In this issue of Blood, Casasnovas et al demonstrate that among diffuse large B-cell (DLBCL) patients with age-adjusted International Prognostic Index (aaIPI) 2-3, the dose-dense immunochemotherapy (IC) regimen rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone (R-ACVBP) was no better than rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP14). Using an interim positron emission tomography (PET)–adapted approach to consolidation therapy, they show that quantitative rather than qualitative PET assessment may better select some patients needing alternative treatments, including autologous stem cell transplantation (ASCT).1
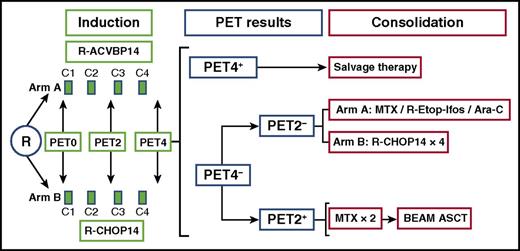
Trial schema. Ara-C, cytarabine; BEAM, carmustine, etoposide, cytarabine, melphalan; MTX, methotrexate; R, rituximab.
Trial schema. Ara-C, cytarabine; BEAM, carmustine, etoposide, cytarabine, melphalan; MTX, methotrexate; R, rituximab.
DLBCL patients with high-risk aaIPI 2-3 have a 5-year progression-free survival (PFS) of only 40% following R-CHOP.2 Intensified induction regimens incorporating etoposide (such as R-CHOEP14) have shown promising results, primarily in those with aaIPI 2, but have not been compared prospectively with R-CHOP14/21 and so have not yet been widely adopted.3 A number of randomized trials incorporating ASCT conducted without PET scans have failed to show an obvious benefit of early high-dose therapy in high-risk aaIPI patients such that upfront ASCT is no longer considered a standard option for all of these patients.4,5 An alternative, more individualized approach is one incorporating interim PET after either 2 or 4 cycles of IC, because this would allow the selection of high-risk patients for treatment intensification while sparing patients unnecessary toxicity in those who are destined to do well with IC alone.
In Casasnovas et al’s study, the first major objective assessed whether the dose-dense R-ACVBP regimen provided superior outcomes in comparison with R-CHOP14, and the second explored the value of interim PET to select patients for ASCT (see figure). The first objective was unconfirmed because PFS and overall survival (OS) of R-ACVBP were not significantly superior to those for R-CHOP14. Casasnovas et al argued that this was in part due to the higher frequency of salvage used in the R-CHOP arm and that perhaps PET4 positivity after R-ACVBP may be associated with a higher risk of refractoriness to salvage therapy.
The use of interim PET in therapeutic monitoring to adjust treatment remains a contentious area. PET was integrated into end-of-treatment lymphoma response assessment in 2007 as part of the International Harmonization Project (IHP). Efforts to standardize PET/computed tomography methods and interpretation meant that response assessment using IHP criteria was replaced by a more robust and reproducible 5-point scale (5-PS).6 Quantitative methods of PET assessment have also been recognized as objective tools for therapeutic monitoring, to reduce the number of false-positive interim PET scans, even when using the 5-PS.7
In this study, interim PET was undertaken to assess responses after 2 (PET2) and 4 (PET4) cycles of either of the 2 IC regimens, such that patients who were PET2−/PET4− completed induction IC; those who were PET2+/PET4− received ASCT; and those who were PET4+ were given investigator’s choice, including salvage chemotherapy ± ASCT. One quarter of the patients were PET2+/PET4− and mostly received ASCT. Because 26% of the patients were double negative (PET2−/PET4−), only a quarter commencing IC actually completed it. The major problem was that because the less discriminatory IHP criteria were used, around half the patients were PET4+ and so received investigator’s choice of salvage ± ASCT.
Accordingly, in an attempt to better address the confounding issue of a high false-positive rate and poor positive predictive value of interim PET, the authors undertook an exploratory analysis using this group’s previously determined quantitative changes in maximum PET positivity according to ΔSUVmax > 66% at PET2 and >70% at PET4.7,8 Notably, upfront ASCT in PET2+/PET4− patients who achieved a ΔSUVmaxPET0-2 > 66% did not improve disease control because PFS and OS were the same as those seen among PET2−/PET4− patients who received IC alone. That is, the patients who were double-PET-negative on the basis of ΔSUVmax reduction (the vast majority of PET2−/PET4−) exhibited very favorable outcomes irrespective of the consolidation they received, thereby suggesting that IC is sufficient for this group. For the group who were PET2+/PET4− and failed to reach PET0-2 ΔSUVmax > 66%, the numbers were too small to conclude that upfront ASCT or salvage therapy was needed. Notably, for this latter group of patients the recent large prospective randomized phase 3 PETAL study evaluating treatment intensification with a Burkitt Lymphoma regimen showed no improvement in outcomes.9
Second, from among the group of visually positive PET4 patients (approximately half of the patients with readable PET scans), only 21% remained positive using quantitative analysis with a ΔSUVmax ≤ 70%. That is, the use of ΔSUVmax reduced the PET4 positive rate by 79%. As a result, for this group of patients who were not PET2−/PET4− using the quantitative analysis, the role of ASCT also remains unknown from this study. Indeed, those visual PET4+ patients failing to achieve a ΔSUVmaxPET0-4 > 70% (a total of 13% of patients in this study) define a very high risk group of chemorefractory patients in whom any salvage therapy or ASCT failed to improve outcomes. This is somewhat supported by the results of a phase 2 PET-driven study in which patients remaining with a score of 5 on the 5-PS after 4 cycles of R-CHOP14 failed to be rescued by salvage chemotherapy plus ASCT.10 Therefore, this group of highly refractory patients requires novel treatment options other than high-dose therapies.
Taken together, these results lend further support to the concept that ΔSUVmax reduction is a better tool than is IHP to identify the smaller subset of patients for whom ASCT or other novel modalities could be tested. Some, but not all, of these unresolved issues will be further explored in the GAINED study (NCT01659099), in which a small percentage of patients (∼10% to 15%) are expected to reach ΔSUVmaxPET0-2 ≤ 66% but ΔSUVmaxPET0-4 > 70% and will be given upfront ASCT. Until then, it is difficult to advocate any change in treatment strategy based upon interim PET analysis, by IHP criteria, by the 5-PS, or by quantitative ΔSUVmax assessment.
Conflict-of-interest disclosure: The author declares no competing financial interests.