Key Points
P vivax Duffy binding protein associates exclusively with a small population of immature reticulocytes.
Increased DARC-DBP binding site accessibility in immature reticulocytes compared with erythrocytes is a key to infection by P vivax.
Abstract
Plasmodium vivax is the most prevalent parasite species that causes malaria in humans and exclusively infects reticulocytes. Reticulocyte infection is facilitated by P vivax Duffy binding protein (DBP), which utilizes DARC (Duffy antigen receptor for chemokines) as an entry point. However, the selective tropism of P vivax for transferrin receptor (CD71)-positive reticulocytes remained unexplained, given the constitutive expression of DARC during reticulocyte maturation. CD71/RNA double staining of reticulocytes enriched from adult peripheral blood reveals 4 distinct reticulocyte populations: CD71high/RNAhigh (∼0.016%), CD71low/RNAhigh (∼0.059%), CD71neg/RNAhigh (∼0.37%), CD71neg/RNAlow (∼0.55%), and erythrocytes CD71neg/RNAneg (∼99%). We hypothesized that selective association of DBP with a small population of immature reticulocytes could explain the preference of P vivax for reticulocytes. Binding of specific monoclonal anti-DARC antibodies and recombinant DBP to CD71high/RNAhigh reticulocytes was significantly higher compared with other reticulocyte populations and erythrocytes. Interestingly, the total DARC protein throughout reticulocyte maturation was constant. The data suggest that selective exposure of the DBP binding site within DARC is key to the preferential binding of DBP to immature reticulocytes, which is the potential mechanism underlying the preferential infection of a reticulocyte subset by P vivax.
Introduction
Plasmodium vivax is the most prevalent parasite species outside sub-Saharan regions of Africa and accounts for 10 to 100 million malaria cases annually.1-3 Malaria-associated symptoms are mainly due to blood stage infection, which causes morbidity and occasional mortality, especially in the elderly and children.2 P vivax merozoites exclusively invade reticulocytes,4 which depend on the expression of DARC (Duffy antigen chemokine receptor). DARC (officially annotated as ACKR1, atypical chemokine receptor 1) is a transmembrane G protein–coupled chemokine receptor family member that lacks the DRY signaling domain.5,6 DARC interacts with P vivax Duffy binding protein (DBP7,8 ), leading to dimerization of DBP and subsequent P vivax invasion of reticulocytes.5,9 Parasite-induced evolutionary pressure leads to DARC negativity in sub-Saharan Africa, conferring a degree of protection against P vivax (review, see Baird10 ). Cocrystallization of DBP and DARC revealed that DARC residues 19 to 30 (QLDFEDVWNSSY) form the primary DBP binding site.5 Accessibility of the DBP binding site in DARC during reticulocyte maturation is ill defined but relevant to understand P vivax tropism and important for eventual vaccine development.11,12 Malleret et al showed that P vivax prefers immature CD71+ reticulocytes but not CD71− cells and that the invasion is blocked upon anti-DARC treatment.13 However, the mechanism of P vivax reticulocyte tropism remains to be elucidated, especially as DARC is expressed on both reticulocytes and erythrocytes. We hypothesized that the DBP binding site in DARC paramount to P vivax–reticulocyte association is differentially exposed upon reticulocyte maturation. To investigate this, we studied the recognition of specific DARC epitopes during various stages of reticulocyte maturation defined by expression of CD71 and RNA.
Study design
Antibodies and materials
Reticulocyte enrichment
For human blood, informed consent and ethical approval were given in accordance with the Declaration of Helsinki and the Dutch national and Sanquin internal ethic boards. Red cells were isolated by Ficoll-paque (ρ = 1.077 g/mL; 526g for 30 minutes), washed twice with phosphate-buffered saline, and subjected to Percoll-Urografin continuous gradients (48 209.7g; 20 minutes) to obtain reticulocyte enriched fractions.
Flow cytometry and DBP SPR
Cells were supplemented in phosphate-buffered saline–bovine serum albumin (1%) and incubated with antibodies (30 minutes, 4°C) or DBP-biotin (60 minutes, RT) as indicated in the figure legends, followed by secondary fluorochrome-coupled antibodies or Streptavidin-APC for 30 minutes, 4°C. When appropriate, cells were stained with anti-CD71 Pacific blue and TO (100 ng/mL). Cells were measured on FACSCanto II or sorted with a FACS-Aria II/III (BD Biosciences, Oxford, UK), and data were analyzed using FlowJo software (Tree Star). DBP surface plasmon resonance (SPR) was performed as previously described.14,15
Western blotting
Cells were lysed in CARIN lysis buffer (20 mM Tris-HCl pH 8.0, 138 mM NaCl, 10 mM EDTA, 100 mM NaF, 1% Nonidet P-40, 10% glycerol). Following protein quantification (detergent compatible; Bio-Rad Laboratories, Hercules, CA), lysates were boiled in Laemmli sample buffer (2% sodium dodecyl sulfate [SDS] wt/vol, 10% glycerol, 5% 2-mercaptoethanol, 60 mM Tris-HCl pH 6.8, brome-phenol blue; 3 minutes, 95°C), subjected to SDS-polyacrylamide gel electrophoresis , blotted using iBlot-PVDF blotting system (Thermo Fisher Scientific, Bleiswijk, The Netherlands), and stained as indicated in the figure legends.
Results and discussion
DARC protein expression does not change during reticulocyte maturation
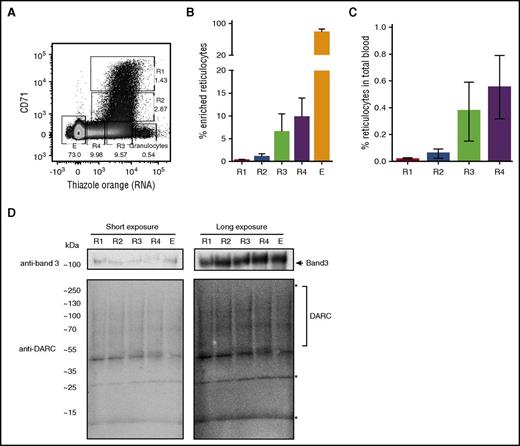
Cord blood reticulocytes can be divided into different maturation stages based on CD71 and RNA content.16 However, this has never been demonstrated for adult peripheral blood. Using CD71 expression and RNA content, we defined 4 subpopulations of circulating reticulocytes in adult peripheral blood. Normalized to the average reticulocyte frequency of 1% in blood, we identified CD71high/TOhigh (R1) at ∼0.016%, CD71low/TOhigh (R2) at ∼0.059%, CD71neg/TOhigh (R3) at ∼0.37%, CD71neg/TOlow (R4) at ∼0.55%, and CD71neg/TOneg erythrocytes (E; Figure 1A-C). These reticulocyte subpopulations, including the CD71high/TOhigh population susceptible to P vivax infection, expressed similar levels of band 3 or DARC total protein (present as a smear on western blot; Figure 1D; specificity of DARC antibodies in supplemental Figure 1A-B).
Total protein expression of DARC does not change significantly during reticulocyte maturation. (A) Reticulocytes were purified from peripheral blood as described in “Study design” and stained with CD71 and TO (RNA). The flow cytometry dot plot shows 4 subpopulations of circulating reticulocytes in adult peripheral blood: CD71high/TOhigh (R1), CD71low/TOhigh (R2), CD71neg/TOhigh (R3), CD71neg/TOlow (R4), and erythrocytes (E). (B-C) Quantification of the dot plot shown in panel A, after reticulocyte enrichment (B; N = 13), and normalized to the actual frequencies in adult peripheral blood (C; N = 13). (D) Reticulocyte subpopulations defined in panel A were sorted, and total protein lysate was subjected to SDS-polyacrylamide gel electrophoresis and western blotting. Blots were stained with anti-band 3 (top) and anti-DARC (clone 2C3; bottom). Note that DARC (calculated peptide mass, 35 kDa) presents as a diffuse pattern of bands as previously described. *a-Specific bands (supplemental Figure 1A).8,19
Total protein expression of DARC does not change significantly during reticulocyte maturation. (A) Reticulocytes were purified from peripheral blood as described in “Study design” and stained with CD71 and TO (RNA). The flow cytometry dot plot shows 4 subpopulations of circulating reticulocytes in adult peripheral blood: CD71high/TOhigh (R1), CD71low/TOhigh (R2), CD71neg/TOhigh (R3), CD71neg/TOlow (R4), and erythrocytes (E). (B-C) Quantification of the dot plot shown in panel A, after reticulocyte enrichment (B; N = 13), and normalized to the actual frequencies in adult peripheral blood (C; N = 13). (D) Reticulocyte subpopulations defined in panel A were sorted, and total protein lysate was subjected to SDS-polyacrylamide gel electrophoresis and western blotting. Blots were stained with anti-band 3 (top) and anti-DARC (clone 2C3; bottom). Note that DARC (calculated peptide mass, 35 kDa) presents as a diffuse pattern of bands as previously described. *a-Specific bands (supplemental Figure 1A).8,19
Immature reticulocytes demonstrate increased binding to DARC-specific antibodies and DBP compared with erythrocytes
Because DARC protein levels did not change between reticulocytes and erythrocytes, we investigated whether the accessibility of the DBP binding pocket within DARC is different between these populations. The reticulocyte populations were stained with antibodies recognizing different DARC epitopes, including the DBP binding site (Figure 2A). Binding of antibodies Fy6 and 2C3, designed as competitive antibodies overlapping the DBP interaction domain, to CD71high/TOhigh reticulocytes was >10-fold increased compared with more mature reticulocytes and erythrocytes (Figure 2B). Binding of Fy3 antibodies, recognizing the last extracellular loop located outside the DBP binding pocket, was modestly increased (<2.5-fold). This difference indicated that accessibility of the FY6/2C3 epitope rather than overall expression decreases during reticulocyte maturation. Antibodies against Duffy blood group polymorphisms (Fyb and Fya), located near the DBP binding site, revealed high binding in CD71high/TOhigh populations, gradually decreasing upon maturation to erythrocytes with Fya seemingly more sensitive to epitope availability than Fyb (Figure 2B). Of note, compared with the 2C3high population, the 2C3low population expressed less CD71 and RNA, whereas overall protein content was similar, indicating that the 2C3high population represents young reticulocytes (supplemental Figure 1C-D). Antibody binding to erythrocyte membrane protein Glycophorin A (CD235a) remained unchanged between the subpopulations (Figure 2C).
DARC-specific antibodies and DBP display significantly higher binding to CD71high/TOhighreticulocytes compared with other reticulocyte subsets or erythrocytes. (A) Schematic representation of 7-transmembrane spanning topology of DARC. N-terminal extracellular domain contains binding site for 2C3 and Fy6 antibodies, which overlap with DBP domain, indicated in gray. The Fy3 binding domain is localized at the third extracellular loop outside the DBP binding pocket. (B) Fold change representation of binding of DARC-specific antibodies to the 4 reticulocyte subpopulations and erythrocytes, as defined in Figure 1A. Enriched fraction of reticulocytes was probed with anti-Fya, anti-Fyb, Fy3, 2C3, and Fy6 antibodies followed by staining with anti-CD71 and TO. Figure is depicted as a fold change normalized to erythrocytes (anti-Fy6; anti-2C3; anti-Fya; anti-Fyb; anti-Fy3; n ≥ 6). (C) Fold change representation CD235a (glycophorin A [GPA]) antibody binding to the different reticulocyte populations and erythrocytes (N = 3). (D) Enriched fraction of reticulocytes was incubated with biotinylated recombinant DBP and counterstained with anti-CD71 and TO. A representable histogram semioffset is depicted, and a quantification of the mean fluorescent intensity (MFI) of DBP-binding (N = 6). Student t test (paired) was used to calculate statistical significance (*P < .05; **P < .01; ***P < .001; ****P < .0001). (E) ImageStream images depicting erythrocytes (top 2 panels), TOhigh/lowCD71neg (middle), and TOhighCD71high (bottom) stained with RNA (TO, green), CD71 (red), and DBP (purple) as well as normal light. (F) Binding of CD71+ reticulocytes on SRP-streptavidine biosensor. CD71+ reticulocytes were isolated as indicated in “Study design” and flowed over biosensor coated with DBP (1000 nM) and anti-GPA (n > 100). The response and sedimentation signals were measured and represented as normalized total response/sedimentation response (T/S). Columns and bars represent means and standard deviation, respectively. One-way analysis of variance was used to calculate statistical significance (*P < .05; **P < .01; ***P < .001; ****P < .0001). BSA, bovine serum albumin; IgG, immunoglobulin G.
DARC-specific antibodies and DBP display significantly higher binding to CD71high/TOhighreticulocytes compared with other reticulocyte subsets or erythrocytes. (A) Schematic representation of 7-transmembrane spanning topology of DARC. N-terminal extracellular domain contains binding site for 2C3 and Fy6 antibodies, which overlap with DBP domain, indicated in gray. The Fy3 binding domain is localized at the third extracellular loop outside the DBP binding pocket. (B) Fold change representation of binding of DARC-specific antibodies to the 4 reticulocyte subpopulations and erythrocytes, as defined in Figure 1A. Enriched fraction of reticulocytes was probed with anti-Fya, anti-Fyb, Fy3, 2C3, and Fy6 antibodies followed by staining with anti-CD71 and TO. Figure is depicted as a fold change normalized to erythrocytes (anti-Fy6; anti-2C3; anti-Fya; anti-Fyb; anti-Fy3; n ≥ 6). (C) Fold change representation CD235a (glycophorin A [GPA]) antibody binding to the different reticulocyte populations and erythrocytes (N = 3). (D) Enriched fraction of reticulocytes was incubated with biotinylated recombinant DBP and counterstained with anti-CD71 and TO. A representable histogram semioffset is depicted, and a quantification of the mean fluorescent intensity (MFI) of DBP-binding (N = 6). Student t test (paired) was used to calculate statistical significance (*P < .05; **P < .01; ***P < .001; ****P < .0001). (E) ImageStream images depicting erythrocytes (top 2 panels), TOhigh/lowCD71neg (middle), and TOhighCD71high (bottom) stained with RNA (TO, green), CD71 (red), and DBP (purple) as well as normal light. (F) Binding of CD71+ reticulocytes on SRP-streptavidine biosensor. CD71+ reticulocytes were isolated as indicated in “Study design” and flowed over biosensor coated with DBP (1000 nM) and anti-GPA (n > 100). The response and sedimentation signals were measured and represented as normalized total response/sedimentation response (T/S). Columns and bars represent means and standard deviation, respectively. One-way analysis of variance was used to calculate statistical significance (*P < .05; **P < .01; ***P < .001; ****P < .0001). BSA, bovine serum albumin; IgG, immunoglobulin G.
Next, we tested binding of recombinant P vivax DBP-biotin (region II, the ligand for DARC5,7,9 ) to the various reticulocyte maturation stages. Notably, the strongest interaction of DBP was observed in the most immature CD71high/TOhigh reticulocyte subset, whereas erythrocytes did not bind DBP (Figure 2D). By ImageStream, binding was only observed in the R1 fraction (Figure 2E). SPR quantifies the affinity of proteins (including antibodies) to specific epitopes.14,15 Flowing CD71+ reticulocytes or erythrocytes over SPR chips coated with DBP or anti-GPA antibody revealed a 3 times higher binding in CD71+ compared with CD71− reticulocytes, whereas the binding affinity of GPA changed minimally (Figure 2F). Of note, Fya-Fyb- reticulocytes/erythrocytes did not bind DBP, anti-Fya, anti-Fyb, 2C3, and Fy6 antibodies (supplemental Figure 1E).
In conclusion, DBP, responsible and required for P vivax reticulocyte invasion, selectively associates with the most immature reticulocytes. The CD71highRNAhigh fraction is estimated to be 0.016% of all enucleated erythroid cells. Assuming a linear age distribution and rate of clearance, this corresponds to reticulocytes during the first 30 minutes after bone marrow egress. Decreased exposure of the DBP epitope on DARC during reticulocyte maturation suggests that the DARC epitope recognized by DBP is remodeled or differentially exposed. A possible reason could be masking of specific epitopes due to protein complex formation. Indeed, protein 4.1 deficiency reduces DARC expression, indicating that DARC expression is dependent on the junctional complex within the erythrocyte membrane.17,18 These protein complexes remodel during reticulocyte maturation, which may lead to DARC epitope masking. Using a monoclonal antibody raised against the whole protein of DARC with unknown epitope specificity, Malleret et al reported no change in anti-DARC binding to specific reticulocyte populations in cord blood samples. This finding can be reconciled with our data showing minimal changes in recognition outside the region recognized by DBP.16 However, the use of monoclonal antibodies to specific epitopes within DARC revealed major recognition differences. It has previously been shown that P vivax preferentially infects CD71pos immature reticulocytes, which represents our CD71high/TOhigh (R1) and CD71low/TOhigh (R2) populations, but not CD71neg cells.13 Here, we provide a reason for this preferential infection, which is due to increased exposure of the DBP binding pocket in DARC, allowing DBP association with CD71high/TOhigh reticulocytes but not in mature reticulocytes or erythrocytes. It provides a plausible explanation to P vivax tropism, potentially leading to new targeting approaches.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Sanquin Central Facility for flow cytometry/sorting expertise (Sanquin Central Facility, Amsterdam, The Netherlands).
This study was supported by funding from Sanquin (PPOC: 11-035) (M.v.L., E.v.d.A., E.O.), the European Union (F.A. H2020-MSCA-ITN-2015, “RELEVANCE”), and National Institute of Allergy and Infectious Diseases, National Institutes of Health grants R56 AI080792 (N.D.S., N.H.T.) and R01 AI064478 (N.D.S., N.H.T.).
Authorship
Contribution: E.O. and F.A. performed the majority of the experiments; A.E.H.B. performed the SPR experiment and N.D.S. produced the recombinant DBP; E.v.d.A., M.v.L., G.V., N.H.T., F.A., and E.O. designed the experiments and analyzed the data; E.v.d.A., N.H.T., N.D.S., G.V., E.O., F.A., and M.v.L. wrote and modified the manuscript. The manuscript was critically revised by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emile van den Akker, Sanquin Research, Department of Hematopoiesis, Plesmanlaan 125, 1066CX Amsterdam, The Netherlands; e-mail: e.vandenakker@sanquin.nl.
References
Author notes
E.O. and F.A. contributed equally to this study.
N.H.T. and E.v.d.A. contributed equally to this study.

![Figure 2. DARC-specific antibodies and DBP display significantly higher binding to CD71high /TOhigh reticulocytes compared with other reticulocyte subsets or erythrocytes. (A) Schematic representation of 7-transmembrane spanning topology of DARC. N-terminal extracellular domain contains binding site for 2C3 and Fy6 antibodies, which overlap with DBP domain, indicated in gray. The Fy3 binding domain is localized at the third extracellular loop outside the DBP binding pocket. (B) Fold change representation of binding of DARC-specific antibodies to the 4 reticulocyte subpopulations and erythrocytes, as defined in Figure 1A. Enriched fraction of reticulocytes was probed with anti-Fya, anti-Fyb, Fy3, 2C3, and Fy6 antibodies followed by staining with anti-CD71 and TO. Figure is depicted as a fold change normalized to erythrocytes (anti-Fy6; anti-2C3; anti-Fya; anti-Fyb; anti-Fy3; n ≥ 6). (C) Fold change representation CD235a (glycophorin A [GPA]) antibody binding to the different reticulocyte populations and erythrocytes (N = 3). (D) Enriched fraction of reticulocytes was incubated with biotinylated recombinant DBP and counterstained with anti-CD71 and TO. A representable histogram semioffset is depicted, and a quantification of the mean fluorescent intensity (MFI) of DBP-binding (N = 6). Student t test (paired) was used to calculate statistical significance (*P < .05; **P < .01; ***P < .001; ****P < .0001). (E) ImageStream images depicting erythrocytes (top 2 panels), TOhigh/lowCD71neg (middle), and TOhighCD71high (bottom) stained with RNA (TO, green), CD71 (red), and DBP (purple) as well as normal light. (F) Binding of CD71+ reticulocytes on SRP-streptavidine biosensor. CD71+ reticulocytes were isolated as indicated in “Study design” and flowed over biosensor coated with DBP (1000 nM) and anti-GPA (n > 100). The response and sedimentation signals were measured and represented as normalized total response/sedimentation response (T/S). Columns and bars represent means and standard deviation, respectively. One-way analysis of variance was used to calculate statistical significance (*P < .05; **P < .01; ***P < .001; ****P < .0001). BSA, bovine serum albumin; IgG, immunoglobulin G.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/12/10.1182_blood-2017-03-774364/4/m_blood774364f2.jpeg?Expires=1769094801&Signature=jBFTfHfnRwgPnYILkRk7IHElyO16IGoNz2D-SUcahQwtQAkDDgDA5xkzoCzjrWNXMiPwN1XGNg33~2yPgf8ppJEmVvI~rRV22GwCZlnuUG84-n1SteqjJJdwc4fWjkf1d4SdGYY8031tDsgE3qyoANj7RhJ~r6EVM-3N21CvBtoB-~DHyR4L61zjCTuNFmBSdoDwwYbswLRapu2vrjg2FL7IxpT9ys8vunyQgGYhsq-YglWovSehFgTAkROQeA64Dyj8dVUTHVEjOy0Wm-ghDCYgayA03tQewQUWTabxBz2EUYd-X0z13zPrM5Dy69qF8FWikQWkIu8rgRvS3r7qJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal