To the editor:
A 46-year-old man was admitted to our hospital because of systemic lymphadenopathy and generalized erythema. White blood cell (WBC) count was 8.3 × 109/L with 8% morphologically abnormal lymphocytes that were positive for CD3, CD4, and CD25. Antibody against human T-cell lymphotropic virus type I (HTLV-1) was positive, and Southern blot analysis (SBA) using his peripheral blood (PB) and a biopsy specimen of erythema revealed monoclonal integration of HTLV-1 provirus. With an elevated serum lactate dehydrogenase level (642 IU/L), he was diagnosed as having acute-type adult T-cell leukemia/lymphoma (ATL).1 His ATL was resistant to chemotherapy, so he then received allogeneic PB stem cell transplantation after administration of high-dose cytarabine and 12 Gy of total body irradiation. Cyclosporine and a short course of methotrexate were given as prophylaxis for graft-versus-host disease. The donor was his HLA-identical brother, an asymptomatic carrier of HTLV-1. Donor PB had no abnormal lymphocytes, and its SBA demonstrated no monoclonal proliferation of HTLV-1–infected cells.
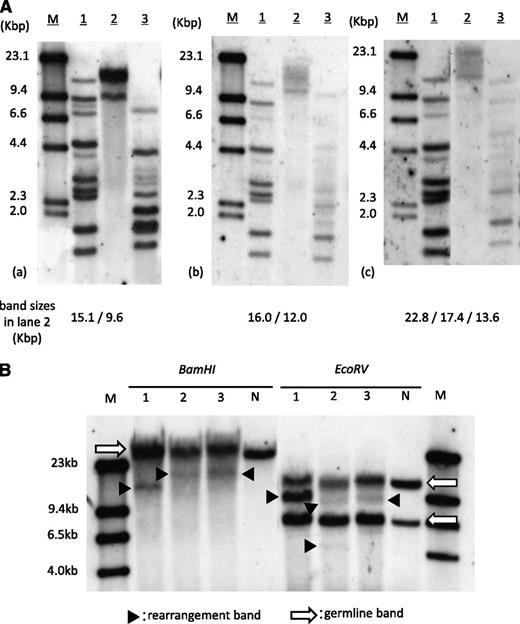
The patient showed neutrophil engraftment on day 14 of transplantation. Simultaneously, his peripheral lymphocyte count started to increase and reached 4.6 × 109/L (45% of WBC count) by day 18. The morphological feature of proliferated lymphocytes resembled that of typical ATL cells, and the surface antigens were positive for CD3, CD4, and CD25. Increased HTLV-1 proviral load of 119.2 copies/100 PB mononuclear cells was detected by quantitative polymerase chain reaction. At this point, we thought this was an early relapse of ATL, but within a week, lymphocytosis rapidly disappeared without additional treatment. The ATL-like lymphocyte count became less than 0.5 × 109/L (5% of WBC count) by day 31, and the proviral load decreased to 14.3 copies/100 PB mononuclear cells on day 71. This phenomenon prompted us to investigate the clonality of expanded lymphocytes. SBA did reveal monoclonal integration of an HTLV-1 provirus; however, band sizes were different from those of pretransplantation (Figure 1A). SBA of T-cell receptor Cβ1 genes also demonstrated different patterns of bands between pre- and posttransplantation (Figure 1B), suggesting the expanded lymphocytes were not from original ATL cells. The result of short tandem repeat polymerase chain reaction using PB showed mixed chimerism, with only 6.3% recipient cells on day 18, and complete donor chimerism on days 31 and 71, implying the expanded lymphocytes were of donor origin.
Clonality analysis of expanded lymphocytes. (A) SBA of HTLV-1. (a) PB of pretransplantation, (b) day 18 of transplantation, and (c) day 71 of transplantation. Band sizes in lane 2 in each panel are shown at the bottom. M, size marker (λDNA/HindIII); lane 1, positive control; lane 2, DNA digested with Eco R1; lane 3, DNA digested with Pst1. (B) SBA of T-cell receptor Cβ1 genes. Lane 1, PB of pretransplantation; lane 2, day 18 of transplantation; lane 3, day 71. Arrows indicate germ-line bands; arrowheads indicate rearranged bands. M, molecular weight marker II; N, negative control (placenta DNA).
Clonality analysis of expanded lymphocytes. (A) SBA of HTLV-1. (a) PB of pretransplantation, (b) day 18 of transplantation, and (c) day 71 of transplantation. Band sizes in lane 2 in each panel are shown at the bottom. M, size marker (λDNA/HindIII); lane 1, positive control; lane 2, DNA digested with Eco R1; lane 3, DNA digested with Pst1. (B) SBA of T-cell receptor Cβ1 genes. Lane 1, PB of pretransplantation; lane 2, day 18 of transplantation; lane 3, day 71. Arrows indicate germ-line bands; arrowheads indicate rearranged bands. M, molecular weight marker II; N, negative control (placenta DNA).
Allogeneic hematopoietic stem cell transplantation (allo-SCT) is increasingly used as a curative option for ATL.2,3 Although HLA-matched related siblings are generally preferred as donors in allo-SCT, 2/3 of the siblings of ATL patients are asymptomatic carriers of HTLV-14 and there are reports demonstrating that transplantations from such donors actually lead to the development of donor-derived ATL.5,6 Our case suggests that allo-SCT from asymptomatic carriers of HTLV-1 may lead to the development of transient, donor-derived, benign HTLV-1–associated monoclonal lymphocytosis very early after transplantation. Such lymphocytosis should not be misdiagnosed as a relapse or a development of donor-derived ATL. To fully understand this phenomenon, further collection of similar cases is necessary.
Authorship
Acknowledgments: Approval was obtained from the Nagasaki University Hospital Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Contribution: M.T., Y.I., J.T., D.I., H.T., and T.H. conceived this report; M.T. collected and analyzed the data; D.S. and H.H. performed Southern blot analysis; M.T., Y.I., and Y.M. wrote the manuscript and created the figures; and all authors contributed to the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshitaka Imaizumi, Department of Hematology, Nagasaki University Hospital, 1-7-1 Sakamoto, Nagasaki 852-8501, Japan; e-mail: y-imaizm@nagasaki-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal