Abstract
Hematopoietic stem cells (HSCs) are responsible for lifelong production of blood cells. At the same time, they must respond rapidly to acute needs such as infection or injury. Significant interest has emerged in how inflammation regulates HSC fate and how it affects the long-term functionality of HSCs and the blood system as a whole. Here we detail recent advances and unanswered questions at the intersection between inflammation and HSC biology in the contexts of development, aging, and hematological malignancy.
Introduction
Given its fundamental roles in immunity and tissue repair, the blood system is highly responsive to inflammatory signaling caused by noxious insults and environmental disturbances such as infection and injury.1 All blood lineages participate in the initiation and resolution of inflammatory events, and inflammatory responses are often accompanied by blood system changes, including overproduction of myeloid cells and, in some cases, platelets.2 These short-lived populations play significant “first responder” roles in host defense and their ongoing replenishment by hematopoietic progenitors is critical during inflammatory responses.3 It has been known for decades that proinflammatory cytokines such as interleukin (IL)-1, tumor necrosis factor-α (TNF-α), interferons (IFNs), and other danger signals such as damage and/or pathogen--associated molecular patterns can increase or, in some cases, suppress, the cellular output of normal and leukemic bone marrow (BM) cells.4 Thus, hematopoietic stem cells (HSCs) are at the root of a highly dynamic blood system, and their biology can be directly affected by inflammatory signaling.4 Identifying how inflammation regulates HSC fate and shapes the blood system during development, aging, chronic inflammatory disease, and hematological malignancy is now crucial to understanding the mechanistic underpinnings of these processes, as well as potential links between them.2 In this Spotlight review, we will discuss the emerging role of inflammatory signals on HSC fate and function. We will highlight important new lines of study that will refine our understanding of the intimate link between inflammation and HSC biology in development, and identify open questions and potential links among hematopoiesis, chronic inflammation, and age-related myeloid malignancies.
Inflammation: a key regulator of HSC fate
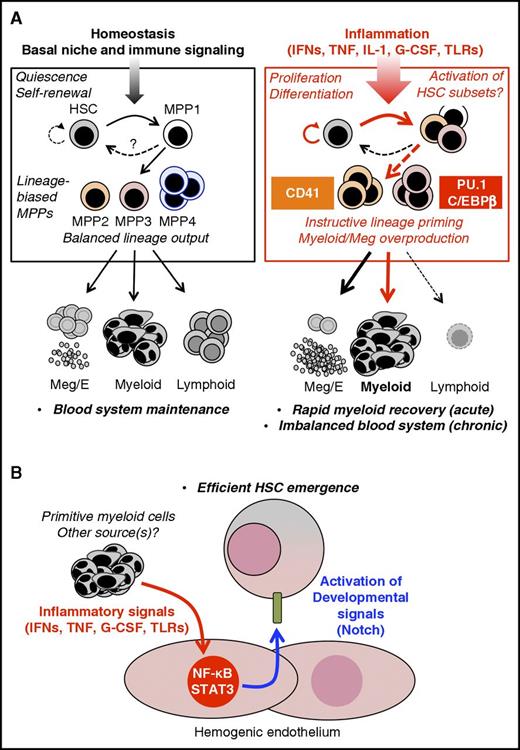
A growing body of work has detailed the effects of specific inflammatory signals, particularly cytokines and Toll-like receptor (TLR) ligands, in shaping HSC fate and blood output. Concurrent advances in model systems such as the mouse and zebrafish, and the use of single-cell analysis approaches have provided detailed mechanistic insights into how inflammation affects HSC proliferation, differentiation, and self-renewal capacity (Figure 1A). HSCs are normally kept in a dormant state via a combination of cell-intrinsic transcriptional and epigenetic regulators5 alongside signals from their niches.6 Despite this, they rapidly lose quiescence and transiently proliferate in response to many inflammatory signals. IFNs have been extensively studied in this setting, and induce HSC proliferation in vivo via suppressing quiescence-enforcing transcription factors such as Foxo3a in addition to stabilizing Myc.7-9 Notably, G-CSF and IL-1 also induce HSC proliferation,10,11 suggesting HSCs may be responding to disruption of homeostasis rather than an instructive cue. Indeed, HSCs localize away from the quiescence-associated arteriolar BM niche during acute IFN-1 stimulation,12 suggesting that inflammation disrupts HSC–niche interactions. Interestingly, the HSC compartment subsequently returns to a quiescent state, even if the inflammatory signal is still present.8,10,13 This implies the existence of a yet-undefined “braking” mechanism that may involve (at least for IFNs) the transcription factor Irf2 and the guanosine triphosphatase Irgm1,14,15 which reestablishes HSC quiescence and prevents exhaustion. Nonetheless, the biological significance of HSC proliferation is unclear; further work is needed to identify the extent to which HSC proliferation drives changes in blood cell output during inflammation vs the contribution of more numerous downstream progenitors.
Inflammatory signals regulate HSC fate. (A) Adult hematopoiesis. Under homeostatic conditions, hematopoietic output is dominated by lineage-biased MPP subsets, particularly the numerically most abundant MPP4 compartment, which generates primarily myeloid and lymphoid output, resulting in balanced blood production. On the other hand, the megakaryocyte/erythroid (Meg/E)-biased MPP2 and myeloid-biased MPP3 are less numerous and may contribute little to normal homeostasis. The phenotypic HSC compartment also includes a subset of metabolically active HSCs, termed MPP1, which likely serves as a “ready” compartment for rapid activation during acute need. Under homeostatic conditions, this system is regulated by BM niche signals and basal levels of proinflammatory cytokines, which maintains a balance between HSC dormancy and lineage priming. In response to proinflammatory signals or during hematopoietic regeneration, the HSC compartment undergoes distinct fate changes, including transient proliferation, expansion of MPP1, and the activation of instructive lineage-specific programs in subsets of HSCs. These include Meg priming in CD41+ cells following IFN, TNF, and IL-1 exposure, as well as activation of C/EBPβ and PU.1 in response to IFN-γ and IL-1, respectively. These lineage-primed HSCs in turn lead to expansion of Meg/E-biased MPP2 and myeloid-biased MPP3, likely resulting in rapid production of platelets and myeloid populations. Meanwhile, lymphoid output is suppressed via inflammatory reprogramming of MPP4, resulting in additional myeloid production. (B) Embryonic development. Proinflammatory factors produced in the AGM by myeloid cells from the primitive hematopoietic wave, as well as from other sources, directly activate NF-κB and STAT3 in hemogenic endothelial cells, leading to increased expression of Notch ligands, hence promoting HSC specification. Thus, inflammatory signals have emerged as central players in controlling developmental pathways required for HSC emergence. These findings suggest a close evolutionary and functional relationship between inflammation and tissue development. G-CSF, granulocyte colony-stimulating factor.
Inflammatory signals regulate HSC fate. (A) Adult hematopoiesis. Under homeostatic conditions, hematopoietic output is dominated by lineage-biased MPP subsets, particularly the numerically most abundant MPP4 compartment, which generates primarily myeloid and lymphoid output, resulting in balanced blood production. On the other hand, the megakaryocyte/erythroid (Meg/E)-biased MPP2 and myeloid-biased MPP3 are less numerous and may contribute little to normal homeostasis. The phenotypic HSC compartment also includes a subset of metabolically active HSCs, termed MPP1, which likely serves as a “ready” compartment for rapid activation during acute need. Under homeostatic conditions, this system is regulated by BM niche signals and basal levels of proinflammatory cytokines, which maintains a balance between HSC dormancy and lineage priming. In response to proinflammatory signals or during hematopoietic regeneration, the HSC compartment undergoes distinct fate changes, including transient proliferation, expansion of MPP1, and the activation of instructive lineage-specific programs in subsets of HSCs. These include Meg priming in CD41+ cells following IFN, TNF, and IL-1 exposure, as well as activation of C/EBPβ and PU.1 in response to IFN-γ and IL-1, respectively. These lineage-primed HSCs in turn lead to expansion of Meg/E-biased MPP2 and myeloid-biased MPP3, likely resulting in rapid production of platelets and myeloid populations. Meanwhile, lymphoid output is suppressed via inflammatory reprogramming of MPP4, resulting in additional myeloid production. (B) Embryonic development. Proinflammatory factors produced in the AGM by myeloid cells from the primitive hematopoietic wave, as well as from other sources, directly activate NF-κB and STAT3 in hemogenic endothelial cells, leading to increased expression of Notch ligands, hence promoting HSC specification. Thus, inflammatory signals have emerged as central players in controlling developmental pathways required for HSC emergence. These findings suggest a close evolutionary and functional relationship between inflammation and tissue development. G-CSF, granulocyte colony-stimulating factor.
Inflammatory signals also have distinct effects on HSC differentiation. For instance, IFN-γ induces myeloid differentiation in a subset of IFN-γ receptor–expressing HSCs via activation of the transcription factors Batf2 and C/EBPβ.16,17 Interestingly, C/EBPβ is also associated with “emergency” granulopoietic responses to IL-3 and granulocyte-macrophage colony-stimulating factor.18 Because all 3 cytokines use the JaK/STAT pathway, C/EBPβ activation may be a common consequence of such signaling. On the other hand, IL-1 initiates myeloid differentiation via NF-κB–dependent PU.1 activation.13 Because IL-1 receptor (IL-1R), TNF receptor, and several TLRs activate NF-κB, PU.1 likely represents a common downstream mechanism and would be consistent with the capacity for signaling via TLRs 2 and 4 to also drive myeloid fate decisions in HSCs.19,20 Notably, IFN-1 and TNF also rapidly activate a posttranscriptional megakaryopoietic program in a subset of HSC-like cells expressing high levels of the megakaryocyte marker CD41.21 Similar expansion of CD41hi cells occurs during chronic IL-1 stimulation, suggesting a common platelet production mechanism that may drive inflammatory thrombosis.13,21 Strikingly, IFN-1–mediated HSC proliferation appears restricted to CD41hi cells,21 suggesting HSCs may respond to inflammatory cues in a heterogenous manner; however, whether CD41hi cells are an exclusive proliferative compartment following other inflammatory stimuli has not been established. Collectively, these findings indicate that inflammatory signals can instruct HSCs to adopt lineage-specific gene programs, thereby influencing blood output (Figure 1A). Further investigation of these mechanisms in human systems, as well as their relevance to hematopoietic changes in complex inflammatory disease environments, remains a critical next step. Moreover, lineage tracing is needed to determine the origin of novel populations in the HSC compartment like CD41hi cells, particularly in relation to established phenotypic definitions for lineage-biased HSC subsets.22-24
Inflammatory signals also have been shown to impair HSC self-renewal. Numerous studies have demonstrated that signaling via TLRs, IFNs, TNF, and IL-1 adversely impacts long-term HSC potential via activation of apoptosis or myeloid differentiation programs, or as recently demonstrated for TLR4 signaling, via activation of p38 MAPK.7,13,16,25-27 Altogether, many of these conclusions are based largely on transplantation assays that force quiescent HSCs into a significant replicative challenge. Although such assays are highly relevant in the context of BM transplantation and should not be overlooked, few studies to date have demonstrated outright BM failure in primary animal models of inflammation absent a concurrent challenge such as chemotherapy or viral infection.8,17 This raises questions as to how and whether impaired HSC self-renewal is manifest, if at all, in human patients with inflammatory disease.
However, these results do reinforce the idea that quiescence prevents HSC depletion during chronic inflammation and suggest that the multipotent progenitor (MPP) compartment, which appears largely responsible for day-to-day blood maintenance,28,29 may buffer ongoing need, thereby minimizing the requirement for continued HSC proliferation. Along these lines, HSCs exhibit a finite replicative potential,30 and serial acute inflammatory episodes that induce HSC proliferation could drive HSC decline. Altogether, these findings have shed significant light and opened new questions on the diverse impacts of inflammation on HSC fate and function.
The study of inflammatory hematopoiesis is occurring in the context of an ongoing redefinition of the hematopoietic hierarchy and has, in some cases, contributed to our understanding of how HSC differentiation programs are translated into blood production. Current evidence favors a “dynamic” model of hematopoiesis in which lineage decisions originate in HSCs, which subsequently route their differentiation through distinct subsets of lineage-biased MPPs23,24,31 that act as shortcuts for rapid cell production in response to stress conditions (Figure 1A).23,32 In the mouse, populations termed MPP2 and MPP3 appear to serve as an emergency pathway that expands during inflammation or regeneration, producing Meg/E- and myeloid-lineage cells, respectively.23 Conversely, the classical lymphoid-primed MPP (termed MPP4), is the most abundant MPP subtype and is presumed to dominate multilineage blood output under homeostatic conditions.23,24 It can be reprogrammed to produce exclusively myeloid output by inflammatory cytokines such as IL-6.33 Molecular investigations of the phenotypic HSC compartment have also defined a metabolically activated compartment with limited self-renewal, termed MPP1, which may represent an initial differentiation step.24 Notably, a similar redefinition is also occurring in the context of human hematopoiesis, although so far only in the context of homeostasis.32 Detailed, in vivo lineage tracing is now needed to assess whether HSCs actively switch between MPP subsets or bypass certain progenitor compartments altogether, during inflammatory or regenerative stress. Furthermore, recent single-cell analyses continue to prompt questions as to whether such MPP populations are truly multipotent, or even represent a defined cellular state.34,35 Such investigations will be critical to understand how cell fate decisions and differentiation pathways are altered in response to inflammation.
Hematopoietic development: the same players in a different game
Although the effect of inflammatory signals on HSC function has been studied largely in adult contexts, new work is defining a key role for proinflammatory cytokines in HSC specification during embryonic development. In vertebrates, hematopoiesis occurs in distinct waves, with primitive erythroid and myeloid cell generation occurring in the yolk sac, followed by emergence of HSCs, with true multilineage potential emerging predominantly from hemogenic endothelial cells in the aorta-gonad-mesonephros (AGM).36 In vertebrates, G-CSF, IL-1, and IL-3 positively regulate definitive hematopoiesis from the AGM, implicating inflammatory signals in hematopoietic development.37-39 In zebrafish, Tnfr2 signaling has been shown to activate NF-κB and Notch ligand jagged1a expression in aortic endothelial cells, promoting HSC specification via interaction with Notch1a, a required developmental signal expressed by neighboring cells.40,41 In a parallel study, HSC specification proceeded via a TLR4/MyD88 dependent pathway, also converging on NF-κB to promote Notch signaling, and knockdown of tnfr2 and gcsfr reduced Notch target gene expression and HSC numbers.42 Interestingly, both studies pinpoint primitive neutrophils as the key cytokine producers, though the ligand source for endothelial TLR4 signaling remains undefined. Moreover, analyses in zebrafish, mouse and fetal human HSPCs demonstrate that IFNγ also plays a role in promoting HSC emergence, and unlike TNF is not produced by primitive myeloid cells43 but instead occurs downstream of Notch, using a STAT3-mediated pathway to drive HSC specification.44
Thus, multiple inflammatory signals converge on Notch signaling to promote HSC emergence (Figure 1B), suggesting that inflammatory and developmental pathways are closely interleaved, perhaps taking advantage of the mitogenic effects of emergency signaling to efficiently drive HSC specification and expansion. It is striking that IFN-γ and TNF, which are often associated with BM failure and impaired self-renewal in the adult setting, promote hematopoiesis in fetal life.45-47 Such distinct effects may relate to the uniquely high self-renewal programming of fetal HSCs, or to dosage of these cytokines. Indeed, tonic levels of IFN-γ appear important for regulating basal HSC activity,26 and despite evidence that it limits HSC self-renewal,47 TNF may positively regulate hematopoiesis in certain contexts.48 Questions also remain about the absolute requirement for individual inflammatory signals in HSC development. Indeed, Tnfr2−/−, Il1r1−/−, and Ifngr−/− mice are viable and have relatively minor alterations in HSC pool size, lineage priming, and/or responsiveness to replicative challenges.13,26,47 On the other hand, animals lacking NF-κB subunits have substantial impairments in hematopoiesis,49,50 suggesting that individual cytokines, but not inflammatory signals in general, are dispensable for hematopoietic development. Similarly, mice bred in gnotobiotic conditions or given antibiotics have smaller myeloid compartments, potentially from lower systemic levels of microbiome-derived TLR ligands or inflammatory cytokines.51,52 These findings support a model in which multiple inflammatory signals may activate overlapping programs that prime the hematopoietic system to develop efficiently and respond effectively to acute needs over the remainder of fetal and adult life. Further work will be needed to identify the relevant stimuli activating proinflammatory signals during embryonic development.
Inflammation, aging, and disease: are they linked?
Inflammation evolved as an acute response that is quickly suppressed upon restoration of tissue homeostasis; failure to efficiently resolve inflammatory insults can have serious consequences for tissue maintenance and function.1 Indeed, in the context of chronic infection, autoimmune diseases such as rheumatoid arthritis and lupus, or chronic autoinflammatory and metabolic diseases including atherosclerosis, obesity and type 2 diabetes, the insult fails to resolve, leading to a persistent inflammatory state. Notably, impaired or otherwise altered blood system function is a common feature of these conditions, characterized by cytopenias in 1 or more lineages, anemia of chronic disease, secondary thrombocytosis, suppression of naïve lymphopoiesis, overproduction of myeloid cell populations that mediate further damage, or suppression of BM function.53-55 These effects have since been recapitulated in mouse models of sepsis, arthritis, and long-term cytokine or pathogen–associated molecular pattern stimulation,7,8,13,26,56-60 including work implicating TNF, IFN, and/or IL-1 signaling in HSC dysfunction during rheumatoid arthritis,54 chronic granulomatous disease,11 lupus,61 and obesity,62 reinforcing the role of these factors in blood deregulation. Interestingly, autoimmune conditions such as rheumatoid arthritis and lupus are associated with increased risk of myeloid malignancies,63,64 and whether disease-related effects on HSCs, such as telomere attrition,65 are caused by inflammatory signals and contribute directly to the development of hematological malignancy, remains to be formally demonstrated.
The natural aging process is also associated with a chronic inflammatory phenotype characterized by anemia, immunosenescence, and thrombocytosis66 as well as systemic overabundance of proinflammatory cytokines such as IL-1, TNF, and IL-6. Notably, this cytokine network, termed the senescence-associated secretory phenotype (SASP),67 may be initiated by senescent cells producing IL-1α in the BM microenvironment.68 The features of aged blood systems have been interpreted as evolution of the HSC pool toward a myeloid-biased phenotype, although studies demonstrating an expansion of Mk-biased HSCs, identified based on CD41 or Vwf expression,69,70 suggest a revised model in which aged HSCs lose lymphoid potential and overproduce Meg-lineage cells in the context of normal myeloid output. Notably, SASP factors likely play at least some role in the reduced functionality of HSCs during aged hematopoiesis. Old IL37 and α-antitrypsin transgenic mice, which have reduced inflammatory cytokine activity, exhibit partial restoration of lymphocyte production,71 suggesting that the aged HSC phenotype is related at least in part to inflammation. Further studies will be needed to more directly pinpoint the contribution (if any) of inflammation to age-related HSC defects in DNA replication, cell polarity, and/or impaired BM niche function.66,72
Recently, significant interest has emerged in the potential interplay between chronic inflammation, aging, and hematological disease. SASP-associated cytokines can play a supporting role in aging-related hematological diseases, with TNF, IFNs, and IL-6 all playing pathogenic roles in myeloproliferative neoplasms, myelodysplastic syndrome (MDS), and acute myelogenous leukemia (AML).33,64,73-76 Recent interest has also centered on the role of the IL-1R signaling pathway in myeloid malignancy, including downstream activation of p38 MAPK in AML,77 and increased expression of the IL-1R accessory protein in chronic myeloid leukemia and MDS leukemic stem cells.78,79 Moreover, hyperactive innate immune signaling via the MyD88/IRAK pathway and TRAF6, downstream signaling mechanisms shared by multiple innate immune inputs including TLRs and IL-1, has emerged as a key driver of MDS expansion as well as a potential therapeutic target.80,81 Altogether, these findings suggest a key role for hyperactive innate immune signaling mechanisms in myeloid leukemia progression. Identifying the cellular source(s) of these signals and the deregulations underlying their production are critical next steps.
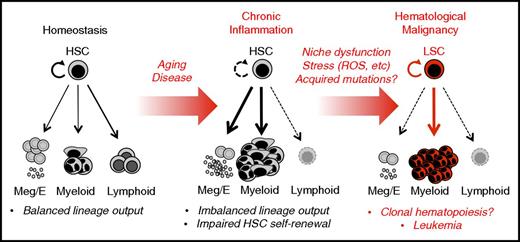
Whether and how chronic inflammation directly induces leukemic transformation or attrition of HSCs remains an open question, particularly if HSCs are able to retain their quiescence in the face of ongoing inflammatory signaling. Notably, HSC attrition or development of a preleukemic state has been observed when inflammation is accompanied by cell-autonomous DNA repair defects in the HSC compartment or dysregulation of the BM niche.17,73,82-85 Altogether, these findings underscore the importance of the BM niche in maintaining HSC pool integrity. They also point to aberrant production of inflammatory signals such as IFNs, IL-1 and the alarmin S100a8 (a damage-associated molecular pattern that signals via TLR4) by injured or mutant BM niche cells as potential mechanisms that induce HSC transformation via excessive ROS production,85,86 and/or depletion of normal HSC clones (Figure 2).73 Hence, senescence or other dysfunctions in the BM niche caused by chronic disease, infection, aging, or exposure to genotoxic therapies87 may lead to overproduction of pathogenic inflammatory signals that subsequently destabilize HSC pool integrity and otherwise alter the fitness “playing field” of the marrow to select for transformed HSC clones.71,73 Thus, elimination of senescent cells88 or transplantation of mesenchymal stromal cells89 may hold promise for resolving inflammation and restoring normal HSC function. Further study is necessary to clarify the role of aging-related inflammation and SASP cytokines in BM niche impairment, as well as the generation and/or expansion of HSCs with mutations in DNMT3A, TET2, SF3B1, and other genes associated with clonal hematopoiesis of indeterminate potential.90
Chronic inflammation in hematological malignancy. Unlike homeostatic blood production, in which HSCs generate a balanced lineage output, chronic inflammation related to disease and/or physiological aging is characterized by continuous production of proinflammatory signals that can lead to significant alteration in HSC function and output. In particular, chronic overproduction of myeloid cells and platelets occurs, often accompanied by loss of lymphoid output (immunosenescence) and impaired erythroid production (anemia of chronic disease). Moreover, chronic inflammation, or even serial inflammatory episodes, may create a maladaptive context in which continued exposure to stress conditions brought about by continued proliferation, BM niche dysfunction, and exposure to stressors such as reactive oxygen species (ROS) promotes genomic instability and potentially the acquisition of somatic mutations, including those characteristic of clonal hematopoiesis of indeterminate potential. In the context of chronic inflammation, normal hematopoiesis may also be impaired in a manner such that preexisting HSC clones carrying leukemogenic mutations may have increased potential to expand and evolve. Hence, chronic inflammation may function as an initiator, as well as a driver, of hematological malignancy. Further investigation is required to identify the source(s) of inflammatory signals, particularly in the bone marrow niche, and whether the effects of chronic inflammation on HSCs play a causative role in the development of hematological malignancies.
Chronic inflammation in hematological malignancy. Unlike homeostatic blood production, in which HSCs generate a balanced lineage output, chronic inflammation related to disease and/or physiological aging is characterized by continuous production of proinflammatory signals that can lead to significant alteration in HSC function and output. In particular, chronic overproduction of myeloid cells and platelets occurs, often accompanied by loss of lymphoid output (immunosenescence) and impaired erythroid production (anemia of chronic disease). Moreover, chronic inflammation, or even serial inflammatory episodes, may create a maladaptive context in which continued exposure to stress conditions brought about by continued proliferation, BM niche dysfunction, and exposure to stressors such as reactive oxygen species (ROS) promotes genomic instability and potentially the acquisition of somatic mutations, including those characteristic of clonal hematopoiesis of indeterminate potential. In the context of chronic inflammation, normal hematopoiesis may also be impaired in a manner such that preexisting HSC clones carrying leukemogenic mutations may have increased potential to expand and evolve. Hence, chronic inflammation may function as an initiator, as well as a driver, of hematological malignancy. Further investigation is required to identify the source(s) of inflammatory signals, particularly in the bone marrow niche, and whether the effects of chronic inflammation on HSCs play a causative role in the development of hematological malignancies.
Conclusions
Inflammatory signaling is deeply intertwined with the function and maintenance of the blood and other tissues by regulating fate decisions at stem cell level. In the blood system, it has become increasingly clear that inflammation acts as a double-edged sword, promoting normal HSC development and function in specific settings while promoting HSC deregulation and functional decline in others. Thus, fully understanding how inflammation affects HSC biology requires careful study of the partnership and antagonism of these 2 systems in developmental and disease contexts. A critical next step is the translation of these findings into clinical practice. Effective use of cytokine blockade and/or anti-inflammatory and antisenescence therapies to restore normal hematopoiesis and/or uniquely target malignant cells requires a deeper biological understanding of how inflammatory signals lead to blood system dysfunction and participate in the pathogenesis of hematological malignancy. Altogether, our current understanding of the impact of inflammation on hematopoiesis has painted a fascinating if incomplete picture of this complex interaction; significant room exists to continue expanding and refining investigations into these fascinating and clinically important questions.
Acknowledgments
This work was supported by a grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK98315) and a Boettcher Foundation Webb-Waring Early Career Investigator Award (E.M.P.).
Authorship
Contribution: E.M.P. devised and wrote the paper.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Eric M. Pietras, Division of Hematology, Department of Medicine, University of Colorado Anschutz Medical Campus, Mail Stop F754, 12700 E. 19th Ave, Aurora, CO 80045; e-mail: eric.pietras@ucdenver.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal