In this issue of Blood,Choi et al describe the clinical efficacy of histone deacetylase inhibitor (HDACi) vorinostat in the prevention of severe acute graft-versus-host disease (aGVHD) in a high-risk setting: myeloablative, unrelated-donor allogeneic hematopoietic cell transplantation (HCT).1
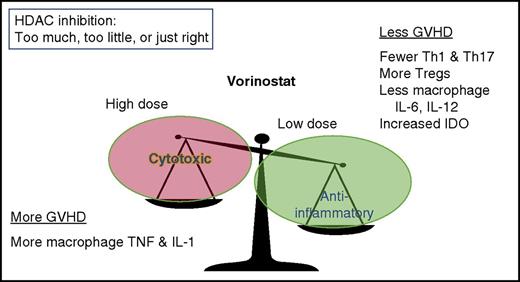
Relationship between vorinostat dose and response. Low doses are anti-inflammatory, whereas high doses of vorinostat are cytotoxic. IDO, indoleamine 2,3-dioxygenase; IL-1, interleukin-1; TNF, tumor necrosis factor; Tregs, regulatory T cells.
Relationship between vorinostat dose and response. Low doses are anti-inflammatory, whereas high doses of vorinostat are cytotoxic. IDO, indoleamine 2,3-dioxygenase; IL-1, interleukin-1; TNF, tumor necrosis factor; Tregs, regulatory T cells.
In the 1970s and early 1980s, most patients undergoing allogeneic HCT received GVHD prophylaxis: first with the single drug methotrexate and later with our current, predominantly calcineurin inhibitor-based, 2-drug GVHD prophylaxis regimens after the report of cyclosporine plus methotrexate reducing rates of grade II-IV aGVHD, published in 1986.2 Despite advances in our understanding of the pathophysiology of GVHD over the past 30-plus years, a calcineurin inhibitor-containing doublet (methotrexate plus either cyclosporine or tacrolimus) has not yet been replaced as the mainstay of GVHD prophylaxis. Why is it taking so long for the next era of GVHD prophylaxis regimens to change the standard?
The answer may lie in the single-minded emphasis of the majority of GVHD prevention research: T cells. It is clearly possible to markedly reduce GVHD risk by eliminating T cells from the graft ex vivo or by eliminating T cells in vivo using drugs such as antithymocyte globulin or post-HCT cyclophosphamide. However, the costs of such drastic pan–T-cell elimination are high: increased risks of both infection and relapse. As a result, the overall morbidity/mortality of HCT (as is measured by composite endpoints such as graft-versus-host disease-free, relapse-free survival [GRFS]) is not improved.3 Novel approaches to eliminating specific T-cell subpopulations (eg, naive T cells) or to reduce T-cell trafficking to GVHD target organs hold promise.4,5 These newer approaches deal primarily with the T-cell trafficking, expansion, and effector phase of GVHD.6 Yet it is possible that the next big breakthrough in GVHD prophylaxis will need to consider the problems inherent in activation of other immune cell subsets, especially macrophages, that can amplify the damage from initial phases of aGVHD.
Vorinostat can favorably affect the balance of circulating T-cell subsets (decreased Th1 and Th17 cells, along with increased regulatory T cells).7 It can also favorably modulate dendritic cell (DC) and macrophage inflammatory cytokine production. In high doses (the approved dose for cutaneous T-cell lymphoma is 400 mg daily), vorinostat is cytotoxic (see figure) and is associated with fatigue, nausea, anorexia, and diarrhea, among other potential side effects.8 Vorinostat at cytotoxic doses can also exacerbate inflammatory cytokine release in human macrophages, including increasing tumor necrosis factor-α and IL-1β, after challenge with lipopolysaccharide. However, after exposure to lower concentrations of vorinostat, inflammatory cytokine production, especially IL-6 and IL-12, is markedly reduced in macrophages.9 Low-dose vorinostat also modulates DC function through increasing IDO expression, essential for protection from GVHD in a murine model.10
In the current study, a relatively low dose of vorinostat, 100 mg twice daily from day −10 to day +100, was given to patients in conjunction with tacrolimus/methotrexate for GVHD prophylaxis following myeloablative unrelated-donor HCT. Rates of severe aGVHD were encouragingly low, 22% grade II-IV and 8% grade III-IV, in the patients (n = 37) treated in the study. In comparison with a similarly treated historical group of 154 patients, the rates of severe aGVHD were essentially cut in half (historical 48% grade II-IV aGVHD prior to day +100). GRFS at 1 year was also encouraging at 47%, in comparison with 28% in the originally reported Minnesota adult cohort.3 Finally, vorinostat-treated patients had lower levels of inflammatory IL-6 and lower circulating biomarkers of tissue damage (ST2 and REG3a) than did historical controls who underwent myeloablative conditioning with tacrolimus/methotrexate GVHD prophylaxis, suggesting that vorinostat treatment reduced both inflammation and the direct damage to GVHD target organs. Although histone acetylation was increased, as was expected in bulk peripheral blood mononuclear cells in vorinostat-treated patients, differences in IDO expression were not reported.
The inverse therapeutic response of low doses of HDACi reducing inflammation seen in prior laboratory studies appears to be confirmed in this clinical trial. Although the direct mechanisms of its effects are not yet clear, it is possible that limiting lymphocyte, macrophage, and DC activation, possibly through IDO or other mechanisms, may reduce tissue damage and lead to favorable clinical outcomes, as is seen in this study. However, dose modifications were required in 70% of patients, and 65% of patients had to have vorinostat dosing withheld at some point during study treatment. Because so many patients required dose adjustments or temporary delays in dosing, one wonders whether an even lower dose of vorinostat would provide similar benefits with reduced toxicity and with clinically simpler administration involving fewer dose adjustments or interruptions. Further studies are still needed to optimize this regimen, and larger randomized clinical trials are essential to confirm its benefit. This study is an exciting step toward further improvements in GVHD prophylaxis, which have the potential to change standard practice, 30 years in the making. We have sensed the victory, and the enemy is only old habits, old thinking, and thus, us. New approaches, new drugs, and the essential new and disciplined clinical trials will lead us into the promised land of less aGVHD.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal