To the editor:
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare form of acute myeloid leukemia (AML) that has been recognized as a distinct entity in the 2008 World Health Organization classification of hematopoietic tumors.1 BPDCN is characterized by frequent skin and bone marrow involvement and an aggressive course.2 Although acute leukemia-type induction chemotherapy (either AML-type or acute lymphoblastic leukemia-type) may provide high rates of initial response, relapse will eventually happen, and prognosis remains dismal.3-7 Alternatively, long-lasting remissions have been observed after allogeneic hematopoietic stem cell transplantation (allo-HSCT).3-10 In 2013, the European Group for Blood and Marrow Transplantation reported the largest cohort of patients with BPDCN receiving allo-HSCT (n = 34), with a 3-year overall survival (OS) of 41%.8 Of note, only 9 patients in this study had received reduced-intensity conditioning (RIC) regimens, with a very poor outcome and a nonrelapse mortality (NRM) rate above 40%. Thus, the benefit of allo-HSCT seemed to be restricted to patients who could receive a myeloablative conditioning regimen (MAC), and was only observed if patients were transplanted in first complete remission (CR1). However, similar to other types of AML, BPDCN mostly affects elderly patients (median age at diagnosis, ∼65 years), most of whom are not eligible for MAC. Another recent study showed no difference in OS after RIC or MAC, but patient numbers were far too small to draw any firm conclusion (n = 8 for MAC and n = 6 for RIC).9 Therefore, to evaluate the role of RIC or nonmyeloablative (NMA) conditioning regimens in this setting, we conducted a multicenter retrospective study of French patients who underwent allo-HSCT for BPDCN.
All cases were retrospectively collected from the database of the French Society of Bone Marrow Transplantation and Cell Therapy. We excluded pediatric cases (n = 4). From February 2003 to January 2014, 43 adult patients with BPDCN received allo-HSCT in 21 French centers. Six of those cases were previously reported.8 All cases were centrally reviewed to calculate the immunophenotypic diagnostic score.11 In 28 cases (65%), the score was 2 or more. The remaining 15 patients all had CD4+CD56+ disease, but as they were mostly diagnosed before the publication of the immunophenotypic diagnostic score, other markers such as CD123, BDCA-2, and BDCA-4 were not assessed, precluding calculation of a score at least equal to 2. Three patients had only skin without marrow involvement at diagnosis. The study has been approved by the institutional review board of the French Society of Bone Marrow Transplantation and Cell Therapy and was conducted with informed consent.
Categorical variables were compared by Fisher exact tests. Failure time data, except for cumulative incidences, were estimated by the Kaplan-Meier method12 and then compared by the log-rank test, with 95% confidence intervals (CIs) estimated by the Cox model.13 For cumulative incidence of relapse (CIR) and NRM, deaths in remission and relapses were, respectively, taken into account as competing risks, using the cumulative incidence curves, and compared by the Gray test; the Fine and Gray model was used to estimate subdistribution hazard ratio.14 Outcome was also adjusted on the following potential prognostic variables: age (<57 or ≥57 years; 57 years was the median age within the cohort), disease status (CR1 vs others), cytomegalovirus (CMV) status of donor and recipient (double negative vs all other combinations), donor type (sibling or unrelated 10/10 vs others), donor and recipient sex (female into male vs other combinations), type of conditioning regimen (MAC vs RIC/NMA), stem cell source, and total body irradiation or not.
Patients’ characteristics according to the conditioning regimen are shown in Table 1. Median age was 57 years (range, 20-72 years), and 17 patients (40%) were aged 60 years or older. Fourteen patients (33%) were transplanted after MAC, and 29 (67%) after RIC/NMA. Patients in the MAC group were significantly younger and received TBI more frequently than those in the RIC/NMA group. Other variables, as defined here, were not statistically different between the 2 groups.
Patients’ characteristics according to the conditioning regimen for HSCT
| . | Total . | MAC . | RIC/NMA . |
|---|---|---|---|
| N | 43 | 14 | 29 |
| Age, y | 57 (20-72) | 36 (20-50) | 61 (32-72) |
| Sex (M/F) | 29/14 | 8/6 | 21/8 |
| F → M | 11 (26%) | 3 (21%) | 8 (28%) |
| Other | 32 (74%) | 11 (79%) | 21 (72%) |
| Time from diagnosis, d | 170 (107-1050) | 147.5 (110-520) | 193 (107-1050) |
| Disease status at HSCT | |||
| CR1 | 34 (79%) | 12 (86%) | 23 (79%) |
| CR2 | 5 (12%) | 1 (7%) | 4 (14%) |
| No CR | 2 (5%) | 1 (7%) | 0 |
| Unknown | 2 (5%) | 0 | 2 (7%) |
| Donor | |||
| Sibling | 19 (44%) | 8 (57%) | 11 (38%) |
| MUD | 10 (23%) | 1 (7%) | 9 (31%) |
| MMUD | 7 (16%) | 4 (29%) | 3 (10%) |
| Cord blood | 6 (14%) | 1 (7%) | 5 (17%) |
| Mismatch relative | 1 (2%) | 0 | 1 (3%) |
| Conditioning regimen | |||
| MAC | 14 (33%) | 14 (100%) | NA |
| Cy/TBI-based | 11 (26%) | 11 (79%) | NA |
| Cy/Bu | 3 (7%) | 3 (21%) | NA |
| RIC/NMA | 29 (67%) | NA | 29 (100%) |
| Flu/Bu/ALG | 10 (23%) | NA | 10 (34%) |
| Flu/2 Gy-TBI | 10 (23%) | NA | 10 (34%) |
| Sequential* | 5 (12%) | NA | 5 (17%) |
| Other† | 4 (9%) | NA | 4 (14%) |
| TBI-based | 23 (53%) | 11 (79%) | 12 (41%) |
| Cell source | |||
| Bone marrow | 7 (16%) | 5 (36%) | 2 (7%) |
| Peripheral blood | 30 (70%) | 8 (57%) | 22 (76%) |
| Cord blood | 6 (14%) | 1 (7%) | 5 (17%) |
| CMV status (D/R) | |||
| −/− | 18 (42%) | 6 (43%) | 12 (41%) |
| −/+ | 9 (21%) | 1 (7%) | 8 (28%) |
| +/− | 4 (9%) | 2 (14%) | 2 (7%) |
| +/+ | 12 (28%) | 5 (36%) | 7 (24%) |
| GVHD prophylaxis | |||
| Ciclo/MTX | 15 (35%) | 12 (86%) | 3 (10%) |
| Ciclo/MMF | 19 (44%) | 1 (7%) | 18 (62%) |
| Ciclo alone | 5 (12%) | 0 | 5 (17%) |
| Other | 2 (5%) | 0 | 2 (7%) |
| Unknown | 2 (5%) | 1 (7%) | 1 (3%) |
| . | Total . | MAC . | RIC/NMA . |
|---|---|---|---|
| N | 43 | 14 | 29 |
| Age, y | 57 (20-72) | 36 (20-50) | 61 (32-72) |
| Sex (M/F) | 29/14 | 8/6 | 21/8 |
| F → M | 11 (26%) | 3 (21%) | 8 (28%) |
| Other | 32 (74%) | 11 (79%) | 21 (72%) |
| Time from diagnosis, d | 170 (107-1050) | 147.5 (110-520) | 193 (107-1050) |
| Disease status at HSCT | |||
| CR1 | 34 (79%) | 12 (86%) | 23 (79%) |
| CR2 | 5 (12%) | 1 (7%) | 4 (14%) |
| No CR | 2 (5%) | 1 (7%) | 0 |
| Unknown | 2 (5%) | 0 | 2 (7%) |
| Donor | |||
| Sibling | 19 (44%) | 8 (57%) | 11 (38%) |
| MUD | 10 (23%) | 1 (7%) | 9 (31%) |
| MMUD | 7 (16%) | 4 (29%) | 3 (10%) |
| Cord blood | 6 (14%) | 1 (7%) | 5 (17%) |
| Mismatch relative | 1 (2%) | 0 | 1 (3%) |
| Conditioning regimen | |||
| MAC | 14 (33%) | 14 (100%) | NA |
| Cy/TBI-based | 11 (26%) | 11 (79%) | NA |
| Cy/Bu | 3 (7%) | 3 (21%) | NA |
| RIC/NMA | 29 (67%) | NA | 29 (100%) |
| Flu/Bu/ALG | 10 (23%) | NA | 10 (34%) |
| Flu/2 Gy-TBI | 10 (23%) | NA | 10 (34%) |
| Sequential* | 5 (12%) | NA | 5 (17%) |
| Other† | 4 (9%) | NA | 4 (14%) |
| TBI-based | 23 (53%) | 11 (79%) | 12 (41%) |
| Cell source | |||
| Bone marrow | 7 (16%) | 5 (36%) | 2 (7%) |
| Peripheral blood | 30 (70%) | 8 (57%) | 22 (76%) |
| Cord blood | 6 (14%) | 1 (7%) | 5 (17%) |
| CMV status (D/R) | |||
| −/− | 18 (42%) | 6 (43%) | 12 (41%) |
| −/+ | 9 (21%) | 1 (7%) | 8 (28%) |
| +/− | 4 (9%) | 2 (14%) | 2 (7%) |
| +/+ | 12 (28%) | 5 (36%) | 7 (24%) |
| GVHD prophylaxis | |||
| Ciclo/MTX | 15 (35%) | 12 (86%) | 3 (10%) |
| Ciclo/MMF | 19 (44%) | 1 (7%) | 18 (62%) |
| Ciclo alone | 5 (12%) | 0 | 5 (17%) |
| Other | 2 (5%) | 0 | 2 (7%) |
| Unknown | 2 (5%) | 1 (7%) | 1 (3%) |
Age and time from diagnosis are shown as median (range). Other variables are shown as number (percentage).
ALG, antilymphocyte globulin; Bu, busulfan; Ciclo, ciclosporin; CR, complete remission; Cy, cyclophosphamide; D, donor; F, female; Flu, fludarabine; GVHD, graft-versus-host disease; Gy, Gray; M, male; MMF, mycophenolate mofetil; MMUD, mismatched unrelated donor; MTX, methotrexate; MUD, (10/10) matched unrelated donor; NA, not applicable; R, recipient; TBI, total-body irradiation.
Sequential conditioning regimen consisted of amsacrine/aracytine/Flu/Cy/ALG combined either with Bu in 4 patients or 2 Gy-TBI in 1.
Other conditioning regimen consisted of Flu/aracytine/2 Gy-TBI (1), Flu/Bu/ALG/Thiotepa (1), Cy/total lymphoid irradiation (1), and Flu/melphalan (1).
Graft failure occurred in 4 patients (9%), with 3 of those having received cord blood units. All received a second transplant 2 to 17 weeks after the first transplant. Acute (grade 2-4) and chronic GVHD occurred in 17 and 16 patients, respectively. After a mean follow-up of 668 days, 22 patients (51%) were alive, 19 of whom were disease-free (44%). Eleven patients relapsed, at a median of 225 days posttransplant. With a mean follow-up of 1050 days for alive patients, the probabilities of CIR, NRM, disease-free survival (DFS), and OS were 26% (95% CI, 13%-40%), 33% (95% CI, 19%-48%), 45% (95% CI, 29%-59%), and 52% (95% CI, 36%-66%) at 2 years posttransplant, respectively.
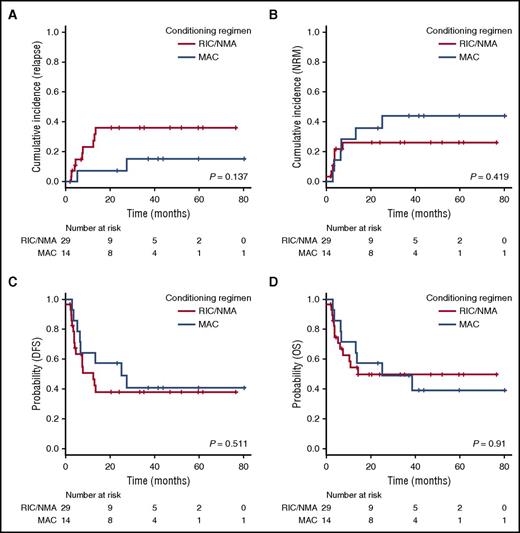
The type of conditioning regimen did not affect either the CIR (7% [95% CI, 4%-29%] vs 36% [95% CI, 17%-55%] at 2 years after MAC or RIC/NMA, respectively; P = .137; Figure 1A) or NRM (44% [95% CI, 17%-68%] vs 26% [95% CI, 11%-44%] at 2 years after MAC or RIC/NMA, respectively; P = .42; Figure 1B). As a consequence, DFS and OS were comparable after MAC or RIC/NMA (2-year DFS, 57% [95% CI, 28%-78%]) vs 38% [95% CI, 20%-56%; P = .51] and 2-year OS, 57% [95% CI, 28%-78%] vs 50% [95% CI, 29%-67%; P = .91; Figure 1C-D). All 17 patients aged 60 years or older had received RIC/NMA. Among them, 10 (59%) were alive at last follow-up, 9 (53%) of whom were disease-free. Six of these 10 alive patients had been followed for more than 2 years.
Transplant outcome for patients transplanted after a MAC vs a RIC/NMA conditioning regimen. (A) CIR. (B) NRM. (C) DFS. (D) OS.
Transplant outcome for patients transplanted after a MAC vs a RIC/NMA conditioning regimen. (A) CIR. (B) NRM. (C) DFS. (D) OS.
By univariate analysis, the only factor significantly affecting NRM was donor type, with a 2-year incidence of NRM of 22% (95% CI, 9%-39%) for siblings/MUD vs 45% (95% CI, 17%-69%) for others (P = .04), whereas CIR was only influenced by disease status at transplant (CR1 vs other, P = .008), although there was also a trend for donor/recipient CMV status (higher risk if double-negative vs other, P=.06). Acute and/or chronic GVHD occurrence did not affect CIR. No factor had any significant effect on DFS or OS. We only observed a trend for a better outcome in patients transplanted with siblings/MUD vs other donor types (P = .08 for DFS and P = .05 for OS).
Overall, we report the largest cohort of patients treated with allo-HSCT for BPDCN, with a median age notably higher than in previous reports,3,4,8 which reflects more accurately the general population affected by this disorder. The group of patients receiving RIC/NMA had an older median age but a very close outcome as compared to patients receiving MAC. This was made possible thanks to good disease control after RIC/NMA regimens.
Recent encouraging data suggested redefining the place of autologous HSCT (auto-HSCT) compared with allo-HSCT for treatment of BPDCN, particularly in patients without marrow infiltration at diagnosis.9 However, the number of patients was remarkably small in this comparative study.
In conclusion, in this retrospective study of 43 patients with BPDCN, allo-HSCT allowed for good disease control, although NRM was consequent, especially when using nonsibling donors. RIC/NMA regimens are equivalent to MAC in terms of outcome, confirming that long-lasting remissions can be achieved in this disease, even in patients aged 60 years or older. RIC/NMA should thus be proposed to patients not eligible for MAC, because of their age or comorbidities, who represent the majority of patients suffering from BPDCN.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank L. Moukhtari and N. Raus, as well as D. Roos-Weil, for data management, and F. Garnache-Ottou for immunophenotyping reviewing.
Contribution: Conception and design: M.L. and S.M.; provision of study materials or patients: all authors; collection and assembly of data: M.L. and S.M.; data analysis and interpretation: all authors; manuscript writing: M.L. and S.M.; and final approval of the manuscript: all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the French Society of Bone Marrow Transplantation and Cell Therapy appears in the online appendix.
Correspondence: Sébastien Maury, Assistance Publique-Hôpitaux de Paris, Hôpital Henri Mondor, 51 avenue du Mal de Lattre de Tassigny, 94010 Créteil Cedex, France; e-mail: sebastien.maury@aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal