Key Points
RNA interference screen targeted to primary human HSPCs identified CYTH1 as a crucial mediator of cell adhesion.
CYTH1 is required for homing and proper bone marrow localization of HSPCs following transplantation.
Abstract
Adhesion is a key component of hematopoietic stem cell regulation mediating homing and retention to the niche in the bone marrow. Here, using an RNA interference screen, we identify cytohesin 1 (CYTH1) as a critical mediator of adhesive properties in primary human cord blood–derived hematopoietic stem and progenitor cells (HSPCs). Knockdown of CYTH1 disrupted adhesion of HSPCs to primary human mesenchymal stroma cells. Attachment to fibronectin and ICAM1, 2 integrin ligands, was severely impaired, and CYTH1-deficient cells showed a reduced integrin β1 activation response, suggesting that CYTH1 mediates integrin-dependent functions. Transplantation of CYTH1-knockdown cells to immunodeficient mice resulted in significantly lower long-term engraftment levels, associated with a reduced capacity of the transplanted cells to home to the bone marrow. Intravital microscopy showed that CYTH1 deficiency profoundly affects HSPC mobility and localization within the marrow space and thereby impairs proper lodgment into the niche. Thus, CYTH1 is a novel major regulator of adhesion and engraftment in human HSPCs through mechanisms that, at least in part, involve the activation of integrins.
Introduction
Somatic stem cells reside in dynamic specialized microenvironments called niches. Hematopoietic stem cells (HSCs) are distinctive among somatic stem cells for their migratory behavior during development and in the adult mammal. This mobility has enabled the successful harvest and engraftment of transplanted stem cells in the treatment of blood diseases and cancer. The precise mechanisms that regulate the homing and engraftment process of HSCs remain incompletely understood. However, several molecules have been shown to modulate these processes through regulation of HSC adhesion and migration. Examples of such molecules are the selectin family of adhesion molecules (E- and P-selectin); the integrins, in particular α4β1 very late antigen-4 (VLA-4) in association with vascular cell adhesion molecule 1 (VCAM-1); and the chemokine CXCL12 (also known as SDF-1) and its G-protein coupled receptor CXCR4. Although the use of exogenous ligands or blocking antibodies has allowed the identification and characterization of these essential cell-surface molecules in both mouse and human HSCs, intracellular mediators of adhesion have been more challenging to assess, particularly in human cells.1-3 Studies in knockout mice have revealed members of the Rho guanosine triphosphatase family as key effector molecules in HSC adhesion and localization by controlling the transduction of external signals to cytoplasmic and nuclear effectors.4 Intracellular signaling mediators like the Rho guanosine triphosphatases represent attractive therapeutic targets to manipulate the localization of both normal malignant hematopoietic cells.5
Here, to address some of the challenges in studying adhesion in human cells, and in an attempt to define novel key regulators, we have developed a paradigm for RNA interference (RNAi)–based screens in primary human cord blood derived hematopoietic stem and progenitor cells (HSPCs) to assess the function of both cell-surface and intracellular molecules in a broad and unbiased manner. We identify cytohesin 1 (CYTH1) as a novel regulator of human HSPC adhesion in vitro and a critical mediator of homing and engraftment in vivo.
Materials and methods
Human HSPCs and MSC isolation
Human cord blood samples were obtained from uncomplicated births at the maternity wards of Helsingborg General Hospital and Skåne University Hospital in Lund and Malmö, Sweden, after informed consent. Mononuclear cells were obtained by density-gradient centrifugation (Lymphoprep, Medinor). Subsequently, CD34+ cells were magnetically isolated (CD34 MicroBead Kit, Miltenyi Biotec). Mesenchymal stroma cells (MSCs) were kindly provided by Stefan Scheding (Lund Stem Cell Center, Lund, Sweden) or isolated from fresh bone marrow as previously described.6 For adhesion assays, 6000 cells per well were plated in 96-well plates 3 days prior to the experiment. MSCs not older than 2 passages were used in all experiments.
Preparation of shRNA lentiviral library and individual shRNA lentiviruses
For the screen, a predefined set of 1778 short hairpin RNAs (shRNAs) targeting cell adhesion genes cloned in the pLKO1 lentiviral vector was used (Mission shRNA Human Gene Family Set, DNA, Cell Adhesion Genes, SH2221, Sigma-Aldrich). For validation studies, individual shRNAs were cloned into a green fluorescent protein (GFP) expressing version of pLKO1 to facilitate cell tracking. Lentiviruses were produced as previously described.7
Transduction and cell culture
Lentiviral transduction of CD34+ cells was performed as previously described.8
Adhesion assay
Three days following transduction, CD34+ cells were resuspended in Iscove modified Dulbecco medium (Thermo Scientific HyClone), 10% fetal bovine serum (Thermo Scientific HyClone) and 50 000 to 60 000 cells per well were plated onto MSC layers in a 96-well plate. Cells were allowed to adhere for 1 hour at 37°C, after which the plate was carefully immersed in a prewarmed phosphate-buffered saline–filled container and a second 96-well plate with U-shaped wells was aligned on top of the first plate. The 2 aligned plates were inversed allowing nonadherent cells to be separated by gravitation into the U-shaped wells during a 1-hour incubation at 37°C. The nonadherent cell fraction was then resuspended in Iscove modified Dulbecco medium 10% fetal calf serum, and the adhesion-inversion procedure was repeated. Finally, cells that adhered to the first plate with MSCs, and cells that did not adhere throughout 2 inversions were collected, stained with anti-CD34 Ab (clone 8G12, BD Biosciences) and 7-Aminoactinomycin D (Sigma Aldrich, Sweden), and analyzed by flow cytometry (FACSCantoII, BD Biosciences) or sorted (FACSAriaIIu, BD Biosciences). Where indicated, expression of integrin β1 was evaluated with flow cytometry (antibody clone MAR4, BD Pharmingen).
Deep-sequencing analysis of shRNAs
Genomic DNA was extracted (High Pure PCR Template Preparation Kit, Roche Diagnostics) from sorted cell pellets. Individual shRNAs were identified from the deep-sequencing reads (Illumina Genome Analyzer II), and the counts were normalized to the total number of reads for the sample.
Gene expression analysis
Unmanipulated CD34+ cells after 2 days expansion were used in adhesion assay as described previously. Viable, CD34+ cells were sorted (FACSAriaIIu, BD Biosciences) directly in RLT buffer, and total RNA was extracted (RNeasy Mini Kit, Qiagen). Samples were analyzed with Affymetrix Human Genome U133plus Array.
In vivo transplantations
To assess long-term engraftment, CD34+ cells transduced with scrambled (SCR) and CYTH1-shRNA1 were sorted for GFP and intravenously injected into sublethally (3 Gy) irradiated NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice (30 000 cells per mouse).
Intravital imaging
Fluorescence-activated cell sorter (FACS) purified Lin−CD34+CD38− cells were prestimulated in StemSpan (StemCell Technologies, Vancouver, BC, Canada) supplemented with 300 ng/mL stem cell factor, 300 ng/mL Flt3-L, and 20 ng/mL thrombopoietin for 4 to 6 hours before addition of lentiviral supernatatnt. Four days later, GFP+ cells were purified by FACS, and intravital imaging and image analysis were performed as described previously.9
Statistical analysis
Statistical significance was calculated in GraphPad Prism 6.0 using Mann-Whitney U test. Error bars show standard deviation.
Accession numbers
The Gene Expression Omnibus accession number for the microarray data reported in this paper is GSE78216. The data can be viewed at the reviewers’ access link (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE78216).
For more details on experimental procedures, see the supplemental Materials and Methods (available on the Blood Web site).
Results
A gravity-based adhesion assay to model HSPC-MSC interactions
To model cell-cell interactions relevant in the context of human HSPC homing and engraftment, we developed an adhesion assay using primary human bone marrow–derived MSCs as a relevant component of the bone marrow environment. The assay is based on gravitational force to separate adherent and nonadherent cord blood–derived CD34+ cells that have been seeded on a layer of MSCs (supplemental Figure 1A; see “Materials and methods” for details). The use of gravitational force has been shown to be more specific and reproducible than regular washing steps as it avoids shear stress and nonstandardized forces.10,11
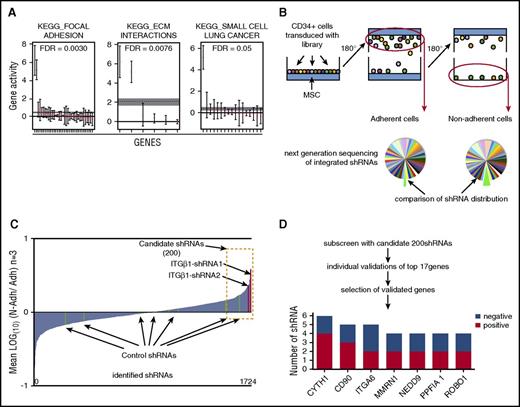
To assess the properties of HSPCs recovered from the gravity-based adhesion assay, we performed global gene expression analysis of the adherent and nonadherent CD34+ cell fractions. As expected, adhesion-associated genes were significantly enriched in the adherent CD34+ cells according to Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation, whereas genes associated with cell cycling and apoptosis, which potentially could affect adhesion properties, did not differ significantly between the 2 fractions (Figure 1A; supplemental Table 1).12 Therefore, the gravity-based assay distinguishes HSPCs with differential adhesive properties.
Gravity adhesion assay to screen for adhesion mediators in human HSPCs. (A) Quantitative set analysis for gene expression showing significantly enriched gene sets, according to KEGG annotations, in adherent vs nonadherent CD34+ cells. The dotted horizontal line indicates the average log fold change for each gene set. (B) Overview of the screening procedure where the gravity adhesion assay was used to isolate adherent and nonadherent human HSPC populations. (C) Ratios of normalized shRNA counts in nonadherent vs adherent cell fractions for all shRNAs detected in the primary screen. (D) Steps to identify candidate genes. The bottom diagram shows all genes for which 2 or more shRNAs scored positively in the validation assays (ie, reduced adhesion). ITGβ1, integrin β1.
Gravity adhesion assay to screen for adhesion mediators in human HSPCs. (A) Quantitative set analysis for gene expression showing significantly enriched gene sets, according to KEGG annotations, in adherent vs nonadherent CD34+ cells. The dotted horizontal line indicates the average log fold change for each gene set. (B) Overview of the screening procedure where the gravity adhesion assay was used to isolate adherent and nonadherent human HSPC populations. (C) Ratios of normalized shRNA counts in nonadherent vs adherent cell fractions for all shRNAs detected in the primary screen. (D) Steps to identify candidate genes. The bottom diagram shows all genes for which 2 or more shRNAs scored positively in the validation assays (ie, reduced adhesion). ITGβ1, integrin β1.
Next, for evaluation of the assay’s sensitivity to detect functional perturbations of key adhesion molecules in HSPCs, we decided to target ITGβ1, which has a well-established role in HSC-niche interactions and is a crucial mediator of engraftment of transplanted HSCs.1,13-15 Silencing of ITGβ1 expression by lentivirally delivered shRNA decreased the adhesion of CD34+ cells to the MSC layer as visualized by a strong enrichment of the transduced GFP+ cells within the nonadherent cell fraction (supplemental Figure 1B, left). This finding was recapitulated with an independent shRNA against ITGβ1 (supplemental Figure 1B, right). On the contrary, no effect on adhesion was observed for any of 4 shRNAs with validated knockdown of the chemokine receptor CXCR4 (data not shown). This suggests that directly adhesive mechanisms rather than chemoattractive interactions are assessed in the gravity assay.
Taken together, we developed an adhesion assay based on gravity force to recover adherent and nonadherent HSPC populations enabling detection of perturbed adhesion molecules in a highly sensitive manner. We reasoned that the assay would provide a suitable platform to broadly screen for novel modifiers of adhesion and HSC-niche interactions.
A pooled shRNA screen uncovers adhesion regulators in human HSPCs
Having established the gravity assay as a reliable method to read out adhesive interactions in human HSPCs, we next decided to apply the assay in a forward screen for new adhesion regulators using RNAi.16 To this end, we generated a pooled lentiviral library of preselected shRNAs targeting broadly defined adhesion-associated genes (1778 shRNAs targeting 336 genes) to screen for regulators of HSPC adhesion to MSCs.
In brief, the pooled lentiviral shRNA library was transduced into cord blood–derived CD34+ cells at an average efficiency of 20% to 30% to avoid multiple shRNA insertions in a single cell. Seventy-two hours after transduction, the cells were seeded onto MSCs and subjected to the gravity-based adhesion assay. Genomic DNA from the adherent and nonadherent cell fractions was analyzed by deep sequencing to identify and quantify integrated shRNAs (Figure 1B). Importantly, 97% of the shRNAs from the library were identified in the sequencing, demonstrating a high representation of the shRNA constructs across 3 replicate screens. Ratios between normalized shRNA counts in nonadherent vs adherent fractions were then calculated with the increasing ratios representing shRNAs enriched in the nonadherent fraction (Figure 1C; supplemental Table 2). By analogy to the effect observed for knockdown of ITGβ1 (supplemental Figure 1B), we expected that shRNAs targeting genes critical for the adhesive interactions between HSPCs and MSCs would be enriched in the nonadherent HSPC fraction.
To get an indication if the screen had selected for relevant outcomes, we first assessed the 2 ITGβ1 shRNAs, previously used to test the assay. We observed a strong enrichment of both shRNAs in the nonadherent fraction supporting the feasibility of the screening strategy. In contrast, a random distribution of control shRNAs lacking on-target knockdown activity showed the background inherent to the screen (Figure 1C).
To increase the accuracy and specificity of the screen, we generated a pooled sublibrary containing the 200 shRNAs showing the highest enrichment in the nonadherent cells (Figure 1C; supplemental Table 2) and performed secondary screens using the previously established screening procedure. From the secondary screen, we identified 17 genes from the top-scoring shRNAs and individually validated them using the gravity adhesion assay (supplemental Table 3). Further, for the most promising candidates (genes encoding nonsecreted proteins or genes highly expressed in HSCs), we additionally tested several shRNAs to confirm target specificity (supplemental Table 3). Genes for which 2 or more shRNAs reduced adhesion, as determined by an increased GFP proportion among nonadherent cells, were considered hits in the screen (Figure 1D). Among these hits, we found several genes previously not implicated in regulation of HSPC adhesion and some known regulators such as integrin α6 (ITGα6), a compound of the VLA-6 integrin complex.17,18
CYTH1 mediates adhesion of CD34+ cells to MSCs
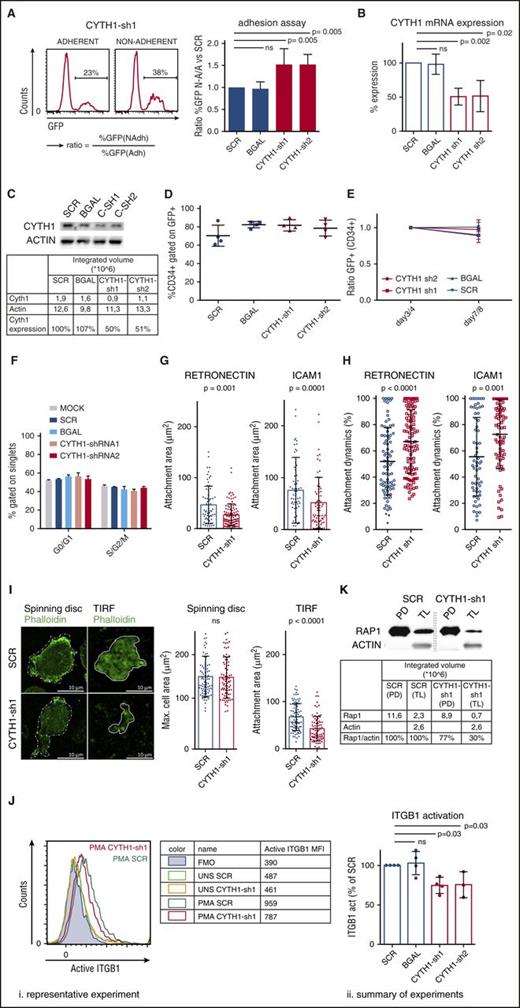
From the screen hits, we decided to focus on CYTH1 as it had not previously been implicated in regulation of HSPC adhesion and showed a highly consistent phenotype in the adhesion assay from 2 independent shRNAs (Figure 2A), and knockdown of CYTH1 was confirmed at the messenger RNA and protein level (Figure 2B-C). Furthermore, knockdown of CYTH1 impaired adhesion of CD34+ cells to layers of human fetal osteoblasts and human umbilical vein endothelial cells (supplemental Figure 2). This indicates a broad role of CYTH1 in regulation of adhesion of CD34+ cells to different niche components within the bone marrow. Because cellular effects associated with cell cycling or differentiation theoretically could have influenced the outcome of the adhesion assays, we first assessed these parameters in CYTH1 targeted cells. We did not detect any signs of altered proliferation, cell cycle status, or differentiation in CYTH1 targeted cells compared with 2 independent control shRNAs (Figure 2D-F), which supports a direct adhesion-mediating role of CYTH1. Taken together, targeting of CYTH1 in human HSPCs by independent shRNAs points toward a highly specific and critical role of CYTH1 in regulation of adhesion to MSCs.
CYTH1 regulates adhesion of human HSPCs through integrins. (A) CD34+ cells were transduced with CYTH1 shRNAs from a GFP-expressing vector and subjected to the adhesion assay. Representative FACS histograms showing GFP levels in adherent and nonadherent cells (left) and quantification of the ratios GFP frequencies expressing cells (right) from the adherent and nonadherent cell fractions; n = 4. (B) CYTH1 knockdown efficiency from CYTH1-sh1 and CYTH1-sh2 by quantitative polymerase chain reaction (n = 3). (C) CYTH1 knockdown efficiency from CYTH1-sh1 and CYTH1-sh2 by western blot (n = 1). (D) CD34+ cells transduced with SCR, β-galactosidase (BGAL), CYTH1-sh1, and CYTH1-sh2 were followed up with flow cytometry for maintenance of CD34 and GFP expression on day 4 after transduction (n = 4). (E) CD34+ cells transduced with SCR, BGAL, CYTH1-sh1, and CYTH1-sh2 were followed up with flow cytometry for maintenance of CD34 and GFP expression on day 3 or 4 and 7 or 8 after transduction (n = 5). (F) Nontransduced (mock), SCR, BGAL, CYTH1-sh1, and CYTH1-sh2 transduced CD34+ cells were assessed for cell cycle status (n = 2). (G) Average attachment area of transduced CD34+ cells plated onto RN and ICAM1-covered surface analyzed with IRM (n = 3). (H) Attachment dynamics (difference between maximum and minimum attachment area over time) analyzed with IRM (n = 3). (I) Representative photos from spinning disc confocal and total internal reflection fluorescence (TIRF) microscope of SCR and CYTH1-sh1 transduced CD34+ cells plated on RN (left) and summary of cell area analysis (n = 2) (right). (J) Representative FACS analysis of active ITGβ1 cell-surface expression in unstimulated (UNS) and PMA-stimulated (PMA) CD34+ cells transduced with SCR and CYTH1-sh1 (i). Table on the right summarizes the data from this experiment. “FMO” row depicts fluorescence minus 1 (FMO) control. A summary of 4 experiments on ITGβ1 activation including the representative experiment is shown on the right (ii). (K) Activation of Rap1 was analyzed in PMA-stimulated SCR and CYTH1-sh1 transduced cells. CYTH1-sh1, CYTH1-shRNA1; CYTH1-sh2, CYTH1-shRNA2; MFI, mean fluorescence intensity; PD, pull down; TL, total lysate.
CYTH1 regulates adhesion of human HSPCs through integrins. (A) CD34+ cells were transduced with CYTH1 shRNAs from a GFP-expressing vector and subjected to the adhesion assay. Representative FACS histograms showing GFP levels in adherent and nonadherent cells (left) and quantification of the ratios GFP frequencies expressing cells (right) from the adherent and nonadherent cell fractions; n = 4. (B) CYTH1 knockdown efficiency from CYTH1-sh1 and CYTH1-sh2 by quantitative polymerase chain reaction (n = 3). (C) CYTH1 knockdown efficiency from CYTH1-sh1 and CYTH1-sh2 by western blot (n = 1). (D) CD34+ cells transduced with SCR, β-galactosidase (BGAL), CYTH1-sh1, and CYTH1-sh2 were followed up with flow cytometry for maintenance of CD34 and GFP expression on day 4 after transduction (n = 4). (E) CD34+ cells transduced with SCR, BGAL, CYTH1-sh1, and CYTH1-sh2 were followed up with flow cytometry for maintenance of CD34 and GFP expression on day 3 or 4 and 7 or 8 after transduction (n = 5). (F) Nontransduced (mock), SCR, BGAL, CYTH1-sh1, and CYTH1-sh2 transduced CD34+ cells were assessed for cell cycle status (n = 2). (G) Average attachment area of transduced CD34+ cells plated onto RN and ICAM1-covered surface analyzed with IRM (n = 3). (H) Attachment dynamics (difference between maximum and minimum attachment area over time) analyzed with IRM (n = 3). (I) Representative photos from spinning disc confocal and total internal reflection fluorescence (TIRF) microscope of SCR and CYTH1-sh1 transduced CD34+ cells plated on RN (left) and summary of cell area analysis (n = 2) (right). (J) Representative FACS analysis of active ITGβ1 cell-surface expression in unstimulated (UNS) and PMA-stimulated (PMA) CD34+ cells transduced with SCR and CYTH1-sh1 (i). Table on the right summarizes the data from this experiment. “FMO” row depicts fluorescence minus 1 (FMO) control. A summary of 4 experiments on ITGβ1 activation including the representative experiment is shown on the right (ii). (K) Activation of Rap1 was analyzed in PMA-stimulated SCR and CYTH1-sh1 transduced cells. CYTH1-sh1, CYTH1-shRNA1; CYTH1-sh2, CYTH1-shRNA2; MFI, mean fluorescence intensity; PD, pull down; TL, total lysate.
CYTH1 regulates adhesion of human HSPCs through integrins
CYTH1 is a member of a family of guanine nucleotide exchange factors and has emerged as an important regulator of signal transduction in several contexts. CYTH1 has been linked to activation of ITGβ2 in a complex with the αL chain, forming the functional integrin dimer lymphocyte function-associated antigen 1 (LFA-1)19 Although integrins have a well-established role in HSPC adhesion and engraftment, it is only recently that mechanisms behind their regulation have begun to be uncovered.20,21 We therefore asked whether the effect of CYTH1 on cell adhesion in HSPCs was specifically mediated by integrins.
First, we functionally assessed the connection between CYTH1 and integrin-dependent adhesion. We monitored the cell adhesive activity on surfaces covered with either retronectin (RN) as a major ligand for ITGβ1 or intercellular adhesion molecule 1 (ICAM1), as a major ligand for ITGβ2. Interference reflection microscopy (IRM) was used to visualize the exact area of cell attachment and its changes in time. Overall, CYTH1-deficient CD34+ cells showed a significantly reduced attachment area on both substrates compared with control cells (Figure 2G). Additionally, the attachment was highly unstable in the CYTH1-deficient cells, as determined by the relative difference between the maximal and minimal cell attachment area over a 3-minute recording time (attachment dynamics) (Figure 2H; supplemental Videos 1-4). To investigate whether global changes in cell morphology could have contributed to the reduced attachment area, we stained cells attaching to a RN-covered surface with phalloidin to visualize the entire cell area through the actin network. Consistent with the IRM data, we found that the attachment area visualized by total internal reflection fluorescence microscopy was significantly decreased in cells with CYTH1 knockdown. However, the maximal whole cell-surface area, as determined by spinning disc confocal microscopy, was unchanged, suggesting that global changes in cell morphology did not contribute to the reduced attachment area (Figure 2I).
Next, we assessed whether CYTH1 deficiency directly influenced the activation status of integrins in CD34+ cells, reasoning that the active forms of integrin are mediating adhesion.22 The integrin inside-out activation pathway can be triggered with phorbol myristate acetate (PMA), a commonly used potent protein kinase C activator.23 Without PMA stimulation, we detected basal levels of activated ITGβ1 similar between CYTH1-deficient and control cells, whereas upon PMA stimulation, the CYTH1-deficient cells showed a significantly lower degree of ITGβ1 activation (Figure 2J). As a complementary approach, we measured activation of Rap1 as one of the major components of the integrin activation machinery.24 We observed less activation of Rap1 in CYTH1-deficient CD34+ cells compared with control cells (Figure 2K), indicating that CYTH1 knockdown abrogates integrin activation upon PMA-triggered stimulation. Together with the functional integrin substrate studies, these findings suggest that CYTH1 regulates human HSPC adhesion as a mediator of integrin activation.
CYTH1 deficiency impairs homing and long-term engraftment of human CD34+ cells in NSG mice
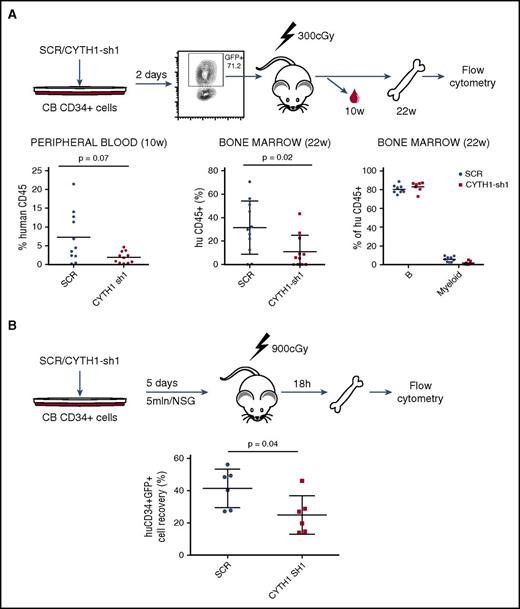
Having identified a critical role of CYTH1 for human HSPC adhesion to MSCs and integrin substrates in vitro, we next sought to determine the relevance of these findings for homing and engraftment in vivo (Figure 3A). To this end, we sorted control SCR and CYTH1-shRNA1 transduced CD34+ cells and transplanted into sublethally irradiated NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice and assessed the cells engraftment capacity (Figure 3A). Human reconstitution measured in peripheral blood at 10 weeks after transplantation showed a reduced engraftment capacity of cells with CYTH1 knockdown (Figure 3A, left graph). A similar decrease was seen in the bone marrow 22 weeks after transplantation (Figure 3A, middle graph) indicating that the CYTH1-deficient cells had an early engraftment defect but otherwise were functional long-term in vivo (Figure 3A, right graph). This is analogous to the phenotype seen in integrin β1 conditional knockout mice, which show normal steady-state hematopoiesis but severe HSC engraftment defects following transplantation.14,15
CYTH1 knockdown decreases long-term engraftment of CD34+ cells in NSG mice and impairs homing to bone marrow. (A) Outline for the in vivo assessment of CD34+ cells long-term engraftment capacity (upper). Analysis of human chimerism in mice transplanted with SCR and CYTH1-sh1 transduced cells (lower). Data accumulated from 2 independent experiments. Lineage distribution is shown only for recipients with at least 1% of human engraftment. (B) Outline for the assessment of CD34+ cells’ homing capacity. Analysis of human chimerism 18 hours following intravenous injection of transduced cells. Data accumulated from 2 independent experiments.
CYTH1 knockdown decreases long-term engraftment of CD34+ cells in NSG mice and impairs homing to bone marrow. (A) Outline for the in vivo assessment of CD34+ cells long-term engraftment capacity (upper). Analysis of human chimerism in mice transplanted with SCR and CYTH1-sh1 transduced cells (lower). Data accumulated from 2 independent experiments. Lineage distribution is shown only for recipients with at least 1% of human engraftment. (B) Outline for the assessment of CD34+ cells’ homing capacity. Analysis of human chimerism 18 hours following intravenous injection of transduced cells. Data accumulated from 2 independent experiments.
To further explore the engraftment defect, we assessed the bone marrow–homing capacity of CYTH1-deficient HSPCs. Transduced CD34+ cells were injected intravenously into lethally irradiated NSG recipients. Analysis of human cells in bone marrow by flow cytometry 18 hours after transplantation showed a significantly decreased recovery of CD34+GFP+ cells transduced with CYTH1 shRNA (Figure 3B). Therefore, the decreased long-term engraftment of CYTH1-deficient HSCs is, at least partly, attributable to a defect in the initial homing to bone marrow.
CYTH1 knockdown affects CD34+CD38− cell mobility and localization within the bone marrow space
The homing assay described previously reflects overall extravasation of transplanted cells into the marrow space, while successful engraftment also depends on proper migration and lodgment into a supportive microenvironment. As the short-term and long-term engraftment defect appeared more severe than what could be reflected by the homing experiment, we studied in more detail whether CYTH1 knockdown also disturbs the final lodgment process. To this end, we took advantage of advanced intravital microscopy developed recently in the particular context of human HSPC homing and lodgment within the niche.9 Recently, we showed that transplanted CD34+CD38− cells decrease their motility within the marrow space and acquire fixed positions close to bone surface and endothelium 4 days after transplantation, indicating a final niche lodgment.9 Consequently, we chose this time point for intravital imaging to study how CYTH1 deficiency affects the niche lodgment (outlined in Figure 4A).
CYTH1 knockdown affects CD34+CD38− cell mobility and localization within the marrow space. (A) Overview of intravital imaging to visualize lodgment of SCR and CYTH1-sh1 transduced CD34+CD38− cells. On the right, representative stills from intravital imaging of CD34+CD38− cells transduced with SCR (left) or CYTH1-sh1 (right). Green (carboxyfluorescein diacetate succinimidyl ester [CFSE]), cell; cyan (second harmonic signal), bone; magenta (Quantum Dots −655), endothelium. The scale bar represents 50 μm. (B) Displacement of the cells in calvaria bone (n = 3). (C) Migration speed of the cells in calvaria bone (n = 3). (D) Sphericity of the cells migrating within calvaria bone (n = 3). (E) Cell distance from the bone surface and endothelium (EC) (n = 3).
CYTH1 knockdown affects CD34+CD38− cell mobility and localization within the marrow space. (A) Overview of intravital imaging to visualize lodgment of SCR and CYTH1-sh1 transduced CD34+CD38− cells. On the right, representative stills from intravital imaging of CD34+CD38− cells transduced with SCR (left) or CYTH1-sh1 (right). Green (carboxyfluorescein diacetate succinimidyl ester [CFSE]), cell; cyan (second harmonic signal), bone; magenta (Quantum Dots −655), endothelium. The scale bar represents 50 μm. (B) Displacement of the cells in calvaria bone (n = 3). (C) Migration speed of the cells in calvaria bone (n = 3). (D) Sphericity of the cells migrating within calvaria bone (n = 3). (E) Cell distance from the bone surface and endothelium (EC) (n = 3).
Strikingly, CD34+CD38− cells transduced with CYTH1 shRNA displayed a significantly higher speed and displacement on day 4 in comparison with control cells (Figure 4B-C; supplemental Videos 5-6). The cells showed a less spherical shape than controls (Figure 4D), which reflects increased polarization and therefore, higher motility. Moreover, the distance to the bone surface, but not to endothelium, was significantly increased for CYTH1-deficient cells, indicating a localization defect specifically toward the endosteal niche (Figure 4A and 4E, right). Overall, the intravital imaging showed that CYTH1 deficiency profoundly affects CD34+CD38− cell mobility and impairs proper lodgment into the niche.
Discussion
Here, we report on the identification of CYTH1 as a critical regulator of human HSPC adhesion and engraftment. Collectively, our results demonstrate a critical and previously unknown role of CYTH1 in regulation of homing and lodgment of human HSPCs. Both these processes are crucially dependent on integrins and our results clearly point to a role of CYTH1 in mediating integrin functions in this context. CYTH1 thus provides a new intracellular target to possibly influence the localization of HSCs in both experimental and therapeutic settings.
In the first part of this work, we established a strategy to broadly screen for modifiers of cell adhesion in primary human HSPCs using pooled lentiviral shRNA libraries. When developing the adhesion assay, our aim was to mimic in vitro, as closely as possible, the cell-cell interactions that occur during the engraftment process of HSPCs in the bone marrow. Overall, the platform reliably detected both known and previously unknown regulators and provides a foundation for larger unbiased screens in the future. Together with other recent studies from our laboratory aimed at identifying modifiers of HSC renewal and expansion, this work illustrates how RNAi screening can be employed as a useful tool to directly target primary human hematopoietic cells and address fundamental questions related to HSC regulation and transplantation.7,8,25
Next, CYTH1, as a promising hit from the screen, was assessed for its role in human HSPC adhesion and engraftment. CYTH1 belongs to a family of cytohesins, which are small guanosine triphosphate exchange molecules involved in diverse cellular processes. Initially, CYTH1 was identified in a protein-protein interaction screen and found to interact specifically with the cytoplasmic part of the integrin β2 chain of LFA-1 and thereby to promote integrin binding to ICAM1.19 Further studies have extended the role of cytohesins in regulating integrin functions to other cell types such as neutrophils and dendritic cells, and the precise adhesion-mediating effect of CYTH1 has been found to be highly context and cell-type dependent.26-28 Our findings now extend the role of CYTH1 to HSPCs in addition to mature blood cells.
Although the previous studies of CYTH1 in mature blood cell had mainly shown a functional association with integrin β2,27 several independent findings in our study suggest that integrin β1 functions are also directly regulated by CYTH1, at least in HSPCs. First, we show in vitro that the adhesive interactions with the integrin β1 ligand RN are severely impaired by CYTH1 knockdown. Secondly, we show that integrin β1 activation by PMA stimulation is reduced in CYTH1-deficient cells. Finally, the in vivo engraftment defect seen upon CYTH1 knockdown is highly compatible with the phenotype of integrin β1 knockout mice where bone marrow homing,15 but not steady-state hematopoiesis, is impaired.14 By contrast, loss of integrin β2 in mice does not impair homing,1 indicating that the in vivo findings are mainly mediated by a disruption of the integrin β1 axis. In vitro, however, it is clear from our IRM experiments that CYTH1 acts as a regulator of both integrin β1 and integrin β2 mediated functions in HSCPs, as visualized by adhesion to RN and ICAM1 respectively. Taken together, we conclude that the function of CYTH1 in HSPCs extends beyond its established role of mediating integrin β2 activity and includes regulation of β1 responses as well, with the latter having the strongest impact on in vivo engraftment. Our findings thus demonstrate a dominant role of CYTH1 in mediating HSPC adhesion via integrins, but it cannot be excluded that it mediates adhesion to nonintegrin substrates as well or that it has a role in other adhesion-associated pathways.
Engraftment of intravenously injected HSCs in the bone marrow is a multistep process that can be subdivided into at least 3 distinct phases.29 The first step, homing, involves transendothelial migration from the blood stream into the bone marrow space. The second step is lodgment, which reflects the selective migration of HSCs within the marrow and their settlement in specific niches. Finally, the last step is the onset of cell proliferation and initiation of hematopoiesis at a given site, which is a reflection of the cells ability to respond to growth and differentiation signals from the niche but not dependent on cell migration. To understand the role of CYTH1 for HSPC function in vivo, we modeled all 3 aspects of the engraftment process in NSG mice, using homing assays (transendothelial migration), intravital microscopy (lodgment), and long-term engraftment assays. Based on these assays, we propose a model where CYTH1 is involved in the initial stages of the engraftment process mediating extravasation and homing to the bone marrow with the most prominent effect on migration and settlement within the marrow space. Consistent with in vitro IRM data, our in vivo imaging indicated a lack of durable adhesive interactions between CYTH1-deficient cells and the niche microenvironment, as the cells were more motile and showed a high degree of displacement. Furthermore, we conclude that CYTH1 is required for efficient niche lodgment especially in areas close to the bone surface, which typically are associated with HSC supportive properties.30 The long-term engraftment studies, on the other hand, suggested that the portion of CYTH1-deficient cells, which had been able to engage within a supportive niche, were in fact maintained normally and showed an intact functional potential. This is consistent with the in vitro assays where adhesion was severely impaired in HSPCs, yet the cells proliferated normally during in vitro expansion cultures. It is thus clear that CYTH1 is essential for normal adhesion capacity both in in vitro and in vivo, whereas it appears to be dispensable for normal proliferation and differentiation potential.
The identification of CYTH1 as an essential regulator of homing and engraftment in human HSPCs opens up several new areas for investigation. One important task will be to further define the molecular basis for its role in integrin activation. Work in this direction may lead not only to a better understanding HSPC adhesion and the engraftment process but possibly also to new strategies that directly can influence the localization of either normal or malignant hematopoietic cells.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE78216).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ann-Margreth Carlsson, Beata Lindqvist, and Ineke de Jong for shRNA cloning and lentivirus preparation.
This work was supported by grants from the Swedish Research Council, the Swedish Cancer Foundation, the Swedish Paediatric Cancer Foundation, and the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant 648894) (J.L.). J.R. was supported by a Marie Curie Intra-European Fellowship (FP7, 255451). The work was further supported by the HematoLinné and StemTherapy programs at Lund University. K.F. was supported by the Francis Crick Institute fellowship; D.B., by the Francis Crick Institute core fund; L.S., by the Swedish Medical Council; and K.P., by Anna-Greta Crafoords postdoctoral fellowship.
Authorship
Contribution: J.R. designed and performed experiments, analyzed data, and wrote the manuscript; K.F. and K.P. designed and performed experiments, analyzed data and edited the manuscript; M.S.T., N.M., and K.K. performed experiments; T.T., A.K., and Å.B. performed deep sequencing and analyzed the data; L.S. and D.B. designed and supervised experiments and edited the manuscript; and J.L. planned the study, designed and supervised experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonas Larsson, Molecular Medicine and Gene Therapy, BMC A12, 221 84, Lund, Sweden; e-mail: jonas.larsson@med.lu.se.
References
Author notes
K.F. and K.P. contributed equally to this study.

![Figure 4. CYTH1 knockdown affects CD34+CD38− cell mobility and localization within the marrow space. (A) Overview of intravital imaging to visualize lodgment of SCR and CYTH1-sh1 transduced CD34+CD38− cells. On the right, representative stills from intravital imaging of CD34+CD38− cells transduced with SCR (left) or CYTH1-sh1 (right). Green (carboxyfluorescein diacetate succinimidyl ester [CFSE]), cell; cyan (second harmonic signal), bone; magenta (Quantum Dots −655), endothelium. The scale bar represents 50 μm. (B) Displacement of the cells in calvaria bone (n = 3). (C) Migration speed of the cells in calvaria bone (n = 3). (D) Sphericity of the cells migrating within calvaria bone (n = 3). (E) Cell distance from the bone surface and endothelium (EC) (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/8/10.1182_blood-2016-06-720649/4/m_blood720649f4.jpeg?Expires=1765894072&Signature=BJjAiiEWE6Lo5Cro0pk3Jyaip5yXkmwFiypDWviUNxyQvYR2BINh0cYvS1EzA-ezHlT~EAuY1YilNoh0dC2GmQYQv~R2C6QsoWI~74l~yQgR5BCgtfDdq2ZE5QtzTOQiPftGvI0i50rtR~6S0R3X58jFySmO2v59SqkRgjMOcfAh04NJRbd-Ex~HCg9EqLB9vnRYsriYH9SU2RIisUMHfvGHyTz7aTcy033mhEJeqTTObZ42SpU2oeLSnMPCIExoViVg-5ZD3Xn8mLNLzDixa6GEeYRckYbDqDBj5Fr7pfflhTvZ~Tqfx~N2V-k7CohlrUPDCPG1ZjBReSDIKaVYxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal