To the editor:
Light-chain (AL) amyloidosis is a rare disease in which an underlying clonal plasma cell population generates aberrant immunoglobulin light chains that misfold and form amyloid fibrils, which are deposited in extracellular tissues and organs, resulting in impairment of vital organ function.1,2 High-dose melphalan and autologous stem cell transplantation (HDM/SCT) can produce both hematologic and clinical remissions and extend survival in selected patients with AL amyloidosis.3-5 However, there are limited data documenting outcomes for those who experience hematologic relapse after an initial hematologic complete response (CR) to HDM/SCT. We report on patients with AL amyloidosis treated with HDM/SCT to review long-term relapse and survival data and to help guide the intricate management and follow-up of this unique patient population.
Our group previously reported on 629 patients with AL amyloidosis who underwent HDM/SCT between 1994 and 2014. These patients were enrolled in several successive institutional review board–approved protocols with inclusion criteria as previously reported.3 Hematologic response was assessed using international consensus criteria,6,7 and 40.3% achieved a CR at 6 to 12 months following HDM/SCT. The CR rate was 34.8% by intention-to-treat. At the time of that report, hematologic relapse had occurred in 40 patients (18.2%), having previously achieved CR with a median time to relapse of 3.97 years (range, 1.89-12.45). The median overall survival (OS) for patients following hematologic relapse was 4.3 years. A retrospective review of 410 patients who underwent HDM/SCT for AL amyloidosis from 1996 to 2009 at Mayo Clinic revealed hematologic relapse or progression in 146 patients (36%) with a median time to relapse/progression of 1.97 years.8 The median OS in this patient group after relapse or progression was 4.31 years, and the most common treatment regimen pursued after progression included lenalidomide or thalidomide. We note however that only 38 of the patients (26%) investigated with relapse/progression had achieved a hematologic CR post-SCT. Others have reported an event-free survival of ∼4 years in patients undergoing SCT for AL amyloidosis independent of hematologic response and superior event-free survival in those individuals achieving CR at 1 year posttransplant.5 Therefore, there remains a scarcity of information available regarding outcomes for relapsed patients after HDM/SCT in AL amyloidosis.
In order to address this imperative gap in the literature, we reviewed 647 patients with AL amyloidosis who were treated with HDM/SCT at our center between 1994 and 2016. The median age of patients was 57 years (range, 28-80). Treatment-related mortality, death occurring within 100 days of SCT, was 7.6% (n = 49/647) and improved to 3.9% (n = 12/301) after 2005. Of the evaluable patients at 6 and 12 months following HDM/SCT, 39.2% (n = 213/543) achieved a CR using international consensus criteria.6,7 By intention-to-treat, the CR rate was 32.9%. Furthermore, 82 of these patients with an initial hematologic CR were found to have subsequently experienced a hematologic relapse, defined as recurrence of a monoclonal protein on serum or urine immunofixation electrophoresis and/or abnormality of the serum free light chain (sFLC) assay corresponding to their original clone, signaling a relapse rate of 38.5% (n = 82/213). This group is the focus of this analysis.
The median time to hematologic relapse was 4.32 years, although the range was quite wide, spanning from 1.49 years to 21.58 years. Interestingly, 13 of the 82 relapsed patients (15.9%) were determined to have a biochemical relapse only, based on abnormal serum free light assay or reappearance of a monoclonal gammopathy on serum or urine immunofixation electrophoreses, without evidence of organ disease progression. Given their overall end-organ stability, these patients with biochemical relapse did not require any additional anti–plasma cell therapy at the time of last follow-up (median follow-up of 6.53 years). Two of the patients with biochemical relapse died during the study period of other causes with no evidence of progressive organ disease due to AL amyloidosis. With elimination of this biochemical relapse group, the relapse rate after HDM/SCT was 32.4% (n = 69/213).
On further evaluation of the 82 patients experiencing hematologic relapse, the majority of patients received full-dose melphalan 200 mg/m2 (70.7%, n = 58/82), whereas a modified dose of 100 to 140 mg/m2 (29.3%, n = 24/82) was received by a smaller proportion. The median time to relapse for the melphalan 200 mg/m2 group was 4.04 years, whereas the group who received modified dose melphalan at 100 to 140 mg/m2 had a comparable median time to relapse of 4.75 years. There was no difference in the number of patients with or without cardiac involvement (51.2% vs 48.4%, n = 42 vs 40) in the relapse cohort. Of the 38 patients with hematologic relapse who had data on sFLC available at the time of diagnosis, 9 individuals (23.7%) demonstrated a difference in free light chains of <50 mg/L prior to their first treatment.
Ten patients experienced a late hematologic relapse >10 years after HDM/SCT. In contrast, 51 patients relapsed within 5 years, and 21 patients experienced relapse between 5 and 10 years after HDM/SCT. Of the patients with late hematologic relapse (>10 years), only 3 (30%) had cardiac involvement, and the majority (80%, n = 8/10) had received full-dose melphalan at 200 mg/m2. Examination of the therapeutic modalities pursued in those requiring treatment after hematologic relapse from HDM/SCT revealed a variety of distinct regimens ranging from older, well-studied medications to those that are more innovative in the realm of AL amyloidosis. The treatment regimens were as follows: bortezomib-based (40.6%, n = 28/69), lenalidomide-based (20.3%, n = 14/69), second cycle of HDM/SCT (11.6%, n = 8/69), thalidomide (7.2%, n = 5/69), melphalan-based (5.8%, n = 4/69), other therapy (2.9%, n = 2/69), and ixazomib-based (1.4%, n = 1/69). The median time from documentation of hematologic relapse to initiation of treatment was 4.56 months. There were 3 patients (4.4%, n = 3/69) with hematologic relapse who did not receive further treatment of their AL amyloidosis, and the first line of treatment after relapse was unknown in 4 patients (5.8%, n = 4/69). Hematologic CR and very good partial response (VGPR) were achieved by 26 patients (37.6%) after additional treatment.
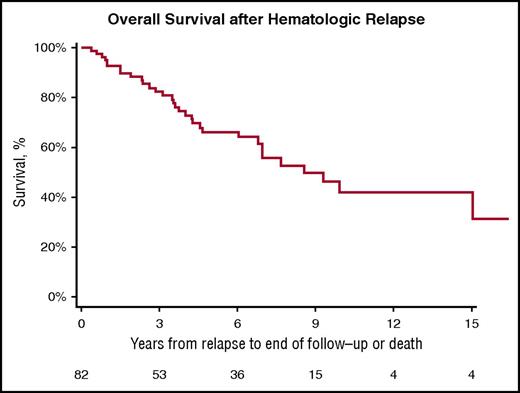
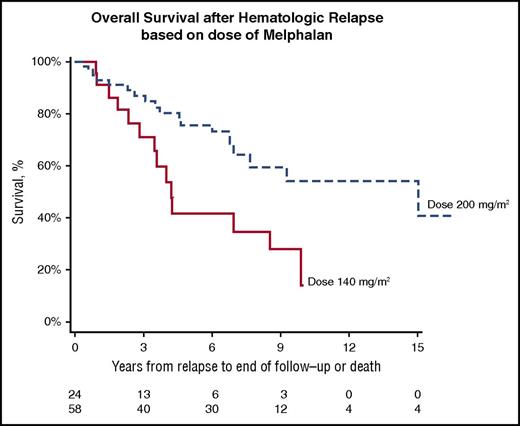
Importantly, the median OS of patients with hematologic relapse after an initial CR to HDM/SCT is 8.54 years from the time of relapse (Figure 1). The median OS after relapse in patients who received 200 mg/m2 of melphalan is 15.02 years compared with 4.23 years for those who received 100 to 140 mg/m2 (log-rank, P = .0080; Figure 2).
Kaplan-Meier estimates of OS from hematologic relapse in patients treated with HDM/SCT for AL amyloidosis. Median OS 8.54 years.
Kaplan-Meier estimates of OS from hematologic relapse in patients treated with HDM/SCT for AL amyloidosis. Median OS 8.54 years.
Kaplan-Meier estimates of OS from hematologic relapse based on dose of HDM prior to SCT. Median OS after HDM 200 mg/m2 of 15.02 years compared to 4.23 years after HDM 100-140 mg/m2 (P =.0080).
Kaplan-Meier estimates of OS from hematologic relapse based on dose of HDM prior to SCT. Median OS after HDM 200 mg/m2 of 15.02 years compared to 4.23 years after HDM 100-140 mg/m2 (P =.0080).
In summary, hematologic relapse, including biochemical relapse only, occurred in 38.5% of patients with AL amyloidosis who achieved an initial CR after HDM/SCT. Hematologic relapse occurred at a median of 4 years, although late relapses >20 years after HDM/SCT were noted as well. Late relapses raise a novel and essential observation of the potential for late hematologic relapse in AL amyloidosis after HDM/SCT and emphasizes the importance of close follow-up with assessment of plasma cell dyscrasia and end-organ function extending to 2 decades after SCT. It is important to note that there may be select patients with an initial CR after HDM/SCT who subsequently display reappearance of a monoclonal gammopathy biochemically, although with firmly stable organ disease. Although it is possible that the monoclonal component observed in these patients at the time of biochemical relapse could represent a nonrelated monoclonal gammopathy of undetermined significance, all individuals displayed a light chain and/or heavy chain gammopathy that coincided with their original amyloidogenic protein type. This group warrants careful and comprehensive evaluation to appropriately determine the need for further anti–plasma cell therapy vs ongoing active surveillance. We did not observe an association between the presence of cardiac involvement and hematologic relapse.
Moreover, the median OS after hematologic relapse following a CR to HDM/SCT is noteworthy at 8.5 years, signifying the effectiveness and benefits of novel agents for AL amyloidosis even when hematologic relapse does occur. Of note, a proportion of these relapsed patients (23.7% of the 38 patients with available sFLC data) were observed to have a difference in free light chains <50 mg/L at the time of diagnosis and therefore may represent a population with a good prognosis at baseline. Patients who received melphalan 200 mg/m2 were found to have significantly prolonged median OS after relapse when compared with the group receiving modified melphalan dosing of 100 or 140 mg/m2. This supports prior investigation indicating that full-dose melphalan conditioning, when appropriate based on patient and disease characteristics, may be superior to reduced-dose melphalan.9
The survival after hematologic relapse is longer than previously described and is a testament to the increasing number of novel therapeutic options available in AL amyloidosis. A limitation to our analysis is the absence of identified risk factors for hematologic relapse given that some of the hematologic parameters that are now commonly used (bone marrow cytogenetics and sFLC values) were not available at the beginning of our study period. Future investigations should explore features that may predict relapse.
This trial was registered at clinicaltrial.gov as #NCT00898235.
Authorship
Acknowledgments: The authors thank the current and past members of the Amyloidosis Center, Cancer Clinical Trials Office, Stem Cell Transplant Program, and Center for Cancer and Blood Disorders.
This research was supported by the Amyloid Research Fund.
Contribution: S.B. and V.S. designed research, performed research, analyzed data, and wrote and edited the manuscript; K.Q., J.M.S., and S.S. performed research and edited the manuscript; and G.D. performed data analysis and provided statistical support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vaishali Sanchorawala, Boston Medical Center, 820 Harrison Ave, FGH 1007, Boston, MA 02118; e-mail: vaishali.sanchorawala@bmc.org.