminal tail.19,20 Despite their pronounced effects on both actin filament assembly and disassembly in vitro, Twfs have so far received only limited attention, and their in vivo function is largely unknown. Previous studies showed that inactivation of Twf in budding yeast and fruit fly results in defects in actin-dependent processes and reduced viability.21-23 In mammals, the ubiquitously expressed Twf1 and Twf2a, as well as the muscle-specific Twf2b, have been identified.24-26 However, physiological functions of the mammalian Twf isoforms have not been reported to date.
Twfs inhibit actin polymerization on barbed ends by capping filament ends with a preference toward adenosine 5′-diphosphate (ADP)–bound actin. Both barbed and pointed end growth are additionally inhibited through Twf-mediated sequestration of ADP–G-actin monomers.21,27 Moreover, Twfs interact with heterodimeric capping proteins, which inhibits both actin filament depolymerization and the addition of new monomers to filament barbed ends. However, the mechanisms of this interaction, as well as its role in cytoskeletal dynamics, have remained unclear.25,28,29 Upon ADF/cofilin–mediated dissociation of G-actin monomers from actin filaments, Twf may compete with ADF/cofilin in binding to ADP–G-actin, thus favoring the release of these monomers from ADF/cofilin.24,30,31 Surprisingly, despite the central role of Twfs in actin dynamics, Twf2a-deficient mice (Twf2a−/−) are viable and apparently healthy. Since Twf2a is dispensable for mouse development, it was speculated that its lack might be compensated by the functionally redundant Twf1 isoform.26
Here, we report that Twf2a−/− mice display mild macrothrombocytopenia associated with a decreased platelet half-life and marked hyperreactivity of these cells in vitro and in vivo. In summary, these studies identify Twf2a as an important negative regulator of platelet reactivity and demonstrate for the first time an in vivo function of Twf-family proteins in mammals.
Materials and methods
Animals
Animal studies were approved by the district government of Lower Franconia (Bezirksregierung Unterfranken). Conditional Twf1-deficient mice were generated by intercrossing Twf1fl/fl mice (exon 3 flanked by loxP sites) with mice carrying the Cre-recombinase under the Pf4 promoter.32 Twf1fl/fl mice were obtained from EUCOMM (The European Conditional Mouse Mutagenesis Program; strain ID EM:05232). Constitutive Twf2a−/− mice have been described earlier.26 For all experiments, 12- to 16-week-old Twf2a−/− mice and the respective Twf2a+/+ littermate controls were used. All mice were derived from the breeding strategy Twf2a+/− × Twf2a+/−, resulting in 25% Twf2a+/+, 50% Twf2a+/−, and 25% Twf2a−/− mice.
Preparation of platelets, determination of platelet lifespan, count, and size,12,33 immunostaining of resting or spread platelets, flow cytometry, platelet adhesion under flow,33 immunoblotting, macrophage depletion and splenectomy,4 platelet terminal galactose levels,34 reticulated platelets, actin polymerization,12 in vivo thrombus formation, and tail bleeding time33 were performed as described previously, and detailed information can be found in supplemental Methods (available on the Blood Web site).
Data analysis
The presented results are mean ± standard deviation (SD) from at least 3 independent experiments per group, if not stated otherwise. Data distribution was analyzed using the Shapiro-Wilk test, and differences between control and Twf2a−/− mice were statistically analyzed using a Student t test or Wilcoxon-Mann-Whitney test. P values <.05 were considered statistically significant (*P < .05; **P < .01; ***P < .001). Results with a P value >.05 were considered not significant (NS).
Results
Twf2a−/− mice display macrothrombocytopenia
Expression profiling revealed that both Twf1/TWF1 and Twf2a/TWF2 are prominently expressed in murine and human platelets with a distinct subcellular localization, while the murine Twf2b isoform could not be detected (supplemental Figure 1). We generated MK- and platelet-specific Twf1-deficient (Twf1fl/fl-Pf4Cre) mice (supplemental Figure 2A) and found that platelet count, size, lifetime, and functionality were unaltered compared with controls, excluding an essential role of Twf1 for platelet production and function (supplemental Figure 2B-J). To test whether Twf2a may compensate for the lack of Twf1 in MKs and platelets, we capitalized on constitutive Twf2a−/− mice.26 Immunoblot analysis on platelet lysates confirmed ablation of Twf2a protein expression, whereas no compensatory upregulation of Twf1 was detectable (supplemental Figure 3A). Twf2a−/− mice displayed macrothrombocytopenia (Figure 1A-B) and, in line with the increased platelet size, a slightly increased surface abundance of major platelet glycoproteins (supplemental Figure 3B). Ultrastructurally, Twf2a−/− platelets showed an enlarged and spherical morphology, with a normal number of α-, δ-granules as well as mitochondria, but an increased presence of vacuoles (supplemental Figure 4). In contrast, leukocyte populations in blood, spleen, lymph nodes, and thymus (supplemental Figure 5) were grossly unaltered in mutant mice as compared with controls. These results suggested a critical and specific role of Twf2a in the regulation of circulating platelet numbers that is nonredundant to that of the closely related Twf1 isoform.
Accelerated platelet clearance accounts for macrothrombocytopenia in Twf2a−/− mice. (A-B) Platelet count (A) and size (B) were determined with an automated cell analyzer (Sysmex). (C) Platelet lifespan was assessed by flow cytometric measurement of the fluorescence-positive platelet population at the indicated time points after injection of a fluorophore-conjugated anti-GPIX antibody derivative. (D-E) Platelet counts were monitored over time after clodronate-encapsulated liposome-mediated macrophage depletion (D) and splenectomy (E). (F-G) The relative platelet content in control and Twf2a−/− spleens was determined by immunostaining (platelet GPIb, cyan; endothelial CD105, magenta) and confocal microscopy of cryosections. Scale bars, 250 μm. Images were acquired with a TCS SP8 confocal microscope (25×/0.95 FLUOTAR VISIR water objective, Leica Microsystems) and are representative of at least 4 individuals. (H-I) The fraction of aged platelets was assessed by platelet lectin-binding (Erythrina crista-galli lectin [ECL] and Ricinus communis agglutinin [RCA]) and the fraction of young (RNA-rich) platelets by thiazole orange (TO) staining and flow cytometry. The overall gated platelet population (based on forward sideward characteristics and GPIX labeling) was set as 100%. Neuraminidase-treated platelets were used as a positive control (H) and RNase-treated platelets as a negative control (I). Values represent mean ± SD (n = 6). Each symbol represents 1 individual. Horizontal lines represent mean (I). ***P < .001, **P < .01, and *P < .05, unpaired Student t test. NS, not significant; WT, wild-type.
Accelerated platelet clearance accounts for macrothrombocytopenia in Twf2a−/− mice. (A-B) Platelet count (A) and size (B) were determined with an automated cell analyzer (Sysmex). (C) Platelet lifespan was assessed by flow cytometric measurement of the fluorescence-positive platelet population at the indicated time points after injection of a fluorophore-conjugated anti-GPIX antibody derivative. (D-E) Platelet counts were monitored over time after clodronate-encapsulated liposome-mediated macrophage depletion (D) and splenectomy (E). (F-G) The relative platelet content in control and Twf2a−/− spleens was determined by immunostaining (platelet GPIb, cyan; endothelial CD105, magenta) and confocal microscopy of cryosections. Scale bars, 250 μm. Images were acquired with a TCS SP8 confocal microscope (25×/0.95 FLUOTAR VISIR water objective, Leica Microsystems) and are representative of at least 4 individuals. (H-I) The fraction of aged platelets was assessed by platelet lectin-binding (Erythrina crista-galli lectin [ECL] and Ricinus communis agglutinin [RCA]) and the fraction of young (RNA-rich) platelets by thiazole orange (TO) staining and flow cytometry. The overall gated platelet population (based on forward sideward characteristics and GPIX labeling) was set as 100%. Neuraminidase-treated platelets were used as a positive control (H) and RNase-treated platelets as a negative control (I). Values represent mean ± SD (n = 6). Each symbol represents 1 individual. Horizontal lines represent mean (I). ***P < .001, **P < .01, and *P < .05, unpaired Student t test. NS, not significant; WT, wild-type.
Markedly reduced platelet lifespan in Twf2a−/− mice
Next, we analyzed the cause of the observed thrombocytopenia and found that Twf2a−/− mice had a markedly decreased platelet lifespan (T1/2 of control platelets: 48.6 ± 2.0 hours vs T1/2 of Twf2a−/− platelets: 22.3 ± 2.11 hours; ***P < .001) in vivo (Figure 1C). Assessment of surface-bound antibodies revealed no significant differences between platelets of control and Twf2a−/− mice (supplemental Figure 6A), suggesting that the accelerated clearance of Twf2a−/− platelets is not caused by autoantibodies. In agreement, depletion of macrophages using clodronate-encapsulated liposomes increased platelet counts in control and similarly, although to a lesser extent, Twf2a−/− mice, demonstrating that Twf2a−/− platelets were only in part removed from the circulation by macrophages (Figure 1D; supplemental Figure 6B). Interestingly, however, splenectomy restored platelet counts in Twf2a−/− mice to those seen in controls (Figure 1E; supplemental Figure 6C), suggesting that macrophage-independent clearance of Twf2a−/− platelets by cells of the reticuloendothelial system also contributes to the thrombocytopenia in these animals. In support of this, we found an expansion of the red pulp (54.42% ± 5.73% of the cross-sectional area in control spleens vs 66.42% ± 2.92% of the cross-sectional area in Twf2a−/− spleens; ***P < .001; supplemental Figure 7) and an increased fraction of platelets in the spleen of Twf2a−/− mice (Figure 1F-G). The accelerated platelet clearance in Twf2a−/− mice was associated with an increased number of young platelets in the circulation, as evidenced by a significantly reduced percentage of desialylated platelets and an increased prevalence of reticulated (RNA-rich) platelets (Figure 1H-I),35 indicating that increased platelet production may partially compensate for the accelerated platelet clearance. In conclusion, these results indicated that the thrombocytopenia in Twf2a−/− mice is, to a large extent, caused by premature clearance of platelets in the spleen.
Twf2a is a negative regulator of platelet reactivity
We next hypothesized that the reduced platelet half-life and hence the resulting thrombocytopenia might be a result of premature platelet activation or consumption and assessed agonist-induced platelet integrin inside-out activation and degranulation (P-selectin exposure) by flow cytometry. Interestingly, Twf2a−/− platelets displayed a pronounced hyperreactivity toward all tested agonists with both increased integrin activation and α-granule release (Figure 2A-B). Of note, Twf2a−/− platelets did not display a reduced sensitivity toward inhibitors of platelet activity, since similar to controls, pretreatment with prostacyclin (PGI2) resulted in reduced platelet integrin activation and degranulation in response to all tested agonists (supplemental Figure 8). The enhanced activation response to agonist stimulation translated into markedly increased adhesion and aggregate formation on collagen I under flow (Figure 2C-D). Furthermore, Twf2a−/− platelets displayed enhanced integrin outside-in signaling as detected by accelerated spreading on fibrinogen (Figure 2E; supplemental Figure 9; supplemental Videos 1 and 2) and clot retraction (Figure 2F).
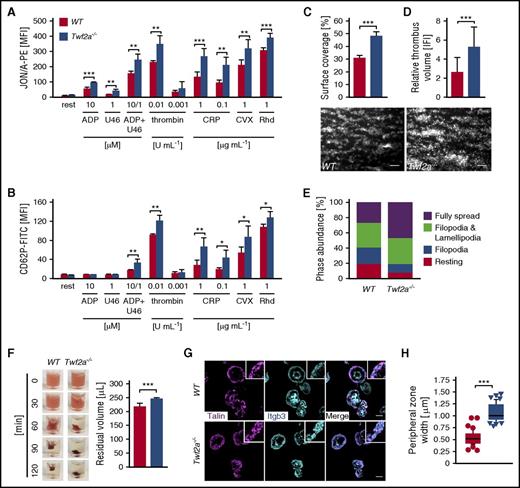
Increased integrin activation in Twf2a−/− platelets. (A-B) Platelet αIIbβ3-integrin (JON/A-PE) activation (A) and P-selectin (anti-CD62P-FITC) exposure (B), serving as a measure for α-granule release, were determined by flow cytometry. (C-D) Platelet adhesion (C) and thrombus formation (D) under flow (1000 s−1) were analyzed in a flow chamber system. Scale bars, 25 μm. Images were acquired with a Zeiss Axiovert 200 inverted microscope (40×/0.6 oil objective). (E) Washed platelets were allowed to spread (15 min) on fibrinogen (100 μg/mL), and phase abundance was determined. (F) Platelet clot retraction in response to stimulation with thrombin (5 U/mL) over time. Residual plasma volume was determined 120 minutes after the addition of thrombin. Images are representative of 4 individuals. (G) Tln recruitment to β3-integrin tails was assessed by immunostaining (talin, magenta; Itgb3, cyan) and confocal microscopy. Scale bars, 3 μm. Images were acquired with a TCS SP8 confocal microscope (100×/1.4 STED WHITE oil objective, Leica Microsystems). (H) Quantification of the peripheral zone (talin and β3-integrin co-localization) width. Values are mean ± SD (n = 6). Images are representative of at least 6 individuals. ***P < .001, **P < .01, and *P < .05, unpaired Student t test (A-G) and Wilcoxon-Mann-Whitney test (H). CRP, collagen-related peptide; CVX, convulxin; rest, resting; Rhd, rhodocytin; U46, U46619, a stable thromboxane A2 analog.
Increased integrin activation in Twf2a−/− platelets. (A-B) Platelet αIIbβ3-integrin (JON/A-PE) activation (A) and P-selectin (anti-CD62P-FITC) exposure (B), serving as a measure for α-granule release, were determined by flow cytometry. (C-D) Platelet adhesion (C) and thrombus formation (D) under flow (1000 s−1) were analyzed in a flow chamber system. Scale bars, 25 μm. Images were acquired with a Zeiss Axiovert 200 inverted microscope (40×/0.6 oil objective). (E) Washed platelets were allowed to spread (15 min) on fibrinogen (100 μg/mL), and phase abundance was determined. (F) Platelet clot retraction in response to stimulation with thrombin (5 U/mL) over time. Residual plasma volume was determined 120 minutes after the addition of thrombin. Images are representative of 4 individuals. (G) Tln recruitment to β3-integrin tails was assessed by immunostaining (talin, magenta; Itgb3, cyan) and confocal microscopy. Scale bars, 3 μm. Images were acquired with a TCS SP8 confocal microscope (100×/1.4 STED WHITE oil objective, Leica Microsystems). (H) Quantification of the peripheral zone (talin and β3-integrin co-localization) width. Values are mean ± SD (n = 6). Images are representative of at least 6 individuals. ***P < .001, **P < .01, and *P < .05, unpaired Student t test (A-G) and Wilcoxon-Mann-Whitney test (H). CRP, collagen-related peptide; CVX, convulxin; rest, resting; Rhd, rhodocytin; U46, U46619, a stable thromboxane A2 analog.
Talin (Tln) recruitment to β-integrin tails represents a key step in integrin activation. In agreement with the enhanced integrin activation, we found increased Tln and β3-integrin colocalization on the leading edge of spread Twf2a−/− platelets compared with controls, as evidenced by a doubling of the width (0.54 ± 0.13 μm for controls vs 1.08 ± 0.18 μm in Twf2a−/− platelets; ***P < .001) of the cortical zone (Figure 2G-H).
Accelerated arterial thrombus formation in Twf2a−/− mice
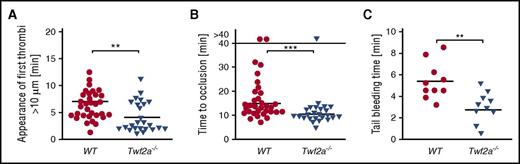
To investigate whether the platelet hyperreactivity observed in vitro can similarly be observed in vivo, we next analyzed arterial thrombus formation and hemostatic function in Twf2a−/− mice. Upon FeCl3-induced damage of the endothelium in mesenteric arterioles, the time to initiation of thrombus formation (7.4 ± 2.9 minutes in control vs 4.8 ± 3.1 minutes in Twf2a−/− mice; **P < .01) and vessel occlusion (15.6 ± 6.3 minutes in control vs 10.6 ± 2.4 minutes in Twf2a−/− mice; ***P < .001) was significantly lower in Twf2a−/− mice than in controls (Figure 3A-B). Similarly, hemostatic plug formation and cessation of bleeding (5.4 ± 1.8 minutes in control vs 2.8 ± 1.5 minutes in Twf2a−/− mice; **P < .01) in a tail bleeding time model was markedly accelerated in Twf2a−/− mice (Figure 3C). These results further corroborated the notion that Twf2a acts as negative regulator of platelet reactivity.
Twf2a is a critical regulator of platelet reactivity in vivo. (A-B) Accelerated adhesion of platelets (A) and occlusion of mesenteric arterioles (B) in Twf2a−/− mice upon FeCl3-induced injury of the endothelial barrier. Each symbol represents 1 mesenteric arteriole (n = 12 individuals). (C) Accelerated hemostatic plug formation in Twf2a−/− mice. Each symbol represents 1 individual (n = 10 individuals). Horizontal lines represent mean. ***P < .001 and **P < .01, Wilcoxon-Mann-Whitney test (A-B) and unpaired Student t test (C).
Twf2a is a critical regulator of platelet reactivity in vivo. (A-B) Accelerated adhesion of platelets (A) and occlusion of mesenteric arterioles (B) in Twf2a−/− mice upon FeCl3-induced injury of the endothelial barrier. Each symbol represents 1 mesenteric arteriole (n = 12 individuals). (C) Accelerated hemostatic plug formation in Twf2a−/− mice. Each symbol represents 1 individual (n = 10 individuals). Horizontal lines represent mean. ***P < .001 and **P < .01, Wilcoxon-Mann-Whitney test (A-B) and unpaired Student t test (C).
Sustained integrin activation contributes to the hyperreactivity of Twf2a−/− platelets
We next sought to identify the cause of the sustained integrin activation in Twf2a−/− platelets. It has previously been reported that sustained activation of platelet αIIbβ3-integrins and the exposure of phosphatidylserine (PS) on the outer platelet membrane leaflet are critically linked. Upon sustained Ca2+ signaling, αIIbβ3-integrins can be switched back to their low-affinity state through Ca2+-dependent activation of the protease calpain and cleavage of the cytoplasmic tails of β3-integrins.36-38
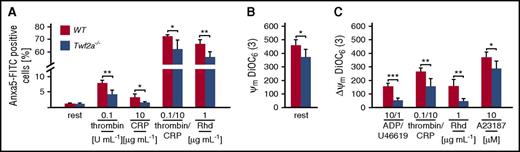
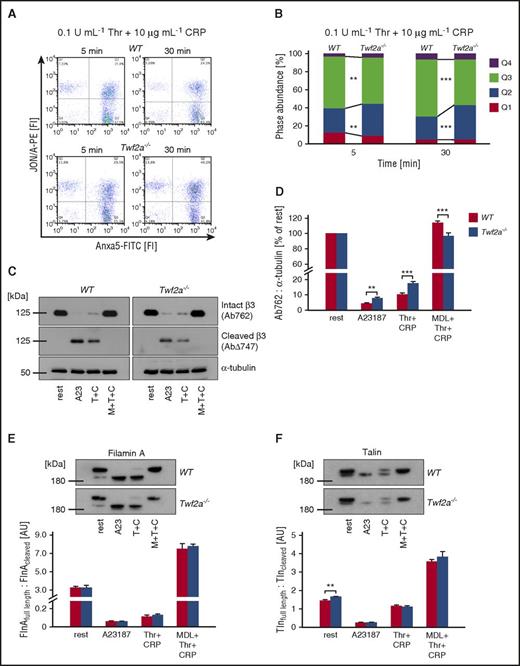
Strikingly, whereas Anxa5 fluorescein isothiocyanate (FITC) binding to individual Twf2a−/− platelets (mean fluorescence intensity of the PS-positive population) was unaltered, fewer mutant platelets exposed PS on their surface (Figure 4A). Moreover, in line with the reduced percentage of PS exposing platelets, activation-induced mitochondrial depolarization was less pronounced in Twf2a−/− platelets (Figure 4B-C). Time-course experiments assessing the distribution of platelets with activated αIIbβ3-integrins (quadrant Q1), PS exposure (Q3), or both (Q2) revealed an increased percentage of Twf2a−/− platelets with activated integrins (events in Q1 and Q2) after both 5 minutes (39.1% ± 3.7% for controls and 43.9% ± 2.9% for Twf2a−/− platelets; *P < .05) and 30 minutes (31.8% ± 1.5% for controls and 43.3% ± 3.6% for Twf2a−/− platelets; ***P < .001) upon simultaneous stimulation with thrombin and collagen-related peptide (Figure 5A-B; supplemental Figure 10). Assessment of calpain-mediated β3-integrin cleavage by immunoblotting revealed a decreased loss of full-length β3-integrin in Twf2a−/− platelets as compared with control (Figure 5C-D). These results strongly suggested altered Ca2+ signaling or calpain activity in Twf2a−/− platelets; however, we did not observe differences in store-operated Ca2+ entry or alterations in the cleavage of further calpain targets such as filamin A or Tln upon stimulation with different agonists (Figure 5E-F; supplemental Figure 11).
Twf2a−/− platelets are less prone to apoptosis. Platelet apoptosis was assessed using Anxa5-FITC binding to exposed phosphatidylserine (A) or the mitochondrial membrane potential sensor 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3); B-C). A23187, Ca2+ ionophore; CRP, collagen-related peptide; rest, resting; Rhd, rhodocytin; U46, U46619, a stable thromboxane A2 analog. Values are mean ± SD (n = 6). ***P < .001, **P < .01, and *P < .05, unpaired Student t test.
Twf2a−/− platelets are less prone to apoptosis. Platelet apoptosis was assessed using Anxa5-FITC binding to exposed phosphatidylserine (A) or the mitochondrial membrane potential sensor 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3); B-C). A23187, Ca2+ ionophore; CRP, collagen-related peptide; rest, resting; Rhd, rhodocytin; U46, U46619, a stable thromboxane A2 analog. Values are mean ± SD (n = 6). ***P < .001, **P < .01, and *P < .05, unpaired Student t test.
Delayed integrin closure accounts for the hyperresponsiveness of Twf2a−/− platelets. (A) Washed platelets were simultaneously stimulated with thrombin (Thr/T) and collagen-related peptide (CRP/C) for the indicated periods of time. Activation of αIIbβ3-integrins (JON/A-PE) and phosphatidylserine exposure (Anxa5-FITC) on the outer leaflet of the platelet membrane was determined by flow cytometry. Flow cytometry plots are representative of at least 6 individuals. (B) Percentage of cells per quadrant. Q1, JON/A+ Anxa5− (top left); Q2, JON/A+ Anxa5+ (top right); Q3, JON/A− Anxa5+ (bottom right); Q4, JON/A− Anxa5− (bottom left). Values are mean (n = 6). (C-F) Platelets were left untreated or preincubated for 10 minutes in the presence of the calpain inhibitor MDL-28170 (MDL/M, 200 μM). Subsequently samples were stimulated with the calcium ionophore A23187 (A23, 10 μM) or thrombin (0.1 U/mL) and CRP (10 μg/mL), lysed, and processed for immunoblotting. Full-length (Ab762) and calpain-cleaved (AbΔ747) β3-integrin (C), as well as filamin A (E), talin (F), and α-tubulin, were probed with the respective antibodies and analyzed by densitometry. Values are mean ± SD (n = at least 4). ***P < .001, **P < .01, and *P < .05, unpaired Student t test.
Delayed integrin closure accounts for the hyperresponsiveness of Twf2a−/− platelets. (A) Washed platelets were simultaneously stimulated with thrombin (Thr/T) and collagen-related peptide (CRP/C) for the indicated periods of time. Activation of αIIbβ3-integrins (JON/A-PE) and phosphatidylserine exposure (Anxa5-FITC) on the outer leaflet of the platelet membrane was determined by flow cytometry. Flow cytometry plots are representative of at least 6 individuals. (B) Percentage of cells per quadrant. Q1, JON/A+ Anxa5− (top left); Q2, JON/A+ Anxa5+ (top right); Q3, JON/A− Anxa5+ (bottom right); Q4, JON/A− Anxa5− (bottom left). Values are mean (n = 6). (C-F) Platelets were left untreated or preincubated for 10 minutes in the presence of the calpain inhibitor MDL-28170 (MDL/M, 200 μM). Subsequently samples were stimulated with the calcium ionophore A23187 (A23, 10 μM) or thrombin (0.1 U/mL) and CRP (10 μg/mL), lysed, and processed for immunoblotting. Full-length (Ab762) and calpain-cleaved (AbΔ747) β3-integrin (C), as well as filamin A (E), talin (F), and α-tubulin, were probed with the respective antibodies and analyzed by densitometry. Values are mean ± SD (n = at least 4). ***P < .001, **P < .01, and *P < .05, unpaired Student t test.
Together, these results reveal sustained integrin activation due to reduced calpain-mediated integrin closure as cause of the pronounced hyperreactivity of Twf2a−/− platelets in different in vitro and in vivo settings.
Twf2a is a negative regulator of actin filament assembly in platelets
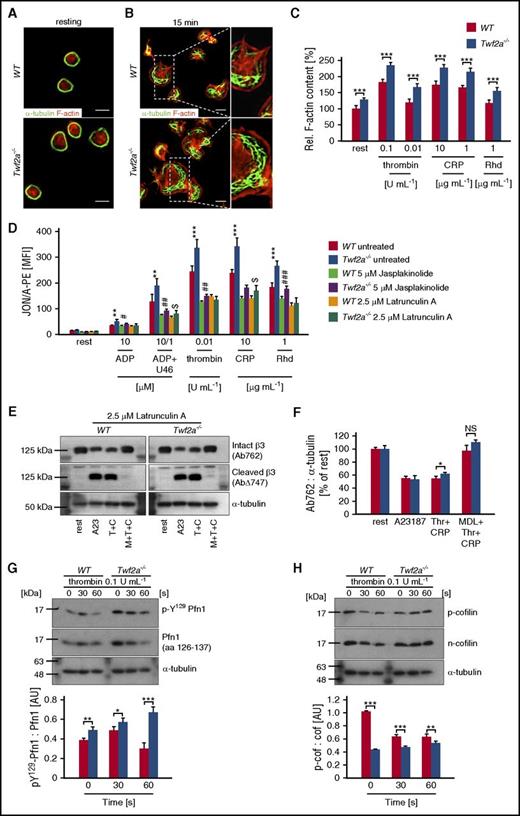
Given the important role of Twfs in actin dynamics and the central role of actin rearrangements for integrin activation, we hypothesized that altered cytoskeletal dynamics may contribute to the observed hyperreactivity of Twf2a−/− platelets by restricting the accessibility of β3-integrin tails to calpain.19,22,30,39 Morphological analyses, however, revealed no gross cytoskeletal alterations but did reveal a mildly enlarged size of resting Twf2a−/− platelets (Figure 6A; supplemental Figure 4). Strikingly, upon spreading, Twf2a−/− platelets displayed an increased size and thickened cortical actin cytoskeleton (Figure 6B) correlating with the enlarged colocalization area of Tln and β3-integrins (Figure 2G-H). This was further supported by an increased content of filamentous actin in resting and activated Twf2a−/− platelets (Figure 6C), which is in agreement with previous reports showing an inhibitory function of fly and yeast Twfs in actin dynamics.19,22,40
Disinhibition of actin assembly prevents integrin closure in Twf2a−/− platelets. (A-B) Resting (A) or fibrinogen-spread (B) platelets were stained for F-actin (red) and α-tubulin (green) after the indicated time periods. Images were acquired with a TCS SP8 confocal microscope (100×/1.4 STED WHITE oil objective, Leica Microsystems). Scale bars, 3 μm. Images are representative of at least 6 individuals. (C) Relative F-actin content of resting and activated platelets was determined by flow cytometry. (D) Control (dimethyl sulfoxide) or toxin-treated (5 μM jasplakinolide, 2.5 μM latrunculin A) platelets remained resting or were activated with the indicated agonists and concentrations. αIIbβ3-integrin activation (JON/A-PE) was determined by flow cytometry. (E-F) Latrunculin A–treated (2.5 μM for 10 minutes) platelets were left untreated (rest) or preincubated for 10 minutes in the presence of the calpain inhibitor MDL-28170 (200 μM). Subsequently, samples were stimulated with the Ca2+ ionophore A23187 (10 μM) or thrombin (0.1 U/mL) and collagen-related peptide (10 μg/mL), lysed, and processed for immunoblotting. Full-length (Ab762) and calpain-cleaved (AbΔ747) β3-integrin, as well as α-tubulin, were probed with the respective antibodies and analyzed by densitometry. (G-H) Platelets were left untreated or stimulated for the indicated time points with 0.1 U/mL thrombin, lysed, and processed for immunoblotting. Total Pfn1 (aa 126-137), phospho-Y129 Pfn1 (G), total n-cofilin, phospho-cofilin (H), and α-tubulin were probed with the respective antibodies and analyzed by densitometry. Values are mean ± SD (n = at least 4). A23, A23187 (Ca2+ ionophore); CRP/C, collagen-related peptide; MDL/M, MDL28170 (calpain inhibitor); Rhd, rhodocytin; Thr/T, thrombin; U46, U46619 (synthetic thromboxane A2 analog). ***,###P < .001, **,##P < .01, and #,$P < .05, unpaired Student t test.
Disinhibition of actin assembly prevents integrin closure in Twf2a−/− platelets. (A-B) Resting (A) or fibrinogen-spread (B) platelets were stained for F-actin (red) and α-tubulin (green) after the indicated time periods. Images were acquired with a TCS SP8 confocal microscope (100×/1.4 STED WHITE oil objective, Leica Microsystems). Scale bars, 3 μm. Images are representative of at least 6 individuals. (C) Relative F-actin content of resting and activated platelets was determined by flow cytometry. (D) Control (dimethyl sulfoxide) or toxin-treated (5 μM jasplakinolide, 2.5 μM latrunculin A) platelets remained resting or were activated with the indicated agonists and concentrations. αIIbβ3-integrin activation (JON/A-PE) was determined by flow cytometry. (E-F) Latrunculin A–treated (2.5 μM for 10 minutes) platelets were left untreated (rest) or preincubated for 10 minutes in the presence of the calpain inhibitor MDL-28170 (200 μM). Subsequently, samples were stimulated with the Ca2+ ionophore A23187 (10 μM) or thrombin (0.1 U/mL) and collagen-related peptide (10 μg/mL), lysed, and processed for immunoblotting. Full-length (Ab762) and calpain-cleaved (AbΔ747) β3-integrin, as well as α-tubulin, were probed with the respective antibodies and analyzed by densitometry. (G-H) Platelets were left untreated or stimulated for the indicated time points with 0.1 U/mL thrombin, lysed, and processed for immunoblotting. Total Pfn1 (aa 126-137), phospho-Y129 Pfn1 (G), total n-cofilin, phospho-cofilin (H), and α-tubulin were probed with the respective antibodies and analyzed by densitometry. Values are mean ± SD (n = at least 4). A23, A23187 (Ca2+ ionophore); CRP/C, collagen-related peptide; MDL/M, MDL28170 (calpain inhibitor); Rhd, rhodocytin; Thr/T, thrombin; U46, U46619 (synthetic thromboxane A2 analog). ***,###P < .001, **,##P < .01, and #,$P < .05, unpaired Student t test.
To test whether increased actin polymerization could contribute to the hyperreactivity, platelets of control and Twf2a−/− mice were pretreated with vehicle (dimethyl sulfoxide) or toxins interfering with actin dynamics (jasplakinolide or latrunculin A). Pretreatment with the actin assembly–promoting toxin jasplakinolide impaired integrin activation overall in control and Twf2a−/− platelets as compared with vehicle-treated samples, but it did not revert the hyperresponsiveness of Twf2a−/− platelets (Figure 6D; supplemental Figure 12). In contrast, pretreatment with the F-actin–destabilizing toxin latrunculin A efficiently inhibited actin polymerization and similarly impaired αIIbβ3-integrin activation and, to a lesser extent, degranulation in control and Twf2a−/− platelets (Figure 6D; supplemental Figures 12 and 13). In addition, latrunculin A pretreatment restored calpain-mediated β3-integrin cleavage in stimulated Twf2a−/− platelets to the levels observed in controls (Figure 6E-F).
Given the central role of Twf2a in actin dynamics,19-23 we next studied the molecular mechanisms leading to increased actin polymerization and hence restriction of integrin inactivation in Twf2a−/− platelets. Assessment of the activation-dependent phosphorylation of Pfn1 on tyrosine 129, which is known to increase Pfn1’s affinity toward G-actin monomers and its actin polymerization activity,41 revealed a significantly higher activity of Pfn1 in Twf2a−/− platelets (Figure 6G). Similarly, Twf2a−/− platelets showed increased levels of dephosphorylated, active n-cofilin that acts in concert with Pfn1 in enhancing actin dynamics by providing free barbed ends and thereby promoting actin polymerization (Figure 6H).42
These data provide the first in vivo evidence for an inhibitory function of mammalian Twf2a in F-actin assembly during platelet activation by fine-tuning Pfn1 as well as n-cofilin function and thereby limiting platelet integrin activation and reactivity.
In conclusion, our results support a critical role of Twf2a and the actin cytoskeleton in integrin activation and strongly suggest that altered cytoskeletal dynamics account for the sustained integrin activation in Twf2a−/− platelets, presumably by sterically limiting calpain-mediated integrin closure.
Discussion
In the present study, we demonstrate for the first time an inhibitory function of mammalian Twf2a in actin dynamics in vivo by limiting Pfn1 and n-cofilin activity, with critical implications for platelet biogenesis, reactivity, and turnover. Our findings point to specific functions of Twf2a in these processes, since the Twf1 isoform could not compensate for the lack of Twf2a (supplemental Figure 3A). In support of this, Twf1fl/fl-Pf4Cre MKs and platelets did not display any overt phenotype in the tested experimental settings (supplemental Figures 2B-J and 14).
Although TWF1 and TWF2 have recently been implicated in human disorders,43-46 so far, TWFs have not been associated with platelet disorders. However, given the relatively moderate macrothrombocytopenia in Twf2a−/− mice, we speculate that humans carrying variants in TWF2 might not show overt clinical manifestations of a platelet disorder. Hence, TWF2-related abnormalities of platelet function could be overseen in routine diagnostic checkups. However, based on our data, it is tempting to speculate that genetic variants in TWF2 could be associated with an increased risk of platelet-dependent thrombotic events, but this will need further investigation.
Our results revealed accelerated clearance of hyperresponsive Twf2a−/− platelets from the circulation by the reticuloendothelial system in the spleen as the cause of macrothrombocytopenia, which might be partially compensated by increased platelet production (Figure 1C-I; supplemental Figures 6 and 7). Based on our findings, we speculate that platelets in the circulation are frequently exposed to trace amounts of agonist such as ADP/adenosine triphosphate released from damaged cells, very small amounts of locally produced thrombin, and, importantly, shear gradients in the vascular system, which may not be sufficient to fully activate them in healthy conditions.47 However, if platelets are hyperreactive, these factors may induce an activation state that leads to their rapid clearance by the reticuloendothelial system. In support of this, we found an increased fraction of platelets in the spleen of Twf2a−/− mice as compared with controls, which was independent of macrophages, since splenectomy, but not clodronate-mediated macrophage depletion, could normalize platelet counts (Figure 1C-G; supplemental Figure 6). Furthermore, we found an elevated fraction of young, reticulated, and sialylated platelets (Figure 1H-I), which have previously been implicated as a marker of increased thrombopoiesis.35,48
The pronounced hyperreactivity of Twf2a−/− platelets in vitro as well as in vivo was characterized by sustained agonist-induced recruitment of Tln to β3-integrin tails (Figures 2 and 3) that coincided with a reduced fraction of PS-exposing platelets (Figure 4A). In addition, despite the strong dependency of PS exposure on sustained Ca2+ signaling, we could not detect altered Ca2+ entry into Twf2a−/− platelets in response to thrombin or collagen-related peptide (supplemental Figure 11). This finding is also of particular interest with regards to the enhanced degranulation, a process strongly dependent on Ca2+ signaling. However, besides Ca2+, the actin cytoskeleton is also critically involved in regulating platelet granule release,49 suggesting that altered actin dynamics may account for the increased α-granule release in Twf2a−/− platelets.
The hyperresponsiveness of Twf2a−/− platelets is different from the described platelet phenotypes of mice lacking immunoreceptor tyrosine-based inhibition motif signaling components such as the leukocyte-associated immunoglobulin-like receptor-1 or the associated signaling molecules, Src-like adapter proteins SLAP and SLAP2, in which platelets showed a (hem) immunoreceptor tyrosine-based activation motif (ITAM)-specific hyperreactivity.50,51 Moreover, hyperreactive Stim1+/Sax platelets (due to a constitutive store-operated Ca2+ entry) showed selectively impaired (hem)ITAM signaling.52 Finally, Ras GTPase-activating protein 3 mutant mice showed increased platelet activation and a markedly accelerated platelet turnover that was independent of splenic clearance.53 Our findings on the first in vivo function of mammalian Twf2a are thus clearly distinct from previously identified negative regulators of platelet activity and highlight the central role of the actin cytoskeleton in regulating platelet function and turnover.
The precise molecular function of Twfs in vivo is still a matter of debate. Twfs were shown to inhibit barbed end growth by capping of actin filaments and pointed end growth by sequestration of G-actin monomers, as well as acceleration of filament depolymerization.27,40 Twfs also interact with capping protein, but whether this interaction contributes to filament assembly, disassembly, or proper subcellular localization of Twfs has remained elusive.25,27,29,31 We found increased actin polymerization in the absence of Twf2a, thus revealing for the first time an inhibitory function of mammalian Twf2a on actin assembly in vivo (Figure 6C). The increase in filamentous actin was particularly prominent at the cell cortex, coinciding with the localization of Twf2a in spread murine and human platelets (Figure 6B; supplemental Figure 1B,D).24 Based on this, we speculate that Twf2a specifically controls actin filament assembly and disassembly in platelets to regulate the width of lamellipodia and thereby regulates integrin activation, cell adhesion, and spreading. This function appears to be specific to Twf2a, since Twf1fl/fl-Pf4Cre platelets showed normal platelet reactivity, cytoskeletal architecture, and dynamics (supplemental Figures 2 and 14).
In Twf2a−/− platelets, the lack of Twf2a’s G-actin sequestering function may increase the pool of free G-actin in mutant platelets, thereby enhancing n-cofilin activity, which generates free barbed ends, favoring actin polymerization, as well as promote Pfn1- and cyclase-associated protein–mediated nucleotide exchange and actin polymerization, particularly at the cell cortex (Figure 6B-C,G-H). In line with this, we have previously observed a defective cortical F-actin meshwork in spread Pfn1-deficient platelets.4 The accelerated spreading and lamellipodia formation of Twf2a−/− platelets further support this hypothesis (Figures 2E,G and 6B; supplemental Figure 9; supplemental Videos 1 and 2). In addition, the importance of Twf2a’s inhibitory function on actin assembly has previously been shown in vitro by Twf2a-mediated limitation of stereocilia elongation in the cochlea, which is a prerequisite for normal audition.54
In agreement with the critical role of the cytoskeleton for integrin inactivation, cleavage of β3-integrin tails and hence dissociation of the integrin–cytoskeleton linkage was reduced in stimulated Twf2a−/− platelets even when using the A23187 ionophore (Figure 5; supplemental Figure 10).37,55 Strikingly, toxin-induced depolymerization of the actin cytoskeleton could restore the hyperreactivity and calpain-mediated cleavage of β3-integrin tails in Twf2a−/− platelets similar to that of controls (Figure 6D-F; supplemental Figures 12 and 13). In addition, our results suggest normal calpain activity in Twf2a−/− platelets, since the cleavage of FlnA and Tln in resting and stimulated Twf2a−/− platelets was unaltered as compared with controls (Figure 5E-F). Moreover, chemical inhibition of calpain activity as well as genetic deletion of calpain-1 resulted in decreased platelet degranulation, aggregation, and spreading, the latter of which was characterized by limited filopodia and lamellipodia formation, which stands in stark contrast to our findings in Twf2a−/− platelets.56-58 Based on these results, we hypothesize that in the absence of Twf2a−/−, enhanced actin dynamics via increased activity of Pfn1 and n-cofilin results in a thickened cortical cytoskeleton, which may sterically limit the accessibility of β3-integrins, hence restricting calpain-mediated cleavage of β3-integrin tails and leading to the unremitting recruitment and association of Tln. Ultimately, the resulting sustained integrin activation manifests as pronounced hyperresponsiveness of Twf2a−/− platelets.
In summary, our study provides the first direct in vivo evidence for an inhibitory function of mammalian Twf2a, but not the closely related Twf1 isoform, in actin dynamics, with implications for thrombopoiesis, platelet function, and turnover. These results point to a novel cytoskeleton-related cause of macrothrombocytopenia that is independent of platelet production and highlight the critical need to monitor platelet function in patients with variants in actin cytoskeleton–associated genes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stefanie Hartmann, Sylvia Hengst, and Jonas Müller for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (NI 556/11-1) (B.N.). S.S. was supported by a grant from the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg, a research fellowship of the Deutsche Forschungsgemeinschaft (STR 1538/1-1), and a nonstipendiary long-term fellowship from the European Molecular Biology Organization (ALTF 86-2017). M.B. is supported by an Emmy Noether grant from the Deutsche Forschungsgemeinschaft (BE5084/3-1).
Authorship
Contribution: S.S. designed research, performed experiments, analyzed data, and wrote the manuscript; S.B., I.C.B., T.V., M.B., and A.B. performed experiments, analyzed data, and commented on the manuscript; M.H., X.D., and P.L. provided vital reagents and commented on the manuscript; K.G.H. provided technical support with imaging and commented on the manuscript; and B.N. supervised research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.S. is Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden.
The current affiliation for T.V. is Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom.
Correspondence: Bernhard Nieswandt, Institute of Experimental Biomedicine I, University Hospital and Rudolf Virchow Center, University of Würzburg, Josef-Schneider-Str 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de; or Simon Stritt, Department of Immunology, Genetics and Pathology, Uppsala University, Dag Hammarskjölds Väg 20, 75185 Uppsala, Sweden; e-mail: simon.stritt@igp.uu.se.

![Figure 1. Accelerated platelet clearance accounts for macrothrombocytopenia in Twf2a−/− mice. (A-B) Platelet count (A) and size (B) were determined with an automated cell analyzer (Sysmex). (C) Platelet lifespan was assessed by flow cytometric measurement of the fluorescence-positive platelet population at the indicated time points after injection of a fluorophore-conjugated anti-GPIX antibody derivative. (D-E) Platelet counts were monitored over time after clodronate-encapsulated liposome-mediated macrophage depletion (D) and splenectomy (E). (F-G) The relative platelet content in control and Twf2a−/− spleens was determined by immunostaining (platelet GPIb, cyan; endothelial CD105, magenta) and confocal microscopy of cryosections. Scale bars, 250 μm. Images were acquired with a TCS SP8 confocal microscope (25×/0.95 FLUOTAR VISIR water objective, Leica Microsystems) and are representative of at least 4 individuals. (H-I) The fraction of aged platelets was assessed by platelet lectin-binding (Erythrina crista-galli lectin [ECL] and Ricinus communis agglutinin [RCA]) and the fraction of young (RNA-rich) platelets by thiazole orange (TO) staining and flow cytometry. The overall gated platelet population (based on forward sideward characteristics and GPIX labeling) was set as 100%. Neuraminidase-treated platelets were used as a positive control (H) and RNase-treated platelets as a negative control (I). Values represent mean ± SD (n = 6). Each symbol represents 1 individual. Horizontal lines represent mean (I). ***P < .001, **P < .01, and *P < .05, unpaired Student t test. NS, not significant; WT, wild-type.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/15/10.1182_blood-2017-02-770768/4/m_blood770768f1.jpeg?Expires=1764961768&Signature=BZp0qm8xiVpOF~NGC2900xfACMGYXXgdF3UFDsXYIgMNiXyRgwRvLqdPL3TdiqMCKX2ALD42z3AsRtADdDFrUf6tLrf2Py5PbL~2OPOn1dDMkUbloxXgs-NoLNp4RJ~Aue1Xx6hI4WlJl29ygWl1OayaTRRqfpbLmdnhMpLOwdRwGgM1s6OZuo7xzZ~qbQ-lkiBWJ05mMG-w9SyfQf1q2lKhIWi94spyh7NNKAltjyvBViENRZmh~vB-oasr~4TzJFCBp0i8kJqS7dBPCgjQg1UflIwckWIeE13gglfLCeaMWECwjy~ODIivvSpxz8Rrlq-agCJpx0pk2ejQDNaaSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal