Key Points
dsDNA production peaks 14 days after HSCT, likely a result of IL-8–driven neutrophil recovery.
dsDNA production may serve as a mechanistic link between endothelial injury, TA-TMA, and GVHD.
Abstract
Transplant-associated thrombotic microangiopathy (TA-TMA) is a common and poorly recognized complication of hematopoietic stem cell transplantation (HSCT) associated with excessive complement activation, likely triggered by endothelial injury. An important missing piece is the link between endothelial injury and complement activation. We hypothesized that neutrophil extracellular traps (NETs) mechanistically link endothelial damage with complement activation and subsequent TA-TMA. Neutrophil activation releases granule proteins together with double-stranded DNA (dsDNA) to form extracellular fibers known as NETs. NETs have been shown to activate complement and can be assessed in humans by quantification of dsDNA in serum. We measured levels of dsDNA, as a surrogate for NETs in 103 consecutive pediatric allogeneic transplant recipients at day 0, +14, +30, +60, and +100. A spike in dsDNA production around day +14 during engraftment was associated with subsequent TA-TMA development. Peak dsDNA production around day +14 was associated with interleukin-8–driven neutrophil recovery. Increased dsDNA levels at days +30, +60, and +100 were also associated with increased mortality and gastrointestinal graft-versus-host disease (GVHD). NETs may serve as a mechanistic link between endothelial injury and complement activation. NET formation may be one mechanism contributing to the clinical overlap between GVHD and TA-TMA.
Introduction
Transplant-associated thrombotic microangiopathy (TA-TMA) is a common but poorly recognized complication following hematopoietic stem cell transplantation (HSCT). TA-TMA is likely the result of complement activation, triggered by endothelial injury from a combination of chemotherapy, infection, graft-versus-host disease (GVHD), or immune dysregulation.1 TA-TMA and GVHD have similar triggers and some manifestations in common and often occur together.1 Manifestations of TA-TMA include anemia, thrombocytopenia, elevated lactate dehydrogenase, presence of schistocytes, severe hypertension, and proteinuria.2,3 The adverse effects of TA-TMA are the result of platelet aggregation with microthrombi formation, leading to tissue ischemia and hypoxemia, which ultimately culminates in multiorgan injury, including kidney, gastrointestinal, pulmonary, cardiac, and/or neurologic dysfunction.4 An important missing piece in our understanding of the pathophysiology of TA-TMA is the link between endothelial injury and complement activation.
Neutrophils are attracted to sites of inflammation and injury by a variety of chemotactic factors, including interleukin-8 (IL-8), leading to neutrophil activation.5,6 Neutrophil activation releases granule proteins, such as a neutrophil elastase, cathepsin G, myeloperoxidase, lactoferrin, and gelatinase together with chromatin, to form extracellular fibers known as neutrophil extracellular traps (NETs).7 NETs serve as a natural defense mechanism with neutrophil-derived double-stranded DNA (dsDNA) acting as the structure for deposition of granule proteins, which can then intercept and kill bacteria extracellularly.7 In vitro, IL-8 is a powerful stimulant of NET production.7 NETs have been associated with many different conditions, including autoimmune disorders, sepsis, pulmonary disease, HSCT, thrombosis, and thrombotic microangiopathies.8-18
NETs activate complement via both alternative and nonalternative pathways.19 Secretion of complement factor P from activated neutrophils is deposited on NETs, which then activates complement through the alternative pathway, leading to formation of the membrane attack complex (C5b-9), which is commonly elevated in TA-TMA.19
The objective of this study was to explore the mechanistic link between endothelial injury and thrombotic microangiopathy after HSCT. We hypothesized that elevated levels of NETs after HSCT link endothelial injury with complement activation and higher levels of NETs would be associated with increased risk of TA-TMA. We tested this hypothesis with an analysis of longitudinal changes in levels of circulating dsDNA (a known surrogate for NETs) and show marked elevation of dsDNA around the time of neutrophil engraftment. Moreover, higher levels of dsDNA were associated with increased incidence of TA-TMA and GVHD. Elevated levels of dsDNA resolved after treatment of TA-TMA with complement blockade.
Methods
Patients
Sera from patients consented to Cincinnati Children’s Hospital Medical Center (CCHMC) Hematopoietic Stem Cell Transplant Biorepository were obtained through a CCHMC Institutional Review Board–approved study. Two separate cohorts were studied. The longitudinal cohort consisted of 103 consecutive pediatric allogeneic HSCT recipients studied at days 0, +14, +30, +60, and +100 following HSCT. A separate and nonoverlapping second cohort included 18 pediatric HSCT patients with severe TA-TMA who we studied at 4 time points: (1) prior to initiation of therapy with complement blockade using eculizumab; (2) 2 weeks into treatment with eculizumab; (3) 4 weeks into treatment with eculizumab; and (4) following discontinuation of therapy because of resolution of TA-TMA. All patients were prospectively monitored for TA-TMA as part of our routine clinical practice. TA-TMA was diagnosed based on either the presence of microangiopathy diagnosed on tissue biopsy or the presence of 5 of the following 7 laboratory and clinical markers: elevated lactate dehydrogenase; proteinuria with a random urine protein/random urine creatinine ratio >2 mg/mg; hypertension; de novo thrombocytopenia; de novo anemia; presence of schistocytes; and evidence of terminal complement activation with an elevated serum concentration of sC5b-9.2,3 Severe TA-TMA was diagnosed if the patient had both proteinuria and elevated sC5b-9 because we have shown previously that these are high-risk features associated with reduced survival.2,3 Only patients with severe TA-TMA were treated with the complement blocking agent eculizumab.
Measurement of circulating dsDNA
NETs are the major source of circulating dsDNA in serum, and circulating dsDNA has previously been used as a surrogate for NET levels.13,20 Serum dsDNA was quantified using the Quant-iT PicoGreen dsDNA Reagent and Kit (Cat. no. P7589; Molecular Probes, Eugene, OR) according to the manufacturer’s instructions, with the exception that 50 µL of patient serum was added to 50 µL of the PicoGreen reagent solution for a total reaction volume of 100 μL in a 96-well plate. Samples were excited at 485 nm, and fluorescence intensity was measured at 520 nm on a fluorometer (SpectraMax M3; Molecular Devices, Sunnyvale, CA).
Serum IL-8 level quantification
Serum IL-8 levels were quantified in sera from patients after HSCT using an enzyme-linked immunosorbent assay (Cat. no. D8000C; R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Neutrophil CXCR1 and CXCR2 flow cytometry
Neutrophils from fresh heparinized blood were stained against Pacific blue–conjugated CD11b, fluorescein isothiocyanate–conjugated CXCR1, phycoerythrin-conjugated CXCR2, and peridinin chlorophyll protein–conjugated CD66b (BD Biosciences, San Jose, CA) followed by red cell lysis with FACS Lyse (BD Biosciences). Samples were washed twice, acquired on a BD FACS Canto II (BD Biosciences), and analyzed using FCS Express version 5 (De Novo Software, Glendale, CA). Time points of samples were obtained from HSCT recipients during the period of neutrophil engraftment (N = 6) and around day +100 after bone marrow transplant (N = 4). The percentage of CD11b(+) neutrophils with dual expression of CXCR1 and CXCR2 was compared between time points of engraftment, day +100, and healthy controls.
Statistical analysis
Continuous variables were compared between 2 groups using the Wilcoxon rank sum test. Associations between 2 continuous variables were assessed using linear regression and are summarized by the R2 value. TA-TMA cumulative incidence curves were estimated using the Kaplan-Meier method; comparisons between groups were assessed using Gray’s method of competing risks, where death was treated as a competing event. Longitudinal data were analyzed for overall survival using proportional hazards regression. Longitudinal data for TA-TMA and GVHD outcomes were analyzed using competing risks regression, where death was treated as a competing event for all outcomes, and additionally for GVHD outcomes, relapse was treated as a competing event.
Results
Longitudinal cohort: patient demographics
Patient demographics for the longitudinal cohort are shown in Table 1. The median age was 9.2 years (range 5 months to 32 years); two-thirds were male and one-third were female patients, and patients were predominantly white. The majority of transplants were performed for nonmalignant conditions (77% nonmalignant and 23% malignant), in keeping with our institutional demographics. Approximately half of the patients received reduced intensity regimens, and most received a calcineurin inhibitor-based GVHD prophylaxis regimen.
Longitudinal cohort: patient demographics
| . | Allogeneic HSCT patients (N = 103) . |
|---|---|
| Age | |
| Median | 9.2 y |
| Range | 5 mo to 32 y |
| Sex | |
| Male | 68 |
| Female | 35 |
| Race | |
| White | 93 |
| African American | 8 |
| Asian | 2 |
| Diagnosis | |
| Immune deficiency | 32 |
| Bone marrow failure | 28 |
| Malignancy | 24 |
| Hemoglobinopathy | 14 |
| Metabolic | 5 |
| Stem cell source | |
| Bone marrow | 84 |
| Peripheral blood | 17 |
| Umbilical cord blood | 2 |
| Donor type | |
| Related | 37 |
| Unrelated | 66 |
| Match | |
| Fully matched | 77 |
| Mismatched | 26 |
| Conditioning regimen | |
| Myeloablative | 53 |
| Busulfan-based | 41 |
| Cyclophosphamide/total body irradiation | 8 |
| Cyclophosphamide/antithymocyte globulin | 4 |
| Reduced intensity | 50 |
| Campath/fludarabine/melphalan | 38 |
| Busulfan/Fludarabine | 6 |
| Other* | 6 |
| GVHD prophylaxis | |
| Calcineurin inhibitor-based | 90 |
| T-cell depletion | 12 |
| None | 1 |
| . | Allogeneic HSCT patients (N = 103) . |
|---|---|
| Age | |
| Median | 9.2 y |
| Range | 5 mo to 32 y |
| Sex | |
| Male | 68 |
| Female | 35 |
| Race | |
| White | 93 |
| African American | 8 |
| Asian | 2 |
| Diagnosis | |
| Immune deficiency | 32 |
| Bone marrow failure | 28 |
| Malignancy | 24 |
| Hemoglobinopathy | 14 |
| Metabolic | 5 |
| Stem cell source | |
| Bone marrow | 84 |
| Peripheral blood | 17 |
| Umbilical cord blood | 2 |
| Donor type | |
| Related | 37 |
| Unrelated | 66 |
| Match | |
| Fully matched | 77 |
| Mismatched | 26 |
| Conditioning regimen | |
| Myeloablative | 53 |
| Busulfan-based | 41 |
| Cyclophosphamide/total body irradiation | 8 |
| Cyclophosphamide/antithymocyte globulin | 4 |
| Reduced intensity | 50 |
| Campath/fludarabine/melphalan | 38 |
| Busulfan/Fludarabine | 6 |
| Other* | 6 |
| GVHD prophylaxis | |
| Calcineurin inhibitor-based | 90 |
| T-cell depletion | 12 |
| None | 1 |
Other: fludarabine/melphalan, 1; cyclophosphamide/antithymocyte globulin, 4; no conditioning, 1.
Patient demographics were analyzed to determine if patient characteristics influenced day 0 dsDNA levels. Age, race, sex, prior therapy, and conditioning regimen had no effect on day 0 dsDNA levels. Disease category, however, did have an effect on day 0 levels, driven by increased levels in the hemoglobinopathy group (P = .03). When this group was further broken down into sickle cell disease and β-thalassemia, 2 patients with sickle cell disease had increased day 0 dsDNA levels, likely related to preexisting endothelial injury due to sickled red cells, which has previously been described in the literature.21
Longitudinal analysis of dsDNA production during the first 100 days after HSCT
Our first goal was to describe changes in dsDNA production during transplant and recovery and to identify associations between dsDNA production and key clinical events, in particular, TA-TMA and GVHD, both of which are associated with endothelial injury. We measured levels of circulating dsDNA at time points day 0, +14, +30, +60, and +100, and median levels ± interquartile range at each time point are summarized in Figure 1A. The day +14 peak was higher and associated with a larger distribution in the groups of patients with only TMA or TMA and GVHD compared with the groups who did not have TMA (Figure 1B-E). Remarkably, elevated dsDNA levels as early as day +14 were associated with increased risk of future TA-TMA development. Recipients with a dsDNA level greater than the median for the cohort had a significantly higher risk for development of TA-TMA compared with those with a level less than the median (Figure 1F; P = .01).
Longitudinal analysis of circulating dsDNA at days 0, +14, +30, +60, and +100. (A) Levels of dsDNA were elevated at day +14 following HSCT for the entire cohort of patients. Median levels of dsDNA for each time point are included in the table below the figure, including range and interquartile range. (B-E) When broken down by development of GVHD and TA-TMA, the day +14 spike in levels of dsDNA persisted for each group. (F) Day +14 levels above the median for the cohort were associated with increased risk of future development of TA-TMA compared with levels less than the median (P = .01).
Longitudinal analysis of circulating dsDNA at days 0, +14, +30, +60, and +100. (A) Levels of dsDNA were elevated at day +14 following HSCT for the entire cohort of patients. Median levels of dsDNA for each time point are included in the table below the figure, including range and interquartile range. (B-E) When broken down by development of GVHD and TA-TMA, the day +14 spike in levels of dsDNA persisted for each group. (F) Day +14 levels above the median for the cohort were associated with increased risk of future development of TA-TMA compared with levels less than the median (P = .01).
Intrigued by the day +14 spike in dsDNA, around the time of neutrophil recovery after HSCT, we looked to see if dsDNA levels correlated with neutrophil count on day +14. In our cohort, the median time to neutrophil engraftment was 12 days after HSCT. We saw a strong correlation between absolute neutrophil count (ANC) on day of sample collection and corresponding level of dsDNA (R2 = 0.24, P < .0001; Figure 2A). Interestingly, we saw no correlation between dsDNA level and ANC when the same analysis was performed at day +100 (R2 = 0.008, P = .36; Figure 2B), leading us to believe that there is something qualitatively different between the neutrophils at day +14 compared with day +100 with respect to propensity to form NETs. We hypothesized that increased levels of dsDNA at day +14 are due to increased levels of IL-8, which is a known stimulant of NET production.
IL-8 and dsDNA levels associated with engraftment. (A) Day +14 dsDNA level is positively correlated with day +14 ANC (R2 = 0.24, P < .0001). (B) Day +100 dsDNA level did not correlate with day +100 ANC (R2 = 0.008, P = .3618). (C) IL-8 level at day +14 is positively correlated with time to engraftment (days) (R2 = 0.31, P < .0001). (D) IL-8 level at day +14 is inversely correlated with ANC at day +14 (R2 = 0.30, P < .0001). (E) IL-8 level at day +14 did not correlate with dsDNA level at day +14 (R2 = 0.0072, P = .40). (F) IL-8 levels at day +14 were significantly higher in patients who had not yet engrafted compared with patients who were engrafted (P < .0001). (G) dsDNA levels at day +14 were significantly higher in patients who were engrafted compared with patients who were not yet engrafted.
IL-8 and dsDNA levels associated with engraftment. (A) Day +14 dsDNA level is positively correlated with day +14 ANC (R2 = 0.24, P < .0001). (B) Day +100 dsDNA level did not correlate with day +100 ANC (R2 = 0.008, P = .3618). (C) IL-8 level at day +14 is positively correlated with time to engraftment (days) (R2 = 0.31, P < .0001). (D) IL-8 level at day +14 is inversely correlated with ANC at day +14 (R2 = 0.30, P < .0001). (E) IL-8 level at day +14 did not correlate with dsDNA level at day +14 (R2 = 0.0072, P = .40). (F) IL-8 levels at day +14 were significantly higher in patients who had not yet engrafted compared with patients who were engrafted (P < .0001). (G) dsDNA levels at day +14 were significantly higher in patients who were engrafted compared with patients who were not yet engrafted.
We measured IL-8 levels at day 14 and found that IL-8 levels were correlated with time to engraftment, with a longer time to engraftment associated with higher IL-8 levels (R2 = 0.31, P < .0001; Figure 2C). Moreover, we found an inverse correlation between IL-8 levels and ANC (higher IL-8 levels associated with lower ANCs, R2 = 0.3, P < .0001; Figure 2D). We compared levels of IL-8 and circulating dsDNA at day +14, but contrary to our hypothesis, we found no association between the two (R2 = 0.0072, P = .40; Figure 2E). We did, however, find that patients who had not yet engrafted had significantly higher levels of IL-8 when compared with those who were engrafted (P < .0001; Figure 2F), and in those patients who were engrafted, there were also significantly higher levels of dsDNA when compared with those who were not yet engrafted (P = .0012; Figure 2G).
Our finding that higher levels of IL-8 correlated with lower ANC and ANC correlated with dsDNA led us to expect to find some correlation between IL-8 level and dsDNA. We hypothesized that higher neutrophil counts at the time of engraftment may be associated with increased binding and internalization of IL-8, leading to a reduction in its level of circulation. If this were the case, IL-8 binding to the neutrophils would stimulate NET production but also reduce the circulating IL-8 level, making a direct correlation hard to detect. To test this hypothesis, we used flow cytometry to assess the surface expression of the IL-8 receptors, CXCR1 and CXCR2, on neutrophils collected at the time of engraftment and at day +100 (Figure 3) in an additional cohort of patients separate from the longitudinal cohort. These data show notable differences between neutrophils studied during engraftment and at day +100. Neutrophils during engraftment showed a significant reduction in CXCR1 and CXCR2 surface expression compared with neutrophils 100 days after transplant (Figure 3B; P = .0095), and a similar reduction was seen in CXCR1 and CXCR2 surface expression compared with healthy controls (Figure 3B; P = .0043). There was no difference in surface expression of CXCR1 and CXCR2 between day +100 samples and healthy controls (Figure 3B; P = .90). This finding supports our hypothesis that IL-8 is bound and the receptors internalized at day +14, supporting a role for IL-8 in the dsDNA peak seen at day +14 after HSCT.
Decreased neutrophil surface expression of CXCR1 and CXCR2 during engraftment. (A) Representative flow plots from a healthy control, a HSCT recipient during engraftment, and an HSCT recipient around day +100. Cells were gated as CD11b(+) neutrophils. Flow plots are shown with CXCR1 on the y-axis and CXCR2 on the x-axis. Coexpression of CXCR1 and CXCR2 is similar between the healthy control sample and HSCT recipient at day +100, but surface expression of both is decreased during engraftment. (B) The % of CD11b-positive neutrophils with coexpression of CXCR1 and CXCR2. CXCR1 and CXCR2 surface expression were compared during engraftment (N = 6), day +100 (N = 4), and in healthy controls (N = 5). There is no difference seen between the surface expression of CXCR1 and CXCR2 in HSCT recipients at day +100 and healthy controls, but the surface expression of both is significantly lower in HSCT recipients during engraftment when compared with HSCT recipients at day +100 and healthy controls (P = .0095 and P = .0043, respectively).
Decreased neutrophil surface expression of CXCR1 and CXCR2 during engraftment. (A) Representative flow plots from a healthy control, a HSCT recipient during engraftment, and an HSCT recipient around day +100. Cells were gated as CD11b(+) neutrophils. Flow plots are shown with CXCR1 on the y-axis and CXCR2 on the x-axis. Coexpression of CXCR1 and CXCR2 is similar between the healthy control sample and HSCT recipient at day +100, but surface expression of both is decreased during engraftment. (B) The % of CD11b-positive neutrophils with coexpression of CXCR1 and CXCR2. CXCR1 and CXCR2 surface expression were compared during engraftment (N = 6), day +100 (N = 4), and in healthy controls (N = 5). There is no difference seen between the surface expression of CXCR1 and CXCR2 in HSCT recipients at day +100 and healthy controls, but the surface expression of both is significantly lower in HSCT recipients during engraftment when compared with HSCT recipients at day +100 and healthy controls (P = .0095 and P = .0043, respectively).
HSCT outcomes associated with NETs
In addition to the association between day +14 dsDNA levels and TA-TMA described above (Figure 1F), we further examined the risk of TA-TMA, GVHD, and mortality after HSCT in association with dsDNA levels at days 0, +14, +30, +60, and +100. Elevated dsDNA levels at days +30, +60, and +100 were associated with increased risk of mortality (N = 16), TA-TMA (N = 37), overall GVHD (N = 32), grade 3 to 4 GVHD (N = 9), and gastrointestinal GVHD at any time (N = 12) (Table 2). We found no association of dsDNA levels with chronic GVHD, but it should be noted that only 4 patients in this cohort developed chronic GVHD, limiting power significantly. We also looked to see if there was an association between dsDNA levels and bloodstream infections between day 0 and engraftment, for which there was no association seen either (P = .27).
Longitudinal association of dsDNA on patient outcomes
| Outcome . | Day 0 . | Day 14 . | Day 30 . | Day 60 . | Day 100 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR Log (NETs day 0) . | P . | OR Log (NETs day 14) . | P . | OR Log (NETs day 30) . | P . | OR Log (NETs day 60) . | P . | OR Log (NETs day 100) . | P . | |
| Mortality | 0.4 | .27 | 0.8 | .64 | 3.7 | .04 | 7 | <.0001 | 8.6 | .0002 |
| TA-TMA | 1.3 | .55 | 3.3 | .0013 | 3.2 | .002 | 1.9 | .02 | 5.5 | <.001 |
| Any GVHD | 1 | .93 | 0.8 | .53 | 2.2 | .08 | 3.1 | <.0001 | 3.1 | .013 |
| Grade 3-4 GVHD | 1.5 | .54 | 0.8 | .7 | 3.5 | .03 | 2.6 | .01 | 2.2 | .24 |
| Gut GVHD | 1 | .98 | 1.3 | .53 | 4.4 | .02 | 3.4 | .002 | 6.2 | .006 |
| Outcome . | Day 0 . | Day 14 . | Day 30 . | Day 60 . | Day 100 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR Log (NETs day 0) . | P . | OR Log (NETs day 14) . | P . | OR Log (NETs day 30) . | P . | OR Log (NETs day 60) . | P . | OR Log (NETs day 100) . | P . | |
| Mortality | 0.4 | .27 | 0.8 | .64 | 3.7 | .04 | 7 | <.0001 | 8.6 | .0002 |
| TA-TMA | 1.3 | .55 | 3.3 | .0013 | 3.2 | .002 | 1.9 | .02 | 5.5 | <.001 |
| Any GVHD | 1 | .93 | 0.8 | .53 | 2.2 | .08 | 3.1 | <.0001 | 3.1 | .013 |
| Grade 3-4 GVHD | 1.5 | .54 | 0.8 | .7 | 3.5 | .03 | 2.6 | .01 | 2.2 | .24 |
| Gut GVHD | 1 | .98 | 1.3 | .53 | 4.4 | .02 | 3.4 | .002 | 6.2 | .006 |
Bold indicates statistically significant values.
Levels of circulating dsDNA and response to therapy for TA-TMA
After identifying the association between circulating dsDNA and TA-TMA, we proceeded to determine whether the level of dsDNA would decrease with resolution of TA-TMA. We addressed this question by measuring dsDNA levels in 18 pediatric patients with severe TA-TMA prior to treatment of TA-TMA, during treatment, and after resolution of TA-TMA when complement-blocking therapy had been discontinued. Serum dsDNA levels 2 and 4 weeks into complement blockade therapy were unchanged from levels at diagnosis of TA-TMA (Figure 4A). Circulating dsDNA levels were significantly decreased after resolution of TA-TMA (defined as termination of complement blockade due to clinical resolution) as compared with levels 4 weeks into treatment and prior to therapy (Figure 4A).
Level of circulating dsDNA and response to therapy for TA-TMA. (A) Levels of dsDNA were significantly elevated 4 weeks into therapy compared with prior to therapy (P = .006), and 2 weeks into therapy in the cohort of patients with TA-TMA (P = .0006). Following resolution of TA-TMA and discontinuation of therapy, levels dropped significantly compared with 4 weeks into therapy (P < .0001) and prior to therapy (P = .04). (B) In the cohort of patients with TA-TMA (N = 18), those who also had GVHD (N = 7) prior to the initiation of therapy had significantly higher levels of dsDNA compared with those without GVHD (N = 11). (C) In the same cohort of patients with TA-TMA (N = 18), those with GVHD following resolution of TA-TMA and discontinuation of therapy (N = 6) had significantly higher levels of dsDNA compared with those without GVHD (N = 12). Tx, therapy.
Level of circulating dsDNA and response to therapy for TA-TMA. (A) Levels of dsDNA were significantly elevated 4 weeks into therapy compared with prior to therapy (P = .006), and 2 weeks into therapy in the cohort of patients with TA-TMA (P = .0006). Following resolution of TA-TMA and discontinuation of therapy, levels dropped significantly compared with 4 weeks into therapy (P < .0001) and prior to therapy (P = .04). (B) In the cohort of patients with TA-TMA (N = 18), those who also had GVHD (N = 7) prior to the initiation of therapy had significantly higher levels of dsDNA compared with those without GVHD (N = 11). (C) In the same cohort of patients with TA-TMA (N = 18), those with GVHD following resolution of TA-TMA and discontinuation of therapy (N = 6) had significantly higher levels of dsDNA compared with those without GVHD (N = 12). Tx, therapy.
TA-TMA and acute GVHD are both associated with endothelial injury and commonly occur together. We proceeded to determine if dsDNA levels would decrease in the patients with treated TA-TMA who also had acute GVHD. To address this question, we examined dsDNA levels in our TA-TMA cohort (N = 18) according to presence or absence of concomitant acute GVHD. Levels of dsDNA were higher in the patients who had both acute GVHD and TA-TMA (N = 7) compared with those with TA-TMA alone (N = 11) prior to the initiation of complement blockade (Figure 4B). Moreover, dsDNA levels remained elevated following resolution of TA-TMA in patients who also had acute GVHD (N = 6), compared with the patients with TA-TMA alone (N = 12) (Figure 4C).
Discussion
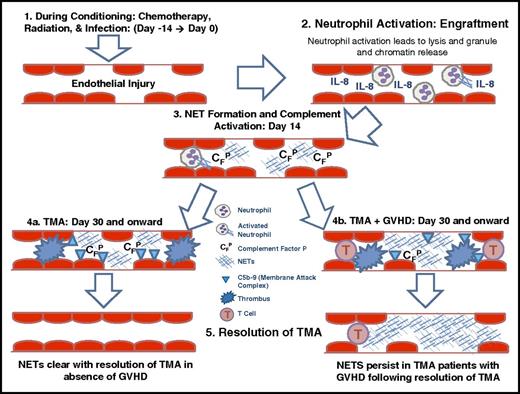
The goal of our study was to investigate the mechanistic link between endothelial injury and complement activation. Our study results demonstrate a strong association between the presence of higher levels of circulating dsDNA following stem cell transplantation and transplant outcomes, including overall survival, TA-TMA, and acute GVHD. We report a decline in circulating dsDNA in children with TA-TMA following therapeutic intervention with complement blockade. Collectively, our data support a possible role for circulating dsDNA, likely largely derived from NETs, as a possible important link between endothelial injury and complement activation. We offer a possible model for the mechanistic link between endothelial injury and TA-TMA (Figure 5). We propose that chemotherapy, radiation, and infections lead to endothelial injury during the early stages of HSCT. Injured endothelium (and perhaps epithelium) releases IL-8, causing neutrophil activation and release of NETs, followed by complement activation with deposition of complement factor P, C5b-9, and microthrombi formation. Treatment with complement blockade leads to clearance of NETs and resolution of TMA in patients without acute GVHD, but in patients with acute GVHD, NETs remain secondary to persistence of endothelial injury.
Proposed mechanistic link between endothelial injury, complement activation, and TA-TMA and GVHD. Based on our results, our proposed mechanism is as follows: 1. Chemotherapy, radiation, and infection lead to endothelial injury during early stages of HSCT. 2. Endothelial injury releases IL-8, causing neutrophil activation with release of NETs. 3. NET formation leads to complement activation with deposition of complement factor P and C5b-9 deposition. 4. Complement activation results in microthrombi formation. 5. Treatment with complement blockade leads to clearance of NETs and resolution of TA-TMA, but in patients with GVHD, NETs remain secondary to persistent endothelial injury. CFP, complement factor P.
Proposed mechanistic link between endothelial injury, complement activation, and TA-TMA and GVHD. Based on our results, our proposed mechanism is as follows: 1. Chemotherapy, radiation, and infection lead to endothelial injury during early stages of HSCT. 2. Endothelial injury releases IL-8, causing neutrophil activation with release of NETs. 3. NET formation leads to complement activation with deposition of complement factor P and C5b-9 deposition. 4. Complement activation results in microthrombi formation. 5. Treatment with complement blockade leads to clearance of NETs and resolution of TA-TMA, but in patients with GVHD, NETs remain secondary to persistent endothelial injury. CFP, complement factor P.
Our longitudinal study revealed for the first time an increased level of dsDNA around the time of engraftment, and this day +14 peak seems to be associated with increased risk of later TA-TMA. Increased levels of dsDNA are strongly associated with TA-TMA and perhaps to a lesser extent with acute GVHD following HSCT, suggesting that early complement activation may initiate later adverse clinical outcomes. In previous work, we have shown that TA-TMA and GVHD commonly occur together, and we believe this overlap is due to the important role played by endothelial injury in both conditions. In recent work, we have shown a close association between elevation of the endothelial injury marker ST2 with TA-TMA, which previously has been considered a biomarker of GVHD, supporting the concept of endothelial injury contributing to both conditions.22
Our data support a role for IL-8 in stimulating release of dsDNA at day +14. This finding supports a potentially important role for IL-8 in neutrophil recovery after HSCT that has not been previously investigated. Endothelial cells are an important source of IL-8, and high levels of IL-8 in the early days after HSCT may be a reflection of endothelial injury.
We found that levels of dsDNA returned to normal after successful treatment of TA-TMA, supporting a mechanistic role for dsDNA in the initiation of TA-TMA. Interestingly, children who had GVHD in addition to TA-TMA did not reduce their dsDNA to normal, suggesting ongoing NET production in this clinical setting despite adequate TA-TMA therapy. We would predict that these children are vulnerable to recurrence of TA-TMA unless both the GVHD and the ongoing endothelial injury are adequately addressed and treated. Outside of the setting of HSCT, NETs are known to expose intracellular antigens, causing autoimmune disorders like psoriasis and systemic lupus erythematosus and could plausibly expose donor alloantigens, causing and sustaining GVHD.23-26 Furthermore, NETS release oxidants and could perpetuate or worsen preexisting endothelial injury in ongoing GVHD.
Our study has strengths and weaknesses. An important strength of our study is that we included multiple measurements of dsDNA in a large cohort of consecutive patients, allowing us to define the kinetics of dsDNA production over the course of HSCT. We used dsDNA as a surrogate for direct measurement of NETs, as reported by others; however, it is possible that the use of this surrogate marker for NETS might introduce inaccuracy, particularly in the setting of extensive tissue injury. In addition, complement activation was not directly measured in our study; however, prior studies done at our institution have shown the association of TA-TMA and complement activation.1,4,27-29 Our patient series was pediatric with a majority of children transplanted for nonmalignant diseases, and different results might be seen in an adult cohort with a majority of patients receiving HSCT for leukemia.
Circulating dsDNA measurement is a quick and easy assay, which could easily be optimized for rapid clinical diagnosis of endothelial injury to aid in the diagnosis of TA-TMA or GVHD. dsDNA should be further investigated and studied in a prospective clinical trial in conjunction with other TA-TMA and acute GVHD biomarkers to further elucidate their prognostic value. Our data offer a mechanistic insight into complement activation and tissue injury after HSCT that might allow development of targeted therapies that would reduce injury and improve survival after HSCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the clinical staff, patients, and families at CCHMC.
Authorship
Contribution: N.J.G. designed and performed experiments, collected data, and wrote the manuscript; P.K., N.L., and D.T.L. designed and performed experiments and edited and approved the manuscript; S.J. and M.N.A. reviewed and interpreted study data and edited and approved the manuscript; A.L. performed statistical analysis and edited and approved the manuscript; A.W., K.E.L., and B.L. collected, annotated, and processed HSCT Biorepository samples; and S.M.D. designed the study, interpreted results, and wrote the manuscript with N.J.G.
Conflict-of-interest disclosure: S.J. and S.M.D. have a US Provisional patent application for methods and compositions related to TA-TMA. The remaining authors declare no competing financial interests.
Correspondence: Stella M. Davies, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 11027, Cincinnati, OH 45229; e-mail: stella.davies@cchmc.org.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal