To the editor:
Human T-cell lymphotropic virus type I (HTLV-I) is a pathogenic retrovirus associated with adult T-cell leukemia/lymphoma (ATLL) and HTLV-I–associated myelopathy.1,2 HTLV-I infection can be transmitted by infected lymphocytes during breastfeeding, sexual contact, needle sharing, and unscreened blood transfusion. Moreover, HTLV-I–associated diseases caused by donor-to-recipient transmission of HTLV-I during solid organ transplantation have been reported.3-6
We describe a case of a 52-year-old Japanese male with acute-type ATLL who developed progressive proliferation of donor-derived HTLV-I–positive T cells after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Written informed consent was obtained from the patient. In our case, the donor was HTLV-I negative and had neither abnormal peripheral blood lymphocytes nor lymphadenopathy. Peripheral blood lymphocyte number and serum immunoglobulins were normal in the donor. Because the tumor cells of the patient were resistant to cytotoxic chemotherapies even in the multidrug setting, he underwent unmanipulated HLA-haploidentical peripheral blood stem cell transplantation (haplo-PBSCT) from his HTLV-I–negative daughter with 3 antigen mismatches in the graft-versus-host direction (Table 1). Mogamulizumab was not used in this case. At the time of transplantation, ATLL cells were still detected in his peripheral blood, and the HTLV-I proviral load by real-time quantitative polymerase chain reaction was 61.8 copies/1000 peripheral blood mononuclear cells (PBMCs). The conditioning regimen consisted of fludarabine (25 mg/m2, days −8 to day −4), melphalan (80 mg/m2, day −3), rabbit antithymocyte globulin (thymoglobulin: 2.5 mg/kg, day −2), and 8 Gy of total body irradiation. Tacrolimus and mycophenolate mofetil (1000 mg/day for 35 days) were used for graft-versus-host disease (GVHD) prophylaxis. After transplantation, complete remission was achieved. Full donor chimera was confirmed by using XY-fluorescence in situ hybridization (XY-FISH). HTLV-I proviral load was undetectable 1 and 5 months after haplo-PBSCT. In contrast, 3 months after transplantation, the Epstein-Barr viral load in his peripheral blood was slightly increased to 430 copies/1 × 106 white blood cells and shortly thereafter decreased to undetectable levels. Eight months after transplantation, the number of CD4+ T cells, CD8+ T cells, and CD4+CD25+CD127–/low regulatory T cells (Tregs) in his peripheral blood were 130/μL, 533/μL, and 52/μL, respectively. Methylprednisolone at a dose of 1 mg/kg per day was used for 2 weeks to treat acute GVHD. Moreover, the patient was treated with low doses of tacrolimus and steroids for 3 years because of chronic GVHD.
HLA status of the patient and donor
| . | Sex . | Relationship . | HTLV-I . | HLA-A . | HLA-B . | HLA-DR . |
|---|---|---|---|---|---|---|
| Recipient | Male | Positive | A2/A26 | B54/B35 | DR4/DR15 | |
| Donor | Female | Daughter | Negative | A2/A2 | B54/B61 | DR4/DR4 |
| . | Sex . | Relationship . | HTLV-I . | HLA-A . | HLA-B . | HLA-DR . |
|---|---|---|---|---|---|---|
| Recipient | Male | Positive | A2/A26 | B54/B35 | DR4/DR15 | |
| Donor | Female | Daughter | Negative | A2/A2 | B54/B61 | DR4/DR4 |
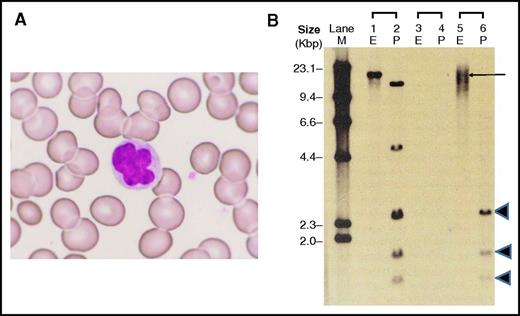
Sixteen months after haplo-PBSCT, abnormal lymphocytes gradually increased in his peripheral blood to a leukocyte count of 7.6 × 109/L with abnormal lymphocytes of 8%. Elevated levels of HTLV-I proviral load (320.6 copies/1000 PBMCs) and serum soluble interlekin-2 receptor (2691 U/mL) were observed. Three years after haplo-PBSCT, the patient’s leukocyte count further increased to 18.5 × 109/L with abnormal lymphocytes of 56%. HTLV-I proviral load and serum soluble interlekin-2 receptor levels also increased to 680.4 copies/1000 PBMCs and 5957 U/mL, respectively. The number of circulating natural killer (CD3–, CD56+) cells was 59/μL. He presented with colitis, but did not show skin lesions or lymphadenopathy. The abnormal lymphocytes were morphologically medium-sized cells with nuclear abnormalities, such as lobulation and notching (Figure 1A), and were positive for CD3, CD4, CD25, and CCR4 and negative for CD8. The absolute number of circulating T cells positive for CD3 was 7.2 × 109/L and 6.7 × 109/L for CD4. CD25 was expressed on 86.4% of CD4-positive T cells. The findings were consistent with ATLL. However, CD7 was still expressed on 76.8% of the abnormal lymphocytes. In XY-FISH analysis on the abnormal lymphocytes, all tested cells showed the typical pattern of female donor cells lacking the Y chromosome. G-band karyotype of the abnormal lymphocytes was 46,XX without additional chromosomal abnormality. Bone marrow examination showed no infiltration of abnormal lymphocytes. Karyotype of bone marrow cells was 46,XX in all analyzed cells (20 metaphases). Furthermore, HTLV-I integration analysis with Southern blotting (Figure 1B) exhibited a dense smear pattern with a faint discrete band by EcoRI digestion (lane 5) and 3 internal bands by PstI digestion (lane 6). No clonal rearrangement of T-cell receptors was detected. Repertoire analysis of T-cell receptor β-variable region complementarity determining region 3 area also failed to demonstrate monoclonal expansion of T cells. In high-sensitive western blotting using an enhanced chemiluminescence ImmunoStar LD detection system (WAKO, Osaka, Japan), the abnormal lymphocytes showed only a weak expression of the HTLV-I viral protein Tax compared with those of primary ATLL cells and MT-4 cells.
Donor-derived abnormal lymphocytes after haplo-PBSCT. (A) Three years after haplo-PBSCT, donor-derived lymphocytes with nuclear abnormalities, such as lobulation, were observed in peripheral blood of the patient (recipient); Wright-Giemsa staining, original magnification ×400. (B) HTLV-I Southern blot analysis of donor-derived abnormal lymphocytes, digested by EcoRI (E) and PstI (P). Lanes 1 and 2, positive control; lanes 3 and 4, negative control; lanes 5 and 6, the patient’s (recipient’s) sample. M, size marker.
Donor-derived abnormal lymphocytes after haplo-PBSCT. (A) Three years after haplo-PBSCT, donor-derived lymphocytes with nuclear abnormalities, such as lobulation, were observed in peripheral blood of the patient (recipient); Wright-Giemsa staining, original magnification ×400. (B) HTLV-I Southern blot analysis of donor-derived abnormal lymphocytes, digested by EcoRI (E) and PstI (P). Lanes 1 and 2, positive control; lanes 3 and 4, negative control; lanes 5 and 6, the patient’s (recipient’s) sample. M, size marker.
Thus, our results showed progressive expansion of HTLV-I–infected CD4+/CD25+ T cells after haplo-PBSCT and further demonstrated that the abnormal lymphocytes originated from the HTLV-I–negative donor. Oral prednisolone was increased to 0.4 mg/kg per day because the national health insurance in Japan has not yet approved antiviral therapy, such as zidovudine, for ATLL. As a result, the patient was in stable condition for several months. However, thereafter, his symptoms of colitis, such as diarrhea and pain, gradually worsened despite the treatment, showing disease progression. Colon biopsy specimens revealed lymphoid infiltrates consisting of abnormal, medium-sized T cells with irregular nuclei. The abnormal lymphocytes in his peripheral blood were also resistant to steroid therapy.
Development of donor-derived ATLL after allo-HSCT has been reported in a small number of ATLL patients, all of whom had received allo-HSCT from HTLV-I–positive donors.7-9 In contrast, Glowacka et al6 reported 3 transplant recipients exposed to HTLV-I by solid organ transplants from an HTLV-I–positive multiorgan donor. They observed the development of primary cutaneous HTLV-I–positive T-cell lymphoma in 2 recipients after transplantation. Rapid development of myelopathy in recipients of solid organ transplants from asymptomatic HTLV-I carrier donors has been also reported.3,4 However, in our case, the donor was HTLV-I–negative. Moreover, a long-term follow-up study of ATLL survivors demonstrated that HTLV-I proviral load became undetectable or decreased to carrier levels after allo-HSCT from HTLV-I–negative donors.10 To our knowledge, this is the first report of an HTLV-I–associated lymphoproliferative disorder of donor T-cell origin after HTLV-I transmission from recipient cells to donor cells.
Recipient immunocompetence has been demonstrated to play an important role in controlling the proliferation of virus-infected cells. Considering the poor clinical outcome resulting from repeated intensive chemotherapy, our patient underwent haplo-PBSCT with active disease.
Xhaard et al reported that the median number of circulating CD4+CD25+CD127–/low Tregs was ∼18/μL (range: 8-28/μL) at 6 months post–allo-HSCT.11 In our case, an increased number of Tregs and a reduced number of natural killer cells were observed in the patient’s peripheral blood after haplo-PBSCT. Low-dose thymoglobulin, in addition to its effector T-cell–depleting properties, stimulates the recovery of Tregs.12 Toulza et al reported a strong negative correlation between the frequency of CD4+/FoxP3+/Tax– Tregs in the circulation and HTLV-I–specific cytotoxic T lymphocyte response.13 Furthermore, in donor-derived ATLL development after allo-HSCT, Tamaki et al14 suggested that downregulated expression of Tax is a potential mechanism for escape of HTLV-I–infected cells from T-cell–mediated immunosurveillance. In addition, dense smears with a discrete band(s) in HTLV-I integration analysis with Southern blotting have been reported in smoldering ATLL patients or in HTLV-I carriers with a high HTLV-I proviral load, showing the existence of growing clones or a cluster of small clones in addition to polyclonal proliferation of HTLV-I–infected cells.15 In our case, there is also the possibility that inflammation in gut GVHD might have stimulated proliferation of HTLV-I–infected T cells.
Our case demonstrates that impaired immune responses against HTLV-I during allo-HSCT provide a potential mechanism for virus transmission from recipient cells to donor cells, as is the case in other viruses, which subsequently allows progressive expansion of HTLV-I–infected donor T cells and development of HTLV-I–associated diseases.
Authorship
Contribution: N.K., T.N., Y.Y., and T.H. were responsible for clinical management of the patient presented and acquisition of data and helped to draft the manuscript; J.T. was responsible for clinical management, interpretation of the data, and scientific revision and drafting of the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junichi Tsukada, Department of Hematology, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8556, Japan; e-mail: jtsukada@med.uoeh-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal