Key Points
Superiority of R-ACVBP over R-CHOP14 was not established, as IHP criteria driving consolidation did not properly reflect disease control.
The 26% PET2−/PET4− patients using IHP criteria increased to 79% using ΔSUVmax, which may help better select those needing an alternative to SIC.
Abstract
Dose-dense induction and up-front consolidation with autologous stem cell transplantation (ASCT) remain controversial issues when treating patients with high-risk diffuse large B-cell lymphoma. GELA designed a randomized phase 2 trial evaluating the efficacy of either rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone (R-ACVBP) or rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP14) induction and a positron emission tomography (PET)-driven ASCT or standard immunochemotherapy (SIC) consolidation in age-adjusted international prognosis index 2 (aaIPI2)-aaIPI3 patients. PET was performed at baseline, after 2 (PET2) and 4 (PET4) induction cycles, and centrally assessed using international harmonization project (IHP) criteria. PET2−/PET4− patients were assigned SIC, PET2+/PET4− patients were assigned ASCT, and PET4+ patients were treated with the investigator’s choice. The primary end-point was the 2007 international working group complete response (CR) rate after induction. Change in maximum standard uptake value (ΔSUVmax) after PET assessment was explored. Two hundred eleven patients were randomly assigned to R-ACVBP (n = 109) or R-CHOP14 (n = 102). PET4−/CR rates were 53%/47% with R-ACVBP and 41%/39% with R-CHOP14 (CR 95% confidence interval [CI], 38%-67% and 28%-54%, respectively; P = .076). Consolidation in the R-ACVBP and R-CHOP14 groups was SIC in 26% and 23% of patients and ASCT in 28% and 18% of patients, respectively. PET4 positivity was higher with R-CHOP14 vs R-ACVBP (54% vs 41%; P = .08), leading to more salvage therapy (37% vs 26%; P = .07) and lower event-free survival (EFS; 4-year EFS, 31% vs 43%; P < .01), but progression-free survival (PFS) and overall survival (OS) were similar in both groups. PET2−/PET4− and PET2+/PET4− patients had similar outcomes. Using ΔSUVmax, 79% of the patients were PET2−/PET4−. ΔSUVmaxPET0-4 >70% was associated with better outcome (4-year PFS, 84% vs 35%; 4-year OS, 91% vs 57%; P < .0001), whatever the consolidation. Superiority of R-ACVBP over R-CHOP14 was not established, as IHP criteria did not properly reflect disease control. ΔSUVmax may help better select patients needing an alternative to SIC, including ASCT.
Introduction
Dose-dense induction and up-front consolidation with autologous stem cell transplantation (ASCT) remain among the most controversial issues in the rituximab era when treating young patients with high-risk diffuse large B-cell lymphoma (DLBCL).1-4
The dose-dense induction regimen R-ACVBP improves progression-free survival (PFS) and overall survival (OS) of patients with 1 risk factor of the age-adjusted international prognosis index (aaIPI) compared with R-CHOP21,5 but the comparison has not been performed in high-risk patients with 2 or 3 risk factors (aaIPI2-aaIPI3). Conversely, 2 cooperative groups5,6 reported no benefit of a high-dose R-chemo14 compared with a conventional dose R-CHOP14 induction treatment, suggesting R-CHOP14 induction might be as efficient as and possibly less toxic than R-ACVBP. Also debated is the outcome benefit with up-front autologous stem cell transplantation (ASCT) compared with standard immunochemotherapy (SIC). In our hands, ASCT after R-ACVBP induction provides 74% 4-year PFS and 76% 4-year OS in this subset of patients, and a historical comparison would suggest better outcome than standard R-CHOP, where the risk for treatment failure reaches 30% to 40%.5,7,8
Recently, 2 trials from a US intergroup9 and the Italian Lymphoma Foundation group5 have reported better disease control with up-front ASCT, but more than half the patients in both studies were probably cured with SIC, underlining that up-front ASCT cannot be considered a standard option for all comers with patients with high-risk DLBCL, and that the real challenge in this setting is the need for a tool selecting patients for ASCT or SIC consolidation. Interim positron emission tomography (PET) after 2 to 4 cycles of chemotherapy10-12 could be such a tool. In 2007, we designed the randomized phase 2 GELA/LYSA trial to evaluate the efficacy of an induction with either R-ACVBP or R-CHOP14 and the value of PET-driven ASCT or SIC consolidation in young patients with aaIPI2-aaIPI3 untreated DLBCL (the study was registered at ClinicalTrials.gov: NCT00498043). A preliminary report focused on the planned interim analysis and the comparison between the predictive value of PET results, using visual and semiquantitative approach analysis, in a subset of 85 patients at a median follow-up of 19 months. Here, we report the final results of this trial on 222 patients with an extended follow-up of 45 months.
Methods
LNH2007-3B study design
Detailed study design is available in our previous report.13 Briefly, patients were randomly assigned to an induction immunochemotherapy with 4 cycles of either R-ACVBP14 or R-CHOP14. Consolidation treatment was driven by centrally reviewed PET assessment according to visual criteria after 2 and 4 cycles (Figure 1). Patients who were classified as PET2 and PET4 negative (double PET negative) received SIC consolidation consisting of, in the R-ACVBP group, 2 cycles of high-dose methotrexate (3 g/m2), and then 4 cycles rituximab (375 mg/m2), ifosfamide (1.5 g/m2), etoposide (300 mg/m2) and 2 cycles cytarabine (100 mg/m2); and in the R-CHOP14 group, 4 additional cycles of R-CHOP14. Patients classified as PET2 positive and PET4 negative received 2 cycles of high-dose methotrexate (3 g/m2) and then a high-dose therapy (carmustine, etoposide, cytarabine, melphal [BEAM] or zevalin, carmustine, etoposide, cytarabine, melphalan [Z-BEAM]), followed by ASCT. For these PET2-positive patients, peripheral stem cell harvest was performed using G-CSF after the third cycle of induction. For patients classified as PET4 positive, the protocol offered some guidance to go for a salvage regimen followed by ASCT in responders to salvage, but the final treatment decision was left to the local investigator.
Eligibility criteria
Eligible patients were 18 to 59 years old with previously untreated histologically proven CD20+ DLBCL and an aaIPI score of 2 or 3. A baseline PET scan (PET0) was mandatory, with at least 1 evaluable hypermetabolic lesion. All patients had to be fit for ASCT. Patients with known positive HIV status, active viral hepatitis B and C, or central nervous system involvement were excluded. This study was approved by the ethics committee of Lyon and the national regulatory agency, according to French regulatory laws. All patients provided written informed consent.
PET restaging
Two PET examinations scheduled 2 weeks after the second (PET2) and the fourth (PET4) induction cycle were required for full assessment. Each patient was scanned on the same camera for baseline and subsequent PET scans. A whole-body acquisition from groin to head was started 60 ± 10 minutes after a 5 MBq/Kg 18F-fluorodeoxyglucose (FDG) injection.
Visual analysis of PET
A blinded central review in real time of the PET images was organized using the positoscope network (Keosys, France). For each patient, PET0, PET2, and PET4 images were sent within 24 hours to 3 central panel PET experts (M.M., A.B.-R., or S.B.). PET2 and PET4 were binary interpreted as positive or negative, as per international harmonization project (IHP) criteria,14 with the previously published precision13,15,16 : the “clearly increased activity relative to the reference background,” which defines positive residual uptake in the IHP criteria, should be at least 25% higher than this background. A central review was performed within 72 hours of receiving PET2 images, and the final result was sent back to the investigator to allow planning of stem cell harvest after cycle 3 in case of positive PET2. A second central review was done within 72 hours of receiving PET4 images, and the final result was sent back to the investigator together with the per protocol recommended consolidation treatment allocation.
Exploratory quantitative PET assessment using the standard uptake value of 18F-FDG
An analysis of ΔSUVmax between PET0 and PET2 (ΔSUVmaxPET0-2) or PET4 (ΔSUVmaxPET0-4) was performed during the central review process, with no influence on the consolidation treatment allocation. To assess ΔSUVmax,17 the hottest tumor in any region or organ on PET2 or PET4 was used for comparison, even if its location differed from the initial hottest tumor in PET0. According to SUV-based assessment, PET2 and PET4 were considered negative when ΔSUVmaxPET0-2 >66% and ΔSUVmaxPET0-4 >70%, respectively.13
Statistical methods
End points and sample size
The primary endpoint was the complete response (CR) rate according to the 2007 International Working Group (IWG) criteria18 after 4 induction cycles of either R-ACVBP14 or R-CHOP14.
To detect a CR rate higher than 50% after 4 cycles of R-ACVBP or R-CHOP14, a sample size of 101 assessable patients in each randomization group was required to provide 85% power at an overall 2.5% (1-sided) significance level. The overall sample size was brought up to 222 patients, including 111 patients in each group, allowing for a 10% drop-out rate. According to Simon’s 2-stage design,19 an interim analysis was performed after the inclusion of 52 assessable patients in each randomization group, and the data and safety monitoring board allowed complete enrollment, as the early stopping rules had not been met. Secondary endpoints included toxicity (National Cancer Institute - Common Terminology Criteria for Adverse Events, version 3.0), PET4 response, overall survival (OS), event-free survival (EFS), and PFS, according to induction treatment group and PET-driven consolidation. The nominal significance level for the secondary endpoints was 5% (2-sided).
Statistical analysis
Response rates and PET results after 2 or 4 cycles in both groups were compared using the χ-squared test. PFS was defined as the time from randomization to first progression, relapse, and either death, whatever the cause, or last follow-up. EFS was measured from the date of randomization to first progression, relapse, and death (whatever the cause); changes of therapy during allocated treatment, including salvage therapy for PET4+ patients; or last follow-up. OS was defined as the time from randomization to death from any cause or last follow-up. Estimates of survival were calculated according to the Kaplan-Meier method and compared with the log-rank test. Endpoints were primarily analyzed on an intention-to-treat basis, but we also looked at outcomes according to the postinduction treatment on an as-treated basis.
Reproducibility of PET2 and PET4 results between on-site and review interpretation was evaluated with κ statistics (k). Agreement for a k value of 0-0.20 is characterized as slight, 0.21-0.4 as fair, 0.41-0.6 as moderate, 0.61-0.8 as substantial, and 0.81-1 as almost perfect.20
All outputs were produced using SAS, version 9.2.
Results
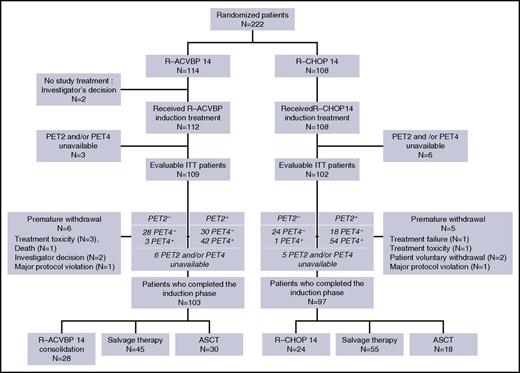
Between October 2007 and March 2010, 222 patients were enrolled in the study. Central pathological review was available for 200 patients (90%), and the diagnosis of DLBCL (World Health Organization 2008 classification) was confirmed for 194 patients (97%). The baseline characteristics of patients are detailed in Table 1 and are similar in both groups: median age was 46 years (range, 18-59 years), 26% of patients had a poor performance status (21% and 31% in the R-ACVBP and the R-CHOP groups, respectively; P = .09), 25% had a bulky mass >10 cm (22% and 28% in the R-ACVBP and the R-CHOP groups, respectively; P = .41), 97% had advanced disease with Ann Arbor stage III or IV, and 95% had elevated lactate dehydrogenase, resulting in an aaIPI of 2 and 3 in 77% and 20% of patients, respectively. Details of patient disposition are shown in Figure 2. There were 114 patients in the R-ACVBP group and 108 in the R-CHOP14 group. Twenty-two patients with missing PET information (n = 9) or premature withdrawal (n = 11) or who did not receive study treatment (n = 2) were deemed nonevaluable, leaving 200 patients who completed induction, including 103 (90%) and 97 (90%) patients in the R-ACVBP and R-CHOP14 groups, respectively.
Characteristics of patients
| . | Group . | All (n = 222) . | ||||
|---|---|---|---|---|---|---|
| R-ACVBP 14 (n = 114) . | R-CHOP 14 (n = 108) . | |||||
| N . | % . | N . | % . | N . | % . | |
| Sex | ||||||
| Male | 64 | 56 | 67 | 62 | 131 | 59 |
| Female | 50 | 44 | 41 | 38 | 91 | 41 |
| Age, years | ||||||
| Median (range) | 47 (19-59) | 44 (18-59) | 46 (18-59) | |||
| Performance status | ||||||
| <2 | 90 | 79 | 74 | 69 | 164 | 74 |
| ≥2 | 24 | 21 | 34 | 31 | 58 | 26 |
| Ann Arbor stage | ||||||
| I-II | 0 | 0 | 7 | 6 | 7 | 3 |
| III-IV | 114 | 100 | 101 | 94 | 215 | 97 |
| Lactate dehydrogenase | ||||||
| ≤Normal | 7 | 6 | 5 | 5 | 12 | 5 |
| >Normal | 107 | 94 | 103 | 95 | 210 | 95 |
| Age-adjusted IPI | ||||||
| 1 | 4 | 4 | 2 | 2 | 6 | 3 |
| 2 | 89 | 78 | 82 | 76 | 171 | 77 |
| 3 | 21 | 18 | 24 | 22 | 45 | 20 |
| Nb of extranodal sites | ||||||
| ≤1 | 44 | 39 | 33 | 31 | 77 | 35 |
| >1 | 70 | 61 | 75 | 69 | 145 | 65 |
| Mass >10 cm | ||||||
| Yes | 25 | 22 | 30 | 28 | 55 | 25 |
| No | 89 | 78 | 77 | 72 | 166 | 75 |
| B symptoms | ||||||
| Yes | 60 | 53 | 52 | 48 | 112 | 50 |
| No | 54 | 47 | 56 | 52 | 110 | 50 |
| BM involvement | ||||||
| Not involved | 85 | 75 | 82 | 76 | 167 | 75 |
| Involved | 20 | 18 | 21 | 19 | 41 | 18 |
| Unspecified | 4 | 4 | 2 | 2 | 6 | 3 |
| Not done | 5 | 4 | 3 | 3 | 8 | 4 |
| Pathology review | 99 | 87 | 101 | 93 | 200 | 90 |
| DLBCL | 81 | 82 | 79 | 78 | 160 | 80 |
| Primary mediastinal B cell | ||||||
| Lymphoma | 16 | 16 | 18 | 18 | 34 | 17 |
| Follicular lymphoma | 1 | 1 | 0 | 1 | 0.5 | |
| Mantel cell lymphoma | 1 | 1 | 1 | 1 | 2 | 1 |
| Burkitt lymphoma | 0 | 1 | 1 | 1 | 0.5 | |
| Other | 0 | 2 | 2 | 2 | 1 | |
| . | Group . | All (n = 222) . | ||||
|---|---|---|---|---|---|---|
| R-ACVBP 14 (n = 114) . | R-CHOP 14 (n = 108) . | |||||
| N . | % . | N . | % . | N . | % . | |
| Sex | ||||||
| Male | 64 | 56 | 67 | 62 | 131 | 59 |
| Female | 50 | 44 | 41 | 38 | 91 | 41 |
| Age, years | ||||||
| Median (range) | 47 (19-59) | 44 (18-59) | 46 (18-59) | |||
| Performance status | ||||||
| <2 | 90 | 79 | 74 | 69 | 164 | 74 |
| ≥2 | 24 | 21 | 34 | 31 | 58 | 26 |
| Ann Arbor stage | ||||||
| I-II | 0 | 0 | 7 | 6 | 7 | 3 |
| III-IV | 114 | 100 | 101 | 94 | 215 | 97 |
| Lactate dehydrogenase | ||||||
| ≤Normal | 7 | 6 | 5 | 5 | 12 | 5 |
| >Normal | 107 | 94 | 103 | 95 | 210 | 95 |
| Age-adjusted IPI | ||||||
| 1 | 4 | 4 | 2 | 2 | 6 | 3 |
| 2 | 89 | 78 | 82 | 76 | 171 | 77 |
| 3 | 21 | 18 | 24 | 22 | 45 | 20 |
| Nb of extranodal sites | ||||||
| ≤1 | 44 | 39 | 33 | 31 | 77 | 35 |
| >1 | 70 | 61 | 75 | 69 | 145 | 65 |
| Mass >10 cm | ||||||
| Yes | 25 | 22 | 30 | 28 | 55 | 25 |
| No | 89 | 78 | 77 | 72 | 166 | 75 |
| B symptoms | ||||||
| Yes | 60 | 53 | 52 | 48 | 112 | 50 |
| No | 54 | 47 | 56 | 52 | 110 | 50 |
| BM involvement | ||||||
| Not involved | 85 | 75 | 82 | 76 | 167 | 75 |
| Involved | 20 | 18 | 21 | 19 | 41 | 18 |
| Unspecified | 4 | 4 | 2 | 2 | 6 | 3 |
| Not done | 5 | 4 | 3 | 3 | 8 | 4 |
| Pathology review | 99 | 87 | 101 | 93 | 200 | 90 |
| DLBCL | 81 | 82 | 79 | 78 | 160 | 80 |
| Primary mediastinal B cell | ||||||
| Lymphoma | 16 | 16 | 18 | 18 | 34 | 17 |
| Follicular lymphoma | 1 | 1 | 0 | 1 | 0.5 | |
| Mantel cell lymphoma | 1 | 1 | 1 | 1 | 2 | 1 |
| Burkitt lymphoma | 0 | 1 | 1 | 1 | 0.5 | |
| Other | 0 | 2 | 2 | 2 | 1 | |
BM, bone marrow; Nb, number.
Primary endpoint
At the end of induction treatment, CR rate according to IWG 2007 criteria was 47% (51 of 109 patients) with R-ACVBP (95% confidence interval [CI], 38%-67%) and 39% (40 of 102) with R-CHOP14 (95% CI, 28%-54%; P = .076), whereas PET4 was negative (complete metabolic response) in 58 (53%) of 109 and 42 (41%) of 102 patients in the R-ACVBP and the R-CHOP14 groups, respectively (P = .08; Table 2). The discrepancies between IWG 2007 CR and complete metabolic response rates were related to missing bone marrow reassessment after 4 cycles of R-ACVBP (n = 7) or R-CHOP14 (n = 2) in patients with unspecified or involved bone marrow at baseline.
Response and outcome according to the randomization group
| . | R-ACVBP (n = 109) . | R-CHOP14 (n = 102) . | All (n = 211) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | % . | 4-year PFS (95% CI) . | P . | 4-year OS (95% CI) . | P . | N . | % . | 4-year PFS (95% CI) . | P . | 4-year OS (95% CI) . | P . | N . | % . | 4-year PFS (95% CI) . | P . | 4-year OS (95% CI) . | P . | |
| Response (IWG 2007) after 4 cycles of R- chemo | ||||||||||||||||||
| Complete response | 51 | 47 | 82.1 (68.4-90.1) | .025 | 94.1 (82.9-98.1) | .12 | 40 | 39 | 82.2 (66.2-91.1) | <.0001 | 87.2 (71.9-94.5) | .0001 | 91 | 43 | 82.2 (72.5-88.7) | <.0001 | 91.1 (83-95.5) | .0045 |
| Partial response | 47 | 43 | 74.3 (59.2-84.5) | 80.8 (66.3-89.5) | 52 | 51 | 78.8 (65.1-87.7) | 82.9 (64.4-92.4) | 99 | 47 | 76.7 (67.1-83.9) | 81.8 (70.7-89) | ||||||
| Stable disease | 3 | 3 | 40 (5.2-75.3) | 80 (20.4-96.9) | 1 | 1 | 0 | 40 (5.2-75.3) | 4 | 2 | 20 (3.1-47.5) | 60 (25.3-82.7) | ||||||
| Progressive disease | 2 | 2 | 5 | 5 | 7 | 3 | ||||||||||||
| Metabolic response | ||||||||||||||||||
| PET4− | 58 | 53 | 80.8 (68-88.9) | 93.1 (82.7-97.4) | 42 | 41 | 78.2 (62.3-88) | 85.4 (70.3-93.1) | 100 | 47 | 79.8 (79.4-86.4) | 88.9 (82.1-94.4) | ||||||
| PET2−/PET4− | 28 | 26 | 78.6 (58.4-89.8) | .71 | 90 (72.1-96.7) | .35 | 24 | 24 | 70.8 (48.4-84.9) | .24 | 83.3 (61.5-93.4) | .69 | 52 | 24 | 75 (60.9-84.6) | .26 | 89.6 (85-92.2) | .21 |
| PET2+/PET4− | 30 | 28 | 82 (63.6-92.5) | 96.4 (77.2-99.5) | 18 | 18 | 88.5 (61.4-97) | 88.2 (60.6-96.9) | 48 | 23 | 85 (71.1-92.6) | 90.4 (81-95.1) | ||||||
| PET4+ | 45 | 41 | 70.8 (55.1-81.9) | .17* | 79.9 (65-89) | .054* | 55 | 54 | 74.5 (60.8-84.1) | .51* | 80.1 (62.4-90.1) | .68* | 100 | 47 | 72.9 (63.1-80.6) | .16* | 80 (69-87.5) | .08* |
| Premature withdrawal | 6 | 6 | 5 | 5 | 11 | 5 | ||||||||||||
| All | 109 | 75 (65.6-82.1) | 85.3 (77.1-90.7) | 102 | 74.2 (64.4-81.6) | 80.7 (70.4-87.7) | 211 | 100 | 74.6 (68.1-80) | 83 (76.8-87.7) | ||||||||
| . | R-ACVBP (n = 109) . | R-CHOP14 (n = 102) . | All (n = 211) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | % . | 4-year PFS (95% CI) . | P . | 4-year OS (95% CI) . | P . | N . | % . | 4-year PFS (95% CI) . | P . | 4-year OS (95% CI) . | P . | N . | % . | 4-year PFS (95% CI) . | P . | 4-year OS (95% CI) . | P . | |
| Response (IWG 2007) after 4 cycles of R- chemo | ||||||||||||||||||
| Complete response | 51 | 47 | 82.1 (68.4-90.1) | .025 | 94.1 (82.9-98.1) | .12 | 40 | 39 | 82.2 (66.2-91.1) | <.0001 | 87.2 (71.9-94.5) | .0001 | 91 | 43 | 82.2 (72.5-88.7) | <.0001 | 91.1 (83-95.5) | .0045 |
| Partial response | 47 | 43 | 74.3 (59.2-84.5) | 80.8 (66.3-89.5) | 52 | 51 | 78.8 (65.1-87.7) | 82.9 (64.4-92.4) | 99 | 47 | 76.7 (67.1-83.9) | 81.8 (70.7-89) | ||||||
| Stable disease | 3 | 3 | 40 (5.2-75.3) | 80 (20.4-96.9) | 1 | 1 | 0 | 40 (5.2-75.3) | 4 | 2 | 20 (3.1-47.5) | 60 (25.3-82.7) | ||||||
| Progressive disease | 2 | 2 | 5 | 5 | 7 | 3 | ||||||||||||
| Metabolic response | ||||||||||||||||||
| PET4− | 58 | 53 | 80.8 (68-88.9) | 93.1 (82.7-97.4) | 42 | 41 | 78.2 (62.3-88) | 85.4 (70.3-93.1) | 100 | 47 | 79.8 (79.4-86.4) | 88.9 (82.1-94.4) | ||||||
| PET2−/PET4− | 28 | 26 | 78.6 (58.4-89.8) | .71 | 90 (72.1-96.7) | .35 | 24 | 24 | 70.8 (48.4-84.9) | .24 | 83.3 (61.5-93.4) | .69 | 52 | 24 | 75 (60.9-84.6) | .26 | 89.6 (85-92.2) | .21 |
| PET2+/PET4− | 30 | 28 | 82 (63.6-92.5) | 96.4 (77.2-99.5) | 18 | 18 | 88.5 (61.4-97) | 88.2 (60.6-96.9) | 48 | 23 | 85 (71.1-92.6) | 90.4 (81-95.1) | ||||||
| PET4+ | 45 | 41 | 70.8 (55.1-81.9) | .17* | 79.9 (65-89) | .054* | 55 | 54 | 74.5 (60.8-84.1) | .51* | 80.1 (62.4-90.1) | .68* | 100 | 47 | 72.9 (63.1-80.6) | .16* | 80 (69-87.5) | .08* |
| Premature withdrawal | 6 | 6 | 5 | 5 | 11 | 5 | ||||||||||||
| All | 109 | 75 (65.6-82.1) | 85.3 (77.1-90.7) | 102 | 74.2 (64.4-81.6) | 80.7 (70.4-87.7) | 211 | 100 | 74.6 (68.1-80) | 83 (76.8-87.7) | ||||||||
PET4+ vs PET4−.
Postinduction treatment according to PET results
Consolidation treatment according to interim PET results in both randomization groups is detailed in Table 3. According to central review, 100 patients (47%) achieved negative PET4 and were assigned SIC when PET2 was negative (n = 52; 26%), or ASCT when PET2 was positive (n = 48; 23%). Ninety-one patients (91%) received the planned treatment; namely, SIC (n = 51 patients; 98%) or up-front ASCT (n = 40 patients; 83%). The concordance between planned and actually received consolidation treatment was similar in the R-ACVBP (53 of 58; 91%) and the R-CHOP14 (38 of 42; 90%) groups. All 9 conflicting cases were related to decision of the local investigator.
Actual consolidation treatment according to PET-driven strategy in both randomization groups
| Actual consolidation treatment . | R-ACVBP 14 (n = 103) . | R-CHOP 14 (n = 97) . | All (n = 200) . | |||
|---|---|---|---|---|---|---|
| N . | % . | N . | % . | N . | % . | |
| PET2−/PET4− | ||||||
| All (n) | 28 | 24 | 52 | |||
| R-chemotherapy | 28 | 100 | 23 | 96 | 51 | 98 |
| High dose therapy + ASCT | 0 | 1 | 4 | 1 | 2 | |
| PET2+/PET4− | ||||||
| All (n) | 30 | 18 | 48 | |||
| High dose therapy + ASCT | 25 | 83 | 15 | 83 | 40 | 83 |
| R-chemotherapy | 5 | 17 | 3 | 17 | ||
| PET4+ | ||||||
| All (n) | 45 | 55 | 100 | |||
| Salvage therapy | 28 | 62 | 38 | 69 | 66 | 66 |
| Salvage therapy followed by ASCT | 21 | 47 | 25 | 45 | 46 | 46 |
| Salvaged not followed by ASCT | 7 | 15 | 13 | 24 | 20 | 20 |
| High dose therapy + ASCT | 17 | 38 | 16 | 29 | 33 | 33 |
| R-chemotherapy | 0 | 1 | 2 | 1 | 1 | |
| Actual consolidation treatment . | R-ACVBP 14 (n = 103) . | R-CHOP 14 (n = 97) . | All (n = 200) . | |||
|---|---|---|---|---|---|---|
| N . | % . | N . | % . | N . | % . | |
| PET2−/PET4− | ||||||
| All (n) | 28 | 24 | 52 | |||
| R-chemotherapy | 28 | 100 | 23 | 96 | 51 | 98 |
| High dose therapy + ASCT | 0 | 1 | 4 | 1 | 2 | |
| PET2+/PET4− | ||||||
| All (n) | 30 | 18 | 48 | |||
| High dose therapy + ASCT | 25 | 83 | 15 | 83 | 40 | 83 |
| R-chemotherapy | 5 | 17 | 3 | 17 | ||
| PET4+ | ||||||
| All (n) | 45 | 55 | 100 | |||
| Salvage therapy | 28 | 62 | 38 | 69 | 66 | 66 |
| Salvage therapy followed by ASCT | 21 | 47 | 25 | 45 | 46 | 46 |
| Salvaged not followed by ASCT | 7 | 15 | 13 | 24 | 20 | 20 |
| High dose therapy + ASCT | 17 | 38 | 16 | 29 | 33 | 33 |
| R-chemotherapy | 0 | 1 | 2 | 1 | 1 | |
One hundred patients did not achieve a complete metabolic response after 4 cycles of immunochemotherapy (Table 2). Most of them (66%) received salvage therapy, including 28 (62%) and 38 (69%) patients in the R-ACVBP and R-CHOP14 groups, respectively, whereas 33% (38% with R-ACVBP and 29% with R-CHOP) received up-front ASCT. On an intent-to-treat basis, there was a trend toward a higher use of salvage therapy in patients included in the R-CHOP14 group (38 [37%] of 102) compared with those of the R-ACVBP group (28 [26%] of 109; P = .07). Postinduction treatment according to IWG 2007 response to induction R-chemotherapy in PET4-positive patients is detailed in supplemental Table 1, available on the Blood Web site.
Outcome of patients according to randomization group
With a median follow-up of 45 months (1-63), 55 (26%) patients progressed or relapsed, and 39 patients died (18%). Deaths were related to lymphoma progression in 22 cases (10%), including 13 (12%) and 9 (8%) patients in the R-ACVBP and R-CHOP14 groups, respectively. Two patients died of toxicity of study treatment in the R-ACVBP group, and 1 patient of subsequent treatment in both groups. Two patients died of a secondary cancer in each randomization group. Six patients died of unrelated disease or trauma. The cause of death was unknown in 3 cases.
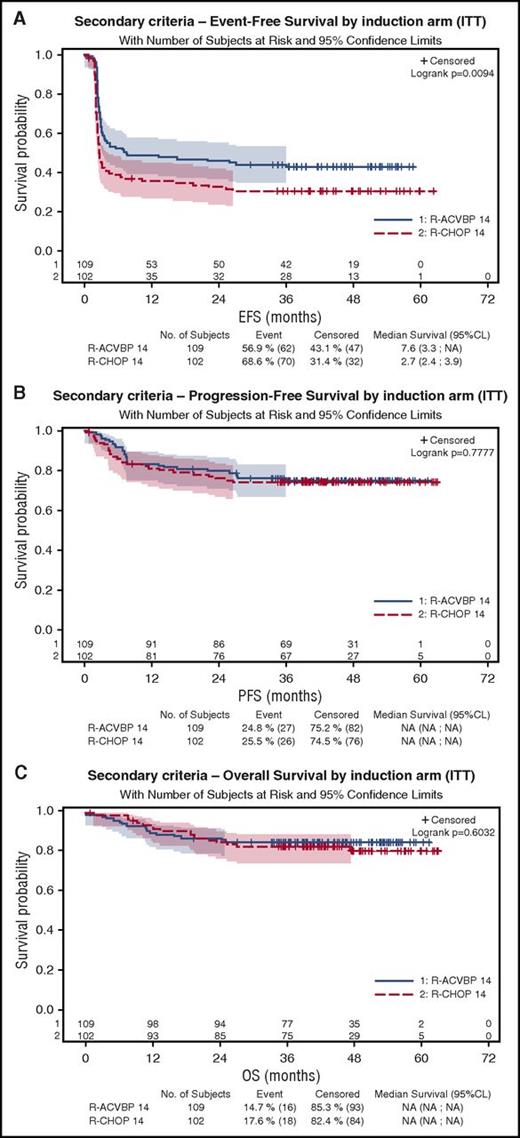
As shown on Figure 3A, 4-year EFS was 43% (95% CI, 34%-52%) and 31% (95% CI, 22%-40%) in the R-ACVBP and the R-CHOP14 groups, respectively (P = .0094), but 4-year PFS and OS were similar in the R-ACVBP (75% and 85%, respectively) and the R-CHOP14 groups (74% [P = .77] and 81% [P = .6]), respectively (Figure 3B-C; Table 2).
Patient’s outcome according to the treatment group. (A) EFS. (B) PFS. (C) OS.
Outcome of patients according to PET-driven strategy
Using IHP criteria, there was no significant difference in terms of PFS or OS between patients with negative or positive PET2 or patients with negative or positive PET4 (Table 2). Outcome of the 26% patients who became double PET negative and received SIC consolidation was nonsignificantly different from the 24% with positive PET2 and negative PET4 who mostly received ASCT: 4-year PFS was 75% vs 85% (P = .28), and 4-year OS was 89.6 vs 90.4% (P = .21) and similar in both randomization groups (P = .09). Focusing on the 40 patients with positive PET2 and negative PET4 who actually received ASCT, outcome remained nonstatistically different compared with those with a double-negative interim PET who received SIC (4-year PFS, 87.2% vs 74.5% [P > .14]; 4-year OS, 89.8% vs 90.2% [P = .9]).
Exploratory analysis of patient’s outcome according to ΔSUVmax
One hundred eighty-three patients were evaluable for quantitative PET assessment, including 99 patients in the R-ACVBP group and 84 in the R-CHOP14 group. SUVmax calculation was not possible for the 17 remaining patients because of insufficient quality of transmitted data. According to ΔSUVmax predefined cutoffs, 84% and 87% of patients were considered PET negative after 2 and 4 cycles of induction therapy, respectively (supplemental Table 2). Patients with a ΔSUVmaxPET0-2 >66% had a significantly better PFS and OS than patients with ΔSUVmaxPET0-2 ≤66% (4-year PFS, 80% vs 56% [P = .0005]; 4-year OS, 87% vs 69% [P = .0034]). PFS and OS of PET2+/PET4− patients who reached a ΔSUVmaxPET0-2 >66% and received ASCT consolidation were similar to those of PET2−/PET4− patients who received SIC (4-year PFS, 88% vs 75% [P = .13]; 4-year OS, 91% vs 90% [P = .87]), whereas the 3 patients with PET2+/PET4− and ΔSUVmaxPET0-2 ≤66% had a trend to a lower 4-year PFS (67%) and OS (50%). Patients with a ΔSUVmaxPET0-4 >70% had significantly better PFS and OS compared with those with a ΔSUVmaxPET0-4 ≤70% (4-year PFS, 84% vs 35% [P < .0001]; 4-year OS, 91% vs 57% [P < .0001]), and the prognostic influence of ΔSUVmaxPET0-4 was similar in both groups. Twenty-three patients (13%), most of whom were salvaged (82%), had a ΔSUVmaxPET0-4 ≤70% associated with a significantly poorer outcome (4-year PFS, 35%; 4-year OS, 57%) compared with those achieving a ΔSUVmaxPET0-4 >70% either with a ΔSUVmaxPET0-2 ≤66% (n = 14 [8%]; 4-year PFS, 77%; 4-year OS, 85%) or a ΔSUVmaxPET0-2 >66% (n = 141 [79%]; 4-year PFS, 86%; 4-year OS, 91%; P < .001; Figure 4A). Within the subset of patients achieving ΔSUVmaxPET0-2 >66% and ΔSUVmaxPET0-4 >70%, the outcome of patients who received SIC (n = 44) or ASCT (n = 36) consolidation was similar (4-year PFS, 79.5% vs 88.7%; P = .29; Figure 5; Table 4).
Outcome of patients according to ΔSUVmax PET0-2/ΔSUVmaxPET0-4 results. (A) PFS. (B) OS.
Outcome of patients according to ΔSUVmax PET0-2/ΔSUVmaxPET0-4 results. (A) PFS. (B) OS.
Outcome of patients with ΔSUVmaxPET02 >66% and ΔSUVmaxPET0-4 >70% according to the consolidation treatment actually received. (A) PFS. (B) OS.
Outcome of patients with ΔSUVmaxPET02 >66% and ΔSUVmaxPET0-4 >70% according to the consolidation treatment actually received. (A) PFS. (B) OS.
Patients’ outcome according to ΔSUVmax reduction after 2 and 4 cycles of chemotherapy and the actual consolidation treatment
| Metabolic response according to ΔSUVmax >66% . | N . | % . | 4-year PFS (95% CI) . | P . | 4-year OS (95% CI) . | P . |
|---|---|---|---|---|---|---|
| All | 153 | 84 | 80* (73-86) | .0005* | 87** (80-92) | .0034** |
| R-chemotherapy | 46 | 30 | 76 (61-86) | .37 | 91 (78-97) | .57 |
| High dose therapy + ASCT | 36 | 23 | 89 (73-96) | 92 (76-97) | ||
| Salvage therapy | 67 | 44 | 80 (69-88) | 83 (68-92) | ||
| Salvage therapy followed by ASCT | 36 | 54 | 92 (76-97) | 91 (51-99) | ||
| Salvaged not followed by ASCT | 31 | 46 | 68 (48-81) | 74 (55-86) | ||
| ΔSUVmax PET0-2 ≤66% | ||||||
| All | 30 | 16 | 56* (37-72) | 69** (49-83) | ||
| R-chemotherapy | 4 | 13 | 100 | .23 | 100 | 42 |
| High dose therapy + ASCT | 3 | 10 | 67 (5.4-94.5) | 50 (1-91) | ||
| Salvage therapy | 23 | 77 | 48 (27-66) | 65 (42-81) | ||
| Salvage therapy followed by ASCT | 6 | 26 | 67 (19-90) | 100 | ||
| Salvaged not followed by ASCT | 17 | 74 | 41 (19-63) | 53 (28-73) | ||
| ΔSUVmax PET0-4 >70% | ||||||
| All | 157 | 87 | 84† (77-89) | <.0001† | 91 (84-95) | <.0001†† |
| R-chemotherapy | 48 | 31 | 79 (65-88) | .62 | 96 (84-99) | .42 |
| High dose therapy + ASCT | 39 | 25 | 87 (71-94) | 89 (75-96) | ||
| Salvage therapy | 70 | 45 | 86 (75-92) | 87 (72-94) | ||
| Salvage therapy followed by ASCT | 38 | 54 | 92 (77-97) | 92 (51-99) | ||
| Salvaged not followed by ASCT | 32 | 46 | 78 (59-89) | 81 (63-91) | ||
| ΔSUVmax PET0-2 >66%/ΔSUVmax PET0-4 >70% | ||||||
| All | 141 | 91 | 85‡ (78-90) | .36‡ | 91‡‡ (83-95) | .37‡‡ |
| R-chemotherapy | 44 | 31 | 79 (64-89) | .5 | 95 (83-99) | .55 |
| High dose therapy + ASCT | 36 | 25 | 89 (73-96) | 92 (76-97) | ||
| Salvage therapy | 61 | 43 | 87 (75-93) | 86 (68-95) | ||
| Salvage therapy followed by ASCT | 35 | 57 | 94 (79-98) | 90 (47-98) | ||
| Salvaged not followed by ASCT | 26 | 43 | 77 (56-89) | 81 (60-91) | ||
| ΔSUVmax PET0-2 ≤66%/ΔSUVmax PET0-4 >70% | ||||||
| All | 14 | 9 | 77‡ (47-93) | 85‡‡ (51-96) | ||
| R-chemotherapy | 2 | 14 | 100 | .73 | 100 | .39 |
| High dose therapy + ASCT | 3 | 21 | 67 (5-94) | 50 (1-91) | ||
| Salvage therapy | 9 | 64 | 78 (36-94) | 89 (43-98) | ||
| Salvage therapy followed by ASCT | 3 | 33 | (5-94) | 100 | ||
| Salvaged not followed by ASCT | 6 | 67 | 83 (27-97) | 83 (27-97) | ||
| ΔSUVmax PET0-4 ≤70% | ||||||
| All | 23 | 13 | 35† (17-54) | 57†† (34-74) | ||
| R-chemotherapy | 4 | 17 | 75 (13-96) | .1 | 75 (13-96) | .38 |
| High dose therapy + ASCT | 0 | 0 | ||||
| Salvage therapy | 19 | 83 | 26 (10-47) | 53 (29-72) | ||
| Salvage therapy followed by ASCT | 4 | 21 | 50 (6-84) | 100 | ||
| Salvaged not followed by ASCT | 15 | 79 | 20 (5-42) | 40 (16-63) |
| Metabolic response according to ΔSUVmax >66% . | N . | % . | 4-year PFS (95% CI) . | P . | 4-year OS (95% CI) . | P . |
|---|---|---|---|---|---|---|
| All | 153 | 84 | 80* (73-86) | .0005* | 87** (80-92) | .0034** |
| R-chemotherapy | 46 | 30 | 76 (61-86) | .37 | 91 (78-97) | .57 |
| High dose therapy + ASCT | 36 | 23 | 89 (73-96) | 92 (76-97) | ||
| Salvage therapy | 67 | 44 | 80 (69-88) | 83 (68-92) | ||
| Salvage therapy followed by ASCT | 36 | 54 | 92 (76-97) | 91 (51-99) | ||
| Salvaged not followed by ASCT | 31 | 46 | 68 (48-81) | 74 (55-86) | ||
| ΔSUVmax PET0-2 ≤66% | ||||||
| All | 30 | 16 | 56* (37-72) | 69** (49-83) | ||
| R-chemotherapy | 4 | 13 | 100 | .23 | 100 | 42 |
| High dose therapy + ASCT | 3 | 10 | 67 (5.4-94.5) | 50 (1-91) | ||
| Salvage therapy | 23 | 77 | 48 (27-66) | 65 (42-81) | ||
| Salvage therapy followed by ASCT | 6 | 26 | 67 (19-90) | 100 | ||
| Salvaged not followed by ASCT | 17 | 74 | 41 (19-63) | 53 (28-73) | ||
| ΔSUVmax PET0-4 >70% | ||||||
| All | 157 | 87 | 84† (77-89) | <.0001† | 91 (84-95) | <.0001†† |
| R-chemotherapy | 48 | 31 | 79 (65-88) | .62 | 96 (84-99) | .42 |
| High dose therapy + ASCT | 39 | 25 | 87 (71-94) | 89 (75-96) | ||
| Salvage therapy | 70 | 45 | 86 (75-92) | 87 (72-94) | ||
| Salvage therapy followed by ASCT | 38 | 54 | 92 (77-97) | 92 (51-99) | ||
| Salvaged not followed by ASCT | 32 | 46 | 78 (59-89) | 81 (63-91) | ||
| ΔSUVmax PET0-2 >66%/ΔSUVmax PET0-4 >70% | ||||||
| All | 141 | 91 | 85‡ (78-90) | .36‡ | 91‡‡ (83-95) | .37‡‡ |
| R-chemotherapy | 44 | 31 | 79 (64-89) | .5 | 95 (83-99) | .55 |
| High dose therapy + ASCT | 36 | 25 | 89 (73-96) | 92 (76-97) | ||
| Salvage therapy | 61 | 43 | 87 (75-93) | 86 (68-95) | ||
| Salvage therapy followed by ASCT | 35 | 57 | 94 (79-98) | 90 (47-98) | ||
| Salvaged not followed by ASCT | 26 | 43 | 77 (56-89) | 81 (60-91) | ||
| ΔSUVmax PET0-2 ≤66%/ΔSUVmax PET0-4 >70% | ||||||
| All | 14 | 9 | 77‡ (47-93) | 85‡‡ (51-96) | ||
| R-chemotherapy | 2 | 14 | 100 | .73 | 100 | .39 |
| High dose therapy + ASCT | 3 | 21 | 67 (5-94) | 50 (1-91) | ||
| Salvage therapy | 9 | 64 | 78 (36-94) | 89 (43-98) | ||
| Salvage therapy followed by ASCT | 3 | 33 | (5-94) | 100 | ||
| Salvaged not followed by ASCT | 6 | 67 | 83 (27-97) | 83 (27-97) | ||
| ΔSUVmax PET0-4 ≤70% | ||||||
| All | 23 | 13 | 35† (17-54) | 57†† (34-74) | ||
| R-chemotherapy | 4 | 17 | 75 (13-96) | .1 | 75 (13-96) | .38 |
| High dose therapy + ASCT | 0 | 0 | ||||
| Salvage therapy | 19 | 83 | 26 (10-47) | 53 (29-72) | ||
| Salvage therapy followed by ASCT | 4 | 21 | 50 (6-84) | 100 | ||
| Salvaged not followed by ASCT | 15 | 79 | 20 (5-42) | 40 (16-63) |
,**indicate comparison of 4-year PFS and 4-year OS between patients with either ΔSUVmax PET0-2 ≤ 66% or >66%, respectively.
,††indicate comparison of 4-year PFS and 4-year OS between patients with either ΔSUVmax PET0-4 ≤70% or >70%, respectively.
,‡‡indicate comparison of 4-year PFS and 4-year OS between patients with either ΔSUVmax PET0-2 ≤66%/ΔSUVmax PET0-4 >70% or ΔSUVmax PET0-2 >66%/ΔSUVmax PET0-4 >70%, respectively.
Reproducibility of PET2 and PET4 results according to visual and ΔSUVmax analysis
The agreement between on-site and review for visual PET2 and PET4 interpretation is detailed in Tables 5 and 6 and was substantial for PET2 (k = 0.731; 95% CI, 0.632-0.83) and almost perfect for PET4 (k = 0.85; 95% CI, 0.777-0.923; Tables 5 and 6).
Agreement between on-site and review PET2 interpretation according to IHP criteria
| . | PET2 review . | All (n = 204) . | ||||
|---|---|---|---|---|---|---|
| PET2 Negative . | PET2 Positive . | |||||
| N = 57 . | Agreement . | N = 147 . | Agreement . | |||
| On-site PET2 | ||||||
| PET2 negative | 53 | 93% | 20 | 73 | 35% | |
| PET2 positive | 4 | 127 | 86% | 133 | 65% | |
| . | PET2 review . | All (n = 204) . | ||||
|---|---|---|---|---|---|---|
| PET2 Negative . | PET2 Positive . | |||||
| N = 57 . | Agreement . | N = 147 . | Agreement . | |||
| On-site PET2 | ||||||
| PET2 negative | 53 | 93% | 20 | 73 | 35% | |
| PET2 positive | 4 | 127 | 86% | 133 | 65% | |
Agreement between on-site and review PET4 interpretation according to IHP criteria
| . | PET4 review . | All (n = 200) . | ||||
|---|---|---|---|---|---|---|
| PET4 Negative . | PET4 Positive . | |||||
| N = 57 . | Agreement . | N = 147 . | Agreement . | |||
| On-site PET4 | ||||||
| PET4 negative | 97 | 97% | 12 | 109 | 55% | |
| PET4 positive | 3 | 88 | 88% | 91 | 45% | |
| . | PET4 review . | All (n = 200) . | ||||
|---|---|---|---|---|---|---|
| PET4 Negative . | PET4 Positive . | |||||
| N = 57 . | Agreement . | N = 147 . | Agreement . | |||
| On-site PET4 | ||||||
| PET4 negative | 97 | 97% | 12 | 109 | 55% | |
| PET4 positive | 3 | 88 | 88% | 91 | 45% | |
As on-site ΔSUVmax evaluation by the local nuclear medicine physician was not required per protocol, only 94 PET2 and 82 PET4 patients were evaluable for studying reproducibility of ΔSUVmaxPET0-2 and ΔSUVmaxPET0-4 between local and central review interpretations. Median ΔSUVmax values were similar between local and central review, with correlation coefficients of 0.93 (95% CI, 0.89-0.95) and 0.96 (95% CI, 0.95-0.98) after 2 and 4 cycles, respectively. The agreement between local and central review was almost perfect for both PET2 and PET4: 81 (97.8%) of 82 for PET2 negativity and 11 (91.6%) of 12 for PET2 positivity (k = 0.9; 95% CI, 0.77-1), based on a 66% ΔSUVmax cutoff; 70 (100%) of 70 for PET4 negativity and 10 (83%) of 12 for PET4 positivity (k = 0.86; 95% CI, 0.66-1), based on a 70% ΔSUVmax cutoff.
Toxicity
The hematological toxicity of induction treatment was greater with R-ACVBP compared with R-CHOP14 and was associated with more frequent infections (supplemental Table 3) and transfusions: 72 patients assigned to R-ACVBP (64%) vs 29 (27%) allocated to R-CHOP14 received at least 1 red blood cell transfusion (P < 10−5), and 20 patients received at least 1 platelet transfusion in the R-ACVBP group compared with 3 in the R-CHOP14 group (P < .0003). Mucositis was also more frequent with R-ACVBP. Four secondary primary cancers were observed; 2 cases in both groups. No secondary acute myeloid leukemia or myelodysplasia was reported.
Discussion
The first goal of this randomized phase 2 trial was to evaluate the efficacy of an induction with either R-ACVBP or R-CHOP14, using IWG 2007 criteria in young patients with high-risk DLBCL. Our first finding was that the primary objective of achieving a higher than 50% CR rate after 4 cycles of induction regimen was not met in both randomization groups (47% vs 39% in the R-ACVBP and R-CHOP14 groups, respectively). These low CR rates, in contrast with reasonably good 4-year outcomes, most probably were because IHP criteria14 did not properly reflect disease control. IHP criteria were selected because they were the most recently available at the time the present study was launched, but they are now known to generate a large amount of false-positive results that may reach 80% of cases when interpreting interim PET.13,21 In addition, in the R-ACVBP group, the low CR rate was also related to missing bone marrow reassessment in 7 patients who were in complete metabolic response after induction treatment. Acknowledging these limitations, findings suggesting R-ACVBP may provide better disease control than R-CHOP may be summarized as follows: first, there was a trend toward a higher complete metabolic response rate (53% vs 41%; P = .08); second, EFS in the R-ACVBP group was significantly better (4-year EFS, 43% vs 31%; P = .0094) because of the higher frequency of salvage therapy on the basis of the PET4 positivity in the R-CHOP14 group (39% vs 27%). However, this higher frequency of salvage therapy in the R-CHOP group possibly contributed to equalizing PFS and OS in both randomization groups (Figure 3; Table 2), leaving unanswered the question of the benefit of a dose-dense induction with ACVBP.
The second major objective of this multicenter prospective phase 2 randomized trial was to assess the value of sequential interim PET to select patients for ASCT or SIC. Our results demonstrate that this PET-driven consolidation strategy based on a real-time central review is feasible in a multicenter setting, as only 4% of the data were missing because of logistical issues. For patients achieving a negative PET4 using IHP criteria, investigators were keen to follow the consolidation therapy allocated on the basis of central interim PET review, as 90% received the planned consolidation therapy in both groups. Importantly, the 75% and 83% 4-year PFS and OS achieved by double PET (PET2 and PET4)-negative patients who received SIC is at least as good as that observed in our previous trial, in which all induction responders received up-front ASCT.8 This suggests that the 26% of patients who become PET2 and PET4 negative using IHP criteria can safely be given SIC without impairment of disease control. The hypotheses to explain why PET2−/PET4− patients receiving standard immunochemotherapy and PET2+/PET4− patients had a similar outcome included a possible equalizing effect of up-front ASCT consolidation and the high proportion of false-positive PET2, using IHP criteria, given the high number of visually PET2+ cases that were finally interpreted as negative according to ΔSUVmax (supplemental Table 1). To further investigate this issue, we analyzed the outcome of PET2+ patients receiving ASCT according to ΔSUVmax result. PFS and OS of PET2+/PET4− patients who reached a ΔSUVmaxPET0-2 >66% are similar to those of PET2−/PET4− patients, whereas the 3 patients with PET2+/PET4− and ΔSUVmaxPET0-2 ≤66% have a trend to a lower PFS and OS. Therefore, it appears that up-front ASCT in patients with ΔSUVmaxPET0-2 >66% does not seem to improve disease control compared with SIC. As only few patients with visual PET2+/PET4− and PET0-2 SUVmax reduction <66% were available in this series, the relevance of ASCT as consolidation in this subset remains to be further studied in a larger cohort.
Postinduction treatment of PET4-positive patients was up to the investigator, and therefore was more diversified: 66% of them were considered primary induction treatment failure and received salvage therapy, whereas PET4 positivity was considered as not justifying salvage in the remaining 34%, leading the investigator to go for up-front ASCT in most cases. A recent uncontrolled prospective series showed that PET-positive patients, on the basis of IHP criteria, may benefit from R–ifosfamide, carboplatin, etoposide salvage therapy, followed by ASCT.22 Still, whether PET4-positive patients using IHP criteria all truly deserve up-front ASCT, or eventually salvage, instead of SIC is unclear, given the limitations of IHP criteria in interim PET interpretation. Other tools such as 5 PS score and ΔSUVmax12,13,23,24 have been evaluated in this setting. In our interim report, we showed that ΔSUVmax score was more predictive than 5 PS to interpret interim PET13 and focused on the former in this final analysis of a larger number of patients (n = 183). Here again, the interobserver reproducibility of ΔSUVmax seems better than visual interpretation of interim PET, particularly after 2 cycles of induction treatment. Moreover, we confirm with 45 months of median follow-up that ΔSUVmax provides a good prognostic stratification of patients, identifying subsets with significantly different outcome (Figure 4). Compared with the 26% double-negative patients with IHP criteria, a substantially higher proportion of patients (79%) were both ΔSUVmaxPET0-2 >66% and ΔSUVmaxPET0-4 >70% (ie, considered as double PET negative using ΔSUVmax), and their outcome was particularly good, with a 4-year PFS and OS of 86% and 91%, respectively. Those double-PET-negative patients, based on ΔSUVmax, had similar 4-year PFS and OS with SIC or up-front ASCT (Table 4). Using interim PET ΔSUVmax reduction, we may speculate that the question of considering ASCT remains open for only about 21% patients who are not double PET negative. Unfortunately, numbers are too small in the subset of patients (9%) who had ΔSUVmaxPET0-2 ≤66% and ΔSUVmaxPET0-4 >70% to come to a conclusion on their need for up-front ASCT or salvage therapy (Table 4). In contrast, ΔSUVmaxPET0-4 ≤70% clearly identifies a subset of chemoresistant patients with a particularly poor outcome, even after salvage therapy, who require new treatment options. In our hands, both ΔSUVmaxPET0-4 and ΔSUVmaxPET0-2 predicted outcome, but ΔSUVmaxPET0-4 appeared more discriminant. Nevertheless, our results are in keeping with those of the prospective PETAL study, in which the 13% subset of patients with ΔSUVmaxPET0-2 ≤66% had a significantly poorer outcome and could not be salvaged by intensified chemotherapy.25,26
In conclusion, we cannot conclude on the superiority of R-ACVBP vs R-CHOP14 induction, primarily because of the choice of IHP criteria as part of response assessment and as a tool to drive consolidation. However, this study shows that a PET-driven consolidation strategy is feasible in a multicenter setting and allows us to adjust the consolidation treatment according to early metabolic response. The proportion of PET2−/PET4− patients was 26% using IHP criteria, and increased to 79% using ΔSUVmax. Our exploratory analysis suggests sequential ΔSUVmax assessment has better reproducibility than IHP and improves the portability of interim PET to better characterize the majority of patients eligible for SIC and select the presumably small subset of patients needing up-front ASCT consolidation and those refractory ones early needing alternative strategies. The final results of LNH2007-3B study laid the groundwork of the phase 3 randomized GAINED trial (NCT01659099), designed to definitely answer the AVCBP vs CHOP14 issue and prospectively validate the ΔSUVmax-driven consolidation in young patients with high-risk DLBCL.
Presented at the 2014 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Lymphoma Academic Research Organisation team, which was greatly committed in the management and the operational support of this study and all the investigators.
This study was funded by the French government (PHRC 2007), Amgen, and Roche.
Authorship
Contribution: R.-O.C. and F.M. designed the research; collected, analyzed, and interpreted data; and wrote the manuscript; and L.Y., C.T., E.B., P.F., A.D., S.T., J.G., M.A., C.F., N.M., R.D., M.M., A.B.-R., S.B., J.-F.E., J.-P.J., C.H., and H.T. collected, analyzed, and interpreted data and approved the manuscript
Conflict-of-interest disclosure: R.-O.C. received honoraria from Roche, Takeda, Gilead, and research funding from Roche and Gilead and had a consulting role for Roche, Takeda, Gilead, AbbVie, BMS, and Merck. L.Y. had a consulting role for Roche. C.T. had a consulting role for Roche, Gilead, and Janssen. E.B. had a consulting role for Roche. P.F. received honoraria and research funding and had a consulting role for Roche. A.D. received research funding from Chugai and had a consulting role for Roche. M.A. had an advisory role for GlaxoSmithKline. S.B. received honoraria from Genzyme. J.-F.E. received honoraria from Roche. C.H. had a consulting role for Roche and received research funding from Roche and Amgen. H.T. received honoraria from Celgene, Roche, Janssen; received research funding from Celgene; and had a consulting role for Takeda, Immunogen, Karyopharm therapeutics, Roche, and Gilead. F.M. had consulting role for Gilead and Roche. The remaining authors declare no competing financial interests.
Correspondence: R.-O. Casasnovas, Department of Hematology, Hôpital Le Bocage and INSERM UMR1231, Bd de Lattre de Tassigny, 21034 Dijon, France; e-mail: olivier.casasnovas@chu-dijon.fr.