To the editor:
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease with distinct immunophenotypes, genetic features, and clinical outcomes. Based on differential gene expression, termed cell of origin (COO), DLBCL can be subcategorized into germinal center B-cell-like (GCB), activated B-cell-like (ABC), and a third subtype (type III), each of which has genetic signatures similar to normal B cells at these stages of differentiation.1 Patients with GCB have superior outcomes compared with ABC when classified using gene expression profiling (GEP).1,,-4
NF-κB, a transcription factor involved in intracellular signaling, is activated from the downstream pathway of the B-cell receptor and is particularly important in the survival of ABC-DLBCL.5,-7 Ibrutinib is an oral covalent inhibitor of Bruton tyrosine kinase, which disrupts signaling from the B-cell receptor to NF-κB, thereby representing a rational therapeutic approach for the ABC subtype.
Because GEP is not available in routine clinical practice, immunohistochemistry (IHC) algorithms are often used to determine COO subtype, the most common of which was established by Hans et al.8 IHC differentiates GCB from non-GCB; the latter includes both ABC and unclassifiable DLBCL. Unfortunately, the correlation between GEP and IHC subtyping is imperfect, so despite the biologic rationale for selective cytotoxicity of ibrutinib for ABC-DLBCL, it is not clear that such preferential activity will be observed when COO is based on IHC. We therefore retrospectively analyzed clinical outcomes of patients with relapsed/refractory (r/r) DLBCL treated with ibrutinib according to COO by Hans IHC algorithm.
We conducted a retrospective cohort study of all r/r DLBCL patients at 6 US cancer centers treated with ibrutinib from 2013 to 2016. COO (GCB vs non-GCB) was determined by local pathology findings and/or the investigator’s application of the Hans algorithm. We included patients with de novo DLBCL as well as those who had transformed from prior indolent lymphoma or chronic lymphocytic leukemia provided that the ibrutinib was given for the DLBCL histology. Patients were excluded if they received ibrutinib for ≤14 days. Group differences were evaluated using χ2 and Kruskal-Wallis tests. Progression-free survival (PFS) was defined as the initiation of ibrutinib to the time of progressive disease, relapse, or death. Overall survival (OS) was defined as the initiation of ibrutinib to time of death. PFS and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. Statistical analyses were performed with SPSS (IBM SPSS, version 23.0, IBM Corporation, Armonk, NY).
Fifty-four patients met inclusion criteria, of whom 36 had de novo and 18 had transformed disease (13 from chronic lymphocytic leukemia). Patients who had transformed from prior indolent lymphoma were included in COO subgroups, whereas patients with Richter transformation (RT) were analyzed separately. By the Hans algorithm, there were 11 patients with GCB and 24 patients with non-GCB, and 6 patients were unknown. The remaining 13 patients had RT. Characteristics, including age, sex, number of prior therapies, and prior use of transplant, did not significantly differ between subgroups (Table 1). Four patients (ages 74, 75, 79, and 80) received only 1 prior treatment, which was R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in all cases. Of these patients, 2 were non-GCB and 2 were transformed DLBCL (1 from indolent lymphoma, 1 RT). Three patients received idelalisib treatment; all 3 were RT patients and received this therapy after ibrutinib.
Characteristics of patients treated with ibrutinib according to subtype
| Characteristic . | All patients (n = 54) . | Non-GCB (n = 24) . | GCB (n = 11) . | RT (n = 13) . | Unknown (n = 6) . | P . |
|---|---|---|---|---|---|---|
| Age at diagnosis, y, median (range) | 62 (38-88) | 61 (38-88) | 61 (47-79) | 61 (47-80) | 68 (40-71) | .97 |
| Sex, no. (%) | ||||||
| Men | 33 (61) | 15 (63) | 4 (36) | 9 (69) | 5 (83) | .27 |
| Women | 21 (39) | 9 (37) | 7 (64) | 4 (31) | 1 (17) | |
| IPI at diagnosis, no. (%) | ||||||
| Low | 14 (26) | 5 (21) | 4 (36) | 4 (31) | 1 (17) | .78 |
| Low intermediate | 11 (20) | 7 (29) | 1 (9) | 2 (15) | 1 (17) | |
| High intermediate | 13 (24) | 7 (29) | 1 (9) | 3 (23) | 2 (33) | |
| High | 16 (30) | 5 (21) | 5 (46) | 4 (31) | 2 (33) | |
| Number of prior treatments | ||||||
| Median | 3 | 3 | 3 | 3.0 | 4 | .61 |
| Range | 1-11 | 1-8 | 2-8 | 1-11 | 2-5 | |
| Prior transplant,* no. (%) | 17 (31) | 6 (25) | 5 (46) | 3 (23) | 3 (50) | .42 |
| CNS disease at diagnosis, no. (%) | 6 (11) | 4 (17) | 0 (0) | 1 (8) | 1 (17) | .53 |
| Characteristic . | All patients (n = 54) . | Non-GCB (n = 24) . | GCB (n = 11) . | RT (n = 13) . | Unknown (n = 6) . | P . |
|---|---|---|---|---|---|---|
| Age at diagnosis, y, median (range) | 62 (38-88) | 61 (38-88) | 61 (47-79) | 61 (47-80) | 68 (40-71) | .97 |
| Sex, no. (%) | ||||||
| Men | 33 (61) | 15 (63) | 4 (36) | 9 (69) | 5 (83) | .27 |
| Women | 21 (39) | 9 (37) | 7 (64) | 4 (31) | 1 (17) | |
| IPI at diagnosis, no. (%) | ||||||
| Low | 14 (26) | 5 (21) | 4 (36) | 4 (31) | 1 (17) | .78 |
| Low intermediate | 11 (20) | 7 (29) | 1 (9) | 2 (15) | 1 (17) | |
| High intermediate | 13 (24) | 7 (29) | 1 (9) | 3 (23) | 2 (33) | |
| High | 16 (30) | 5 (21) | 5 (46) | 4 (31) | 2 (33) | |
| Number of prior treatments | ||||||
| Median | 3 | 3 | 3 | 3.0 | 4 | .61 |
| Range | 1-11 | 1-8 | 2-8 | 1-11 | 2-5 | |
| Prior transplant,* no. (%) | 17 (31) | 6 (25) | 5 (46) | 3 (23) | 3 (50) | .42 |
| CNS disease at diagnosis, no. (%) | 6 (11) | 4 (17) | 0 (0) | 1 (8) | 1 (17) | .53 |
CNS, central nervous system; IPI, International Prognostic Index.
All transplants were autologous except 2, who received allogeneic transplants (1 with GCB subtype and 1 with non-GCB subtype).
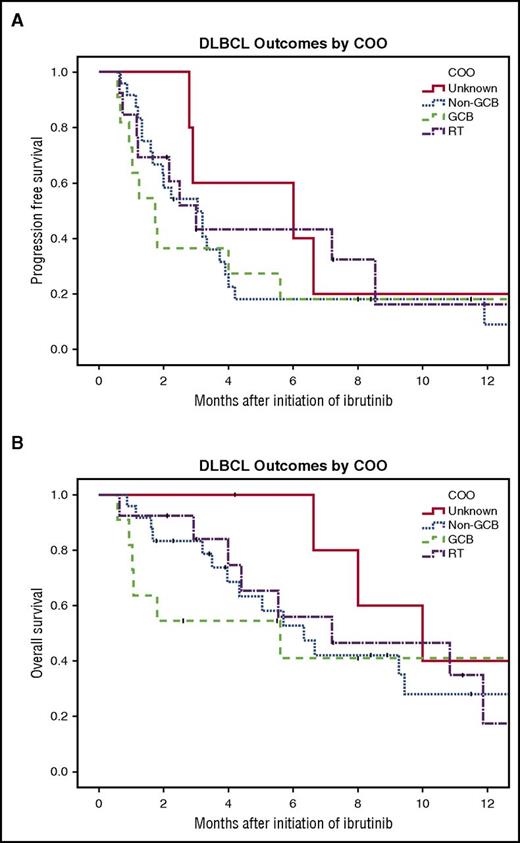
The overall response rate (ORR) for the entire cohort was 28%, with 5 patients achieving a complete response (CR) and 10 achieving a partial response (PR). The ORR by subtype was as follows: 18.2% for GCB patients (2 CR, 0 PR), 21% for non-GCB patients (2 CR, 3 PR), 46.2% for RT (1 CR, 5 PR), and 33.3% for unknown (0 CR, 2 PR). There was no significant difference in ORR between the subtypes (P = .34). The median PFS was 1.7, 3.0, 3.0, and 6.0 months for patients with GCB, non-GCB, RT, and unknown subtype, respectively (P = .85; Figure 1A). The median OS was 5.6, 6.3, 7.2, and 10.0 months for patients with GCB, non-GCB, RT, and unknown subtype, respectively (P = .97; Figure 1B). For the 2 unknown patients with PR, 1 discontinued ibrutinib at 2.3 months secondary to toxicity but was alive at 4.2 months and the other progressed at 13.5 months. For the 2 non-GCB patients with CR, 1 discontinued at 7.1 months secondary to intolerance but later was restarted and remained on ibrutinib at 23.5 months, and the other discontinued for toxicity at 8.3 months and progressed at 11.9 months. For the 3 non-GCB patients with PR, 1 remained on ibrutinib at 8.4 months, the other discontinued for toxicity at 9.5 months and progressed at 11.5 months, and the last progressed at 3.7 months. For the 2 GCB patients with CR, 1 remained on ibrutinib at 8.0 months and the other died at 5.6 months. One RT patient reached CR and then at 2.7 months received chimeric antigen receptor T cells and was alive at last follow-up of 15.6 months. For the 5 RT patients with PR, 3 were alive and still on ibrutinib at 7.2, 2.1, and 3.0 months and the other 2 progressed at 2.2 and 8.5 months.
Survival of patients with r/r DLBCL treated with ibrutinib by COO subtype. (A) PFS (log-rank test, P = .85) and (B) OS (log-rank test, P = .97).
Survival of patients with r/r DLBCL treated with ibrutinib by COO subtype. (A) PFS (log-rank test, P = .85) and (B) OS (log-rank test, P = .97).
In this real world series of DLBCL patients treated with ibrutinib in the r/r setting, we found that the clinical activity of single-agent ibrutinib did not differ when stratified by RT and de novo DLBCL. Importantly, response rates for ibrutinib in GCB and non-GCB subtypes of r/r DLBCL do not differ when using the Hans algorithm to assign subtype. PFS and OS were poor in both groups and not statistically different. The patients with unknown subtype had a trend toward improved PFS and OS likely because of the small sample size. The ORR to ibrutinib in this series of patients with r/r DLBCL (28%) was consistent with that which was previously published.9 Our study has some limitations. Data were collected retrospectively, and response rates were assigned by investigator using clinical criteria. In addition, there was no central pathology review, and the diagnosis and subtyping were assigned using local pathology laboratories. Despite these limitations, this study mimics real-world clinical practice.
Strategies to subclassify DLBCL are useful to identify patients at higher risk for developing r/r disease and to identify mechanisms of oncogenesis, which could be exploited with novel treatments. Several drugs, such as bortezomib,10,,-13 lenalidomide,14,,,-18 and ibrutinib,9,19 all known to affect NF-κB signaling implicated in ABC-DLBCL, are being incorporated into treatment strategies. The only study evaluating the efficacy of single-agent ibrutinib in the r/r setting classified 80 patients with GEP and found improved responses in ABC compared with GCB (ORR 37% vs 5%), but also demonstrated a number of responses in patients with unknown/unclassifiable DLBCL (ORR 22%) with relatively few complete remissions.9
IHC-based algorithms have poor concordance with each other and GEP classification and do not always differentiate PFS and OS outctomes.20,,-23 These inconsistencies question the utility of IHC as a true surrogate of GEP to establish COO for use in clinical practice.
In conclusion, until GEP or other molecular technologies such as Nanostring are in more widespread use for routine subtyping of DLBCL, caution is advised when selecting patients for subtype-specific therapy, because clinical outcomes for patients receiving ibrutinib may not differ by COO as determined by IHC.
Authorship
Contribution: A.M.W. performed research, analyzed the data, and wrote the manuscript; B.T.H. designed and supervised the study, analyzed the data, and wrote the manuscript; and D.J.L., A.R.M., K.I., F.J.H.-I., N.R., S.S., M.S., M.R.S., P.C., and D.J. performed research and edited the paper.
Conflict-of-interest disclosure: D.J.L. received institutional research support from Curis and Takeda and consultancy fees from Curis. A.R.M. received research funding and consultancy fees from Gilead, AbbVie, Pharmacyclics, and TG Therapeutics; research funding from Acerta, Regeneron, Portola, and DTRM Biopharma; and consultancy fees from Janssen, Celgene, and Kite. N.R. maintained membership on advisory boards for Celgene and AbbVie and is on the speakers bureau for Gilead. S.S. received research funding from Seattle Genetics, Merck Sharp and Dohme Corp, Janssen, Acerta, Pharmacyclics, Genentech, and Portola. M.S. maintained membership on advisory boards for Pharmacyclics and AbbVie; received research support and consultancy fees from Gilead and consultancy fess from PRIME Oncology and Genentech; and received research support from Plexxikon, Gilead, and National Institutes of Health, National Cancer Institute. M.R.S. received institutional research support from Gilead; received institutional research support, maintained membership on advisory board, and received honoraria from Seattle Genetics. P.C. is on the speakers bureau for Celgene. B.T.H. received research funding from Pharmacyclics and AbbVie and received honoraria for membership on advisory committee at Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Brian T. Hill, Cleveland Clinic, Taussig Cancer Institute, 9500 Euclid Ave, R35, Cleveland, OH 44195; e-mail: hillb2@ccf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal