Abstract
Racial and ethnic disparities in patients with solid malignancies have been well documented. Less is known about these disparities in patients with hematologic malignancies. With the advent of novel chemotherapeutics and targeted molecular, cellular, and immunologic therapies, it is important to identify differences in care that may lead to disparate outcomes. This review provides a critical appraisal of the empirical research on racial and ethnic disparities in incidence, survival, and outcomes in patients with hematologic malignancies. The review focuses on patients with acute myeloid leukemia, acute lymphocytic leukemia, multiple myeloma, non-Hodgkin lymphoma, Hodgkin lymphoma, myeloproliferative neoplasms, and myelodysplastic syndrome. The review discusses possible causes of racial and ethnic disparities and also considers future directions for studies to help decrease disparities.
Introduction
According to the American Cancer Society, there will be an estimated 1 688 780 new cancer cases diagnosed and 600 920 cancer deaths in the United States in 2017.1 Of these new cancer diagnoses, about 172 910 will be patients with hematologic malignancies. Approximately 58 300 of these patients are expected to die of their disease. With the ever-changing demographics of the country, it is reasonable to anticipate that a large proportion of these diagnoses and deaths will be in racial and ethnic minorities. In fact, it is predicted that by 2055, the United States will not have a single racial or ethnic majority.2 Many studies have documented poor health outcomes in racial and ethnic minority patients diagnosed with cancer,3,4 although it is unclear if these same disparities are observed in patients with hematologic malignancies. This review specifically focuses on empirical research on racial disparities in adults diagnosed with acute myeloid leukemia (AML), acute lymphocytic leukemia ALL), multiple myeloma (MM), non-Hodgkin lymphoma (NHL), Hodgkin lymphoma (HL), myeloproliferative neoplasms (MPNs), and myelodysplastic syndrome (MDS) in the United States.
Definitions
Although there is widespread acknowledgment that general health disparities exist, before 2000 there was no consensus definition of what constitutes a disparity. In 2000, the Minority Health and Health Disparities Research and Education Act, also known as United States Public Law 106-525, was passed, and it provided a legal definition of a health disparity population. It stated that “[a] population is a health disparity population if there is a significant disparity in the overall rate of disease incidence, prevalence, morbidity, mortality, or survival rates in the population as compared to the health status of the general population.”5 For the purposes of this review, we will focus on racial differences in these parameters for patients with hematologic malignancies. We will use the definition of social determinants set forth by the World Health Organization, which defined social determinants as “…the conditions in which people are born, grow, live, work and age,”6 such as cultural and socioeconomic factors.
Leukemia
AML
In 2017, more than 21 000 cases of newly diagnosed AML are expected.1 The treatment of AML can vary, depending on the age and performance status of the patient. For patients who receive intensive therapies, treatment regimens rely heavily on inpatient management, which may make home support and compliance lesser factors for success. Data for AML from 2010 to 2014 suggest that whites have a higher age-adjusted incidence of disease (4.3 per 100 000 persons) compared with blacks (3.5), Asian/Pacific Islanders (3.4), and Hispanics (3.6).7 Despite a lower incidence among minority populations, some groups have worse survival. Patel et al8 analyzed data from more than 39 000 patients in the Surveillance, Epidemiology, and End Results (SEER) database from 1999 to 2008 and found that black and Hispanic patients with AML had increased risk of death by 12% and 6%, respectively, compared with non-Hispanic whites. These disparities were observed despite a higher prevalence of favorable cytogenetics and a younger age at diagnosis in these minority groups.8,9
Although overall survival (OS) and outcomes for AML seem to be improving over the last several years, the improvement has not been equally distributed among different racial and ethnic groups. For non-Hispanic whites with AML, excluding acute promyelocytic leukemia (APL), age-adjusted 5-year relative survival increased from about 12% in 1991-1996 to 16% in 2003-2008.10 Similar statistically significant findings were found for blacks (from 8% to 12%) and Asian/Pacific Islanders (from 11% to 17%), but the improvement for Hispanics (from 13% to 14%) was not statistically significant. Younger African American, Hispanic, and Asian/Pacific Islanders between age 15 and 54 years with AML (excluding APL) demonstrated no statistically significant improvement in age-adjusted 5-year survival from 1991-1996 to 2003-2008. Furthermore, being African American, residing in an area with a high poverty level, and being covered only by Medicaid were found to be independent predictors of worse survival in AML.11 Age-adjusted 5-year relative survival for patients with AML from SEER 18 can be found in Table 1.
Five-year relative survival by race and cancer type
| Cancer type . | Race . | No. of patients . | Relative survival adjusted for age (%) . | 95% CI . |
|---|---|---|---|---|
| NHL | Non-Hispanic white | 141 319 | 69 | 69-70 |
| Black | 15 719 | 59 | 57-60 | |
| Hispanic white | 21 673 | 62 | 61-63 | |
| Asian | 12 479 | 62 | 60-63 | |
| Myeloma | Non-Hispanic white | 39 461 | 46 | 45-47 |
| Black | 12 253 | 43 | 42-44 | |
| Hispanic white | 6 702 | 43 | 41-44 | |
| Asian | 3 453 | 44 | 42-46 | |
| AML | Non-Hispanic white | 23 217 | 18 | 18-19 |
| Black | 2 851 | 15 | 14-17 | |
| Hispanic white | 4 014 | 17 | 16-19 | |
| Asian | 2 677 | 18 | 16-19 | |
| HL | Non-Hispanic white | 20 080 | 84 | 84-85 |
| Black | 3 567 | 77 | 75-79 | |
| Hispanic white | 4 276 | 79 | 77-80 | |
| Asian | 1 412 | 80 | 78-83 | |
| ALL | Non-Hispanic white | 4 520 | 44 | 42-45 |
| Black | 628 | 33 | 29-37 | |
| Hispanic white | 2 808 | 34 | 32-36 | |
| Asian | 657 | 43 | 39-47 |
| Cancer type . | Race . | No. of patients . | Relative survival adjusted for age (%) . | 95% CI . |
|---|---|---|---|---|
| NHL | Non-Hispanic white | 141 319 | 69 | 69-70 |
| Black | 15 719 | 59 | 57-60 | |
| Hispanic white | 21 673 | 62 | 61-63 | |
| Asian | 12 479 | 62 | 60-63 | |
| Myeloma | Non-Hispanic white | 39 461 | 46 | 45-47 |
| Black | 12 253 | 43 | 42-44 | |
| Hispanic white | 6 702 | 43 | 41-44 | |
| Asian | 3 453 | 44 | 42-46 | |
| AML | Non-Hispanic white | 23 217 | 18 | 18-19 |
| Black | 2 851 | 15 | 14-17 | |
| Hispanic white | 4 014 | 17 | 16-19 | |
| Asian | 2 677 | 18 | 16-19 | |
| HL | Non-Hispanic white | 20 080 | 84 | 84-85 |
| Black | 3 567 | 77 | 75-79 | |
| Hispanic white | 4 276 | 79 | 77-80 | |
| Asian | 1 412 | 80 | 78-83 | |
| ALL | Non-Hispanic white | 4 520 | 44 | 42-45 |
| Black | 628 | 33 | 29-37 | |
| Hispanic white | 2 808 | 34 | 32-36 | |
| Asian | 657 | 43 | 39-47 |
Data from SEER 18 (2000-2014) showing 5-year relative survival of patients with selected malignancies stratified by race. Data were age-standardized to the International Cancer Survival Standard 1 (ICSS-1) for age 15 years or older except for ALL and HL which used ICSS-3. Age standardization allows comparison of outcomes for the different races/ethnicities adjusting for different age distributions for those groups.12
Racial disparities for precursor diagnoses such as MDS have also been observed. From an analysis of 2538 patients with MDS, Hispanic patients tend to be younger, have worse thrombocytopenia, and have a higher proportion of therapy-related MDS when compared with other minority groups.13 Interestingly, a higher percentage of Hispanic patients (33%) were treated with transplantation compared with whites (13%) and African Americans (10%). In contrast to patients with AML, Hispanic patients had the best median OS (47 months) compared with whites (37 months) and African Americans (30 months). There was no racial/ethnic difference in transformation rates to AML in this study.
There are limited data on racial differences in patients with MPNs. One study conducted between 1990 and 2012 analyzed 127 patients.14 White patients with polycythemia vera had a lower rate of cardiovascular thrombosis and hemorrhagic complications compared with non-white patients. White patients with polycythemia vera or essential thrombocythemia had a higher likelihood of progressing to myelofibrosis.14
In pediatric AML, survival disparities in children (younger than age 15 years) and adolescent and young adults (AYAs) with AML continue to exist between black, Hispanic, and white patients.15 An analysis of patients with pediatric AML from 2003 to 2007 from the SEER database revealed a nearly 17% difference in 5-year relative survival between white (55%) and black AYAs (38%).15 For children younger than age 15 years, a 17% difference was again observed between whites (71%) and blacks (54%). Relative survival between Hispanic and non-Hispanic white children and AYAs with AML was observed to be similar.
ALL
In 2017, there will be nearly 6000 new cases of ALL in the United States.1 For children, ALL is one of the most common cancers diagnosed and constitutes approximately 25% of childhood malignancies.16 The incidence also seems to be highest in Hispanic children (43 per 1 million).16
ALL treatment regimens can involve significant outpatient time and dose intensification that requires high levels of compliance and social support. Thus, both biologic factors (drug metabolism, sensitivity to chemotherapy, cytogenetic profiles) and nonbiologic factors (social support, financial toxicity, compliance, or access to care) may contribute to the success of treatment.
The analysis of the SEER database from 1999 to 2008 by Patel et al8 found that survival disparities were worse in ALL than in AML, despite the improved outcomes that are usually associated with ALL. The probability of death for black and Hispanic patients with ALL was about 45% and 46% higher, respectively, than for white patients. Asian/Pacific Islanders had a probability of death similar to that of whites.
It is unclear whether the increased probability of death is a result of more aggressive disease or from nonbiologic factors. Some data suggest that African Americans and Hispanics have unfavorable prognostic factors compared with whites. For ALL patients treated on Pediatric Oncology Group multicenter randomized clinical trials, African Americans were more likely to have central nervous system involvement, a white blood cell count >50 × 109/L, and worse cytogenetics at diagnosis when compared with white patients.17 Patients with a Spanish last name also had worse prognostic features at initial diagnosis. However, after controlling for these adverse initial prognostic features, outcomes were still worse for black patients and those with a Spanish last name, suggesting that variable response to chemotherapy might be contributing to disparate outcomes.
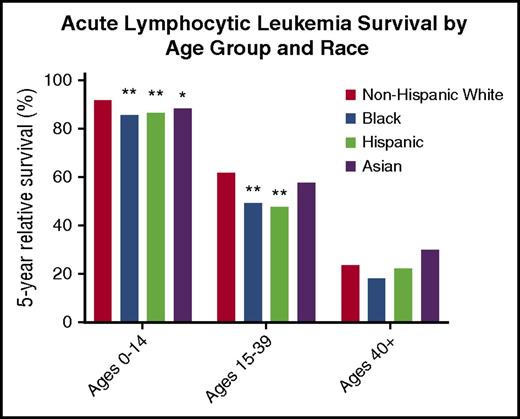
Although survival disparities between black and white children with ALL improved when compared with the children in the 1970s, disparities persist.15 Data on 5-year relative survival stratified by age group and race from SEER 18 (2000-2014) is summarized in Figure 1.12 By using non-Hispanic whites as the reference group, statistically significant differences in survival between races were observed in pediatric patients for blacks, Hispanics, and Asians and in blacks and Hispanics for AYA patients. No differences in survival were observed in patients older than age 40 years with ALL.
Five-year relative survival for patients with ALL by age group and race from SEER 18 (2000-2014). Non-Hispanic whites were used as the reference group. Hispanic categorization was not mutually exclusive from other race categories.17 Reported data used unadjusted P values. **P < .001; *P < .05.
Five-year relative survival for patients with ALL by age group and race from SEER 18 (2000-2014). Non-Hispanic whites were used as the reference group. Hispanic categorization was not mutually exclusive from other race categories.17 Reported data used unadjusted P values. **P < .001; *P < .05.
MM
In 2017, it is expected that there will be more than 30 000 new cases of MM.1 The number of available treatments has increased substantially in the last 15 years. For black Americans, MM represents one of the most commonly diagnosed hematologic malignancies. Waxman et al18 analyzed SEER data from 1973 to 2005 and found the incidence rate for MM was 11.0 per 100 000 person-years for blacks and 4.9 per 100 000 person-years for whites. The increased incidence of MM may be a result of the increased prevalence of monoclonal gammopathy of undetermined significance. The adjusted monoclonal gammopathy of undetermined significance rate for black patients (∼4%) is about double that for whites and Hispanics (∼2%).19 There was no racial survival disparity among black and white patients with MM who were younger than age 50 years, although 5-year disease-specific survival for those age 50 to 69 years was significantly better for black than for white patients (42% vs 36%; P < .001) and patients age 70 years or older (31% vs 26%; P < .001).18 Furthermore, an analysis of MM patients who received an autologous stem cell transplant (ASCT) did not show any difference in survival by race.20
Some data suggest that novel myeloma therapies disproportionately benefited white patients of higher socioeconomic status (SES).21 After the introduction of novel therapies, black patients had half the observed survival improvements of their white counterparts, suggesting a lack of access or biological differences to treatment. Bortezomib use among black patients with MM was less than that for white patients, and this difference persisted even after controlling for potential measures of variable access.22
In an analysis involving more than 37 000 MM patients,23 Hispanics had a significantly worse median OS compared with whites in a multivariable analysis (2.4 vs 2.6 years; P = .006). Asians and African Americans did not have significantly different median OS compared with whites. Asians had the best median myeloma-specific survival of all racial/ethnic groups. African Americans had a significantly better median myeloma-specific survival compared with whites as well. For patients age 75 years or older, Hispanics had the worst median OS at 1.3 years, whereas Asians had the best median OS at 1.8 years. These were both significantly different when compared with OS for whites. Table 1 shows SEER 18 data for 5-year relative survival adjusted for age for myeloma patients stratified by race.12
In summary, in patients with MM, blacks in certain age groups seem to have improved OS compared with whites. However, black, Hispanic, and Asian patients have all had less improvement in survival compared with whites with the introduction of novel immunomodulatory therapies (eg, bortezomib) and the use of ASCT. Interestingly, survival for patients who actually received ASCT did not differ on the basis of race. This may suggest restricted access to innovative antimyeloma therapies, and more studies are needed to elucidate whether social determinants play into the differential survival gains of patients from minority racial/ethnic groups.
Lymphoma
In 2017, it is estimated that there will be more than 80 000 new cases of HL and NHL.1
HL
Similar to other malignancies, there are some data to suggest differences in incidence and OS for HL between racial groups. HL has a bimodal age distribution in incidence with peaks in patients who are in their mid-20s or early 60s, but many of these studies were done on largely white populations. Although modern analyses confirm this distribution for whites and Asians, the incidence for other minority groups is different.24 African American men older than age 30 years have an incidence rate that is more or less stable, whereas Hispanics had only a small incidence peak for patients in their 20s and then the highest incidence rate in patients older than age 65 years.
Black and Hispanic AYAs have a 62% and 35% higher risk of death as a result of HL.25 Black and Hispanic AYAs are also more likely to be diagnosed with HL at an advanced stage when compared with whites and Asian/Pacific Islanders.
One study found inferior 5-year OS rates for black Americans (76%) and Hispanics (75%) compared with whites (82%) and Asian/Pacific Islanders (81%).24 Furthermore, survival for patients with lower SES diagnosed with HL was worse than that for patients with higher SES.26 Patients in the lowest SES categorization had a 64% increased risk of death related to HL compared with patients in the highest category for age 15 to 44 years. Even after adjustments were made for SES, blacks and Hispanics still had a 74% and 43% higher risk of death than whites in this age group. Asian/Pacific Islanders had persistently comparable rates of OS. Table 1 has data from SEER 18 on 5-year relative survival for patients with HL.12
NHL
There are significant racial differences for patients diagnosed with NHL. One study analyzed the SEER database between 1992 and 2010 for patients diagnosed with diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL).27 For patients age 65 years or older, blacks have the lowest incidence rates of DLBCL and FL. Non-Hispanic whites have the highest incidence of CLL/SLL in the same age group. Similar racial disparities exist among the rare NHLs. For patients diagnosed with natural killer T-cell NHLs, Asian/Pacific Islanders along with Hispanic whites had the highest age-adjusted incidence rates.28 For AYA patients, it is notable that blacks and Asian/Pacific Islanders were more likely to be diagnosed at a later stage than other groups. Interestingly, extranodal involvement was a significant adverse risk factor for non-Hispanic whites but not for other racial groups.29
For DLBCL diagnosed between 1992 and 2005, racial differences in survival varied according to stage.27 For stage I disease, non-Hispanic whites had the best 5-year survival rate (67%), whereas blacks had the worst (60%). Asian/Pacific Islanders had the worst survival rate for stage IV disease (35%), and non-Hispanic whites had the best survival rate (41%).
FL differs with regard to racial differences.27 There was no consistent racial pattern predicting survival in patients with stages II to IV disease. Black patients had the best survival rate for stage II disease, Hispanic whites had the best survival rate for stage III disease, and non-Hispanic whites had the best survival rate for stage IV disease.
For CLL/SLL, there was no significant racial difference found for patients with stage II and III disease. Of those with stage I disease, Asian/Pacific Islander had the best OS, whereas non-Hispanic whites had the best OS among patients diagnosed with stage IV disease.27 Table 1 shows 5-year relative survival for patients with NHL stratified by race.12
Hematopoietic cell transplantation
Hematopoietic cell transplantation (HCT) is an important treatment option for patients with hematologic disorders. Disparities with regard to access and outcomes in HCT are discussed below.
The association of race and ethnicity with outcomes for patients with acute leukemias and MDS who have undergone transplantation has been studied extensively.30-33 Analysis of the California Cancer Registry found that black and Hispanic patients with AML had a decreased likelihood of stem cell transplantation compared with whites (odds ratio, 0.64; 95% confidence interval, 0.46-0.87 and odds ratio, 0.74; 95% confidence interval, 0.62-0.89, respectively).34 Interestingly, Asian/Pacific Islanders did not have the same disparities in the receipt of care when compared with whites. Furthermore, when the statistical models were adjusted for the receipt of any treatment (eg, chemotherapy or transplant), the survival disparity for black patients was nearly nullified. Some analyses found contrasting results and reported that black patients who received and seemed to respond to treatment still had worse outcomes compared with white patients.11 A registry study showed that African Americans had worse survival after unrelated donor transplantation, even after adjustment for transplant and socioeconomic factors.31 For Hispanics, trends suggest a higher risk of treatment failure and mortality after HCT.32
Overall, black and Hispanic patients with AML and ALL have worse survival outcomes and decreased likelihood of receiving definitive therapy with HCT when compared with their white counterparts. Studies differ on whether improving access to care completely eliminates inferior outcomes. Furthermore, the underrepresentation of minorities in national bone marrow registries and increased diversity in HLA haplotypes contributes to the difficulty in finding appropriately matched volunteer donors for minorities needing allogeneic transplants. This may, in part, explain disparate minority access to allogeneic HCT. Although expanded access to graft sources (eg, haploidentical and umbilical cord blood grafts) have attempted to increase access for racial/ethnic minorities unable to find appropriately matched allogeneic donors, there are only limited data on whether this has resulted in a meaningful change despite that minorities constitute a disproportionate number of these graft type recipients.
Disparities with regard to ASCT have also been studied. SEER 18 data on ASCT revealed that age-adjusted relative rates of ASCT use for myeloma patients were significantly higher in non-Hispanic whites than in blacks, Hispanics, and Asians.35 The authors of that analysis calculated that if racial/ethnic disparities were eliminated, this would result in a nearly 16% increase in the use of ASCT, with the majority of the increase driven by increased participation by minorities. Unfortunately, there are no significant data on racial disparities in ASCT specific for HL and NHL.
Potential causes for disparities
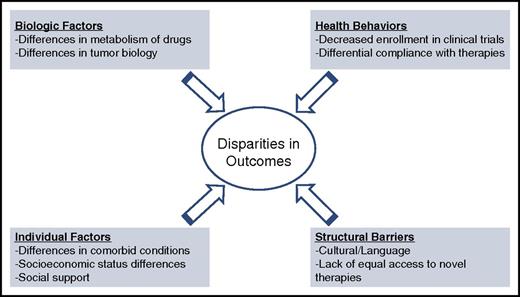
Several studies have attempted to analyze the reasons for the aforementioned disparities. Multiple causes have been posited to explain these disparities, including structural barriers to care, variable access, compliance with intensive therapies, and overall disease heterogeneity. Figure 2 shows a conceptual model for understanding disparities.
Conceptual framework for disparities in care for hematologic malignancies.
Variable access/structural barriers to care
Differential access to care is perhaps the most studied etiology for general health disparities, although little data are available for hematologic malignancies. Much of the literature on solid tumors with regard to access has focused on improving access to screening modalities. Because there are no known screening measures currently approved for hematologic malignancies, the limited data that do exist for access to care focus on access to treatment rather than screening. In MM, for example, blacks are 49% less likely to receive stem cell transplantation than whites.22 Once potential measures for variable access (household income, Medicare enrollment, urban/rural status) were controlled for, blacks were still 37% less likely to undergo stem cell transplantation, suggesting that other structural barriers and decision-making patterns may differ between black and white patients. For DLBCL, black patients are less likely to receive chemotherapy for their disease, which could contribute to disparities in survival.36
Although there are some data about the importance of language/English comprehension as a barrier to accessing care for patients with solid tumor malignancies, to the best of our knowledge, there is no significant information on whether patients with hematologic malignancies without adequate knowledge of English are similarly affected.
Given these findings, more studies should be done to assess why non-white minorities have lower rates of use for widely available therapies compared with their white counterparts.
Compliance
Some studies have demonstrated relatively poor adherence to medications and follow-up care among African Americans, Hispanics, and other minority groups.37-39 Others have studied differences in cancer therapy compliance, although these studies have focused more on solid tumor malignancies.40,41 Chemotherapy for hematologic malignancies can be very cumbersome and difficult to tolerate. A representative example of this is ALL, which requires dose intensification regimens and outpatient compliance with oral therapies. Although it is reasonable to postulate that differential outcomes in hematologic malignancies may be a consequence of worse compliance among racial/ethnic minorities, some studies refute compliance as a cause of worse outcomes. Pollock et al17 found that racial differences in survival in ALL were not the result of differences in compliance or deviations in prescribed therapy. All of the patients in that study were treated on Pediatric Oncology Group studies, and there was little difference between African Americans and whites in how the protocol treatment was administered.
Another study of the differences in postremission therapy between black and white AML patients demonstrated analogous results.42 Although the time to postremission therapy was shorter for whites compared with blacks with AML, dose intensity and number of cycles were similar between the 2 groups. Because black and white patients received similar postremission management, differences in treatment and compliance did not explain differences in outcomes. In contrast, an analysis of patients with childhood ALL enrolled in a Children’s Oncology Group study found that non-white race was an independent predictor of overreporting 6-mercaptopurine (6-MP) intake and that a higher proportion of nonadherent patients were overreporters.43 Because adherence to 6-MP is an important component of maintenance therapy for ALL, it is possible that this nonadherence could differentially increase the risk of relapse for non-white patients. Thus, studies conflict about whether compliance contributes significantly to differences in outcomes for racial/ethnic minorities. More studies in hematologic conditions are needed.
Metabolism/tumor biology
Differential metabolism of chemotherapy drugs has also been hypothesized as a possible reason for disparate outcomes in hematologic malignancies.44,45 For example, higher dihydrofolate reductase levels (a key enzyme that helps metabolize methotrexate, an important component of therapy in ALL) may be associated with higher risk of treatment failure, and African Americans have higher levels of this enzyme when compared with whites.44 Furthermore, cytotoxic metabolites of 6-MP and methotrexate may be associated with a higher risk of relapse,45 and in fact, lower intracellular levels of methotrexate are found in the red blood cells of African American and Hispanic patients compared with their white counterparts.17 More thorough study of pharmacologic and polymorphism differences among minorities would help ascertain whether there is a biological basis for worse outcomes based on different responses to treatments.
Comorbid conditions
An increased number of comorbid conditions has been postulated as a potential reason for racial disparities in cancer care. Although this has been studied more in solid tumors,46 some data exist for hematologic malignancies. In one study of patients with NHL, African American patients had more comorbidities, but there was no difference between them and white patients with regard to all-cause mortality and lymphoma-specific mortality.47 However other studies found a strong overlap between increased comorbidities and low SES. One large population-based study mostly of solid tumors (although it included patients diagnosed with NHL) found that 1-year survival was significantly worse in patients from the lowest SES, and that this difference was partly explained by comorbidities.48 Understanding how the number and type of comorbidities may affect outcomes in hematologic malignancies is of utmost importance because certain comorbid conditions are barriers to accessing novel clinical trials. Given that other barriers already exist for minority recruitment and participation in clinical trials,49 a more thorough analysis of why this occurs and how to overcome these challenges is needed.
Clinical trial participation
It is important to note that recruitment and enrollment of minorities in clinical trials is low compared with white patients.49 For example, many of the trials leading to the approval of nivolumab for non-squamous lung and advanced renal cell carcinoma had study populations involving nearly 90% white patients and less than 10% underrepresented minorities.50,51 Many of the trials involving immunotherapy for hematologic malignancies do not report the racial demographics of their study populations, so it is of some concern that these trials may be plagued by a similar lack of diversity. In MM trials, minorities are known to be underrepresented. This underrepresentation may in turn challenge the generalizability of the results of these clinical trials to minority patients.52 Furthermore, non-white minorities tend to participate less in pharmacogenomics studies than their white counterparts, which makes it even more difficult to determine whether a particular drug has similar metabolism between groups.53
Active recruitment of underrepresented minority groups should be a key aspect of any clinical trial involving hematologic malignancies. For cancer trials specifically, the lack of underrepresented minority enrollment may be a result of a lack of awareness of trial options, low SES, lack of culturally relevant education, and certain cultural factors that predispose some groups to decline participation.54
Summary
As the American population continues to become more diverse, it is of paramount importance that outcomes for all racial and ethnic groups continue to improve. Currently, there are significant racial/ethnic disparities in incidence and survival for patients diagnosed with hematologic malignancies, yet little is known about the basis for these differences. Future disparities research in hematologic malignancies should systematically evaluate the social determinants and biologic hypotheses for these differences. To facilitate this research, future public health endeavors should focus on increasing clinical trial participation of minorities with hematologic malignancies and refining the SEER data on race, ethnicity, and SES to allow these questions to be addressed in the future.
Acknowledgments
The authors thank Lynn Onstad for statistical support.
This work was supported by grant T32CA009515 from the National Institutes of Health, National Cancer Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: K.K. and S.J.L. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kedar Kirtane, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, D5-100, Seattle, WA 98109; e-mail: kkirtane@fredhutch.org.