To the editor:
The risk for venous thromboembolism (VTE) is increased significantly in hospitalized patients, which can lead to morbidity and, rarely, mortality.1,2 Thromboprophylaxis with low-dose anticoagulation for up to 14 days during hospitalization has been shown to decrease the incidence of hospital-related VTE, with meta-analysis confirming decreased rates of asymptomatic, symptomatic, and fatal VTE, but failing to find a reduction in mortality.3-6 Despite the use of standard-course thromboprophylaxis, symptomatic VTE does rarely occur in medical patients postdischarge,7 prompting the evaluation of extended thromboprophylaxis for up to 28 days.8 Extended thromboprophylaxis has proven effective in several cohorts of surgical patients; however, there are limited data in medical patients who have more heterogeneity, and thus different rates and risk factors for VTE.9,10 The initial study investigating extended prophylaxis in medical patients, the EXCLAIM study, randomly assigned patients to 10 vs 28 days of prophylactic-dose low-molecular-weight heparin (LMWH). Although EXCLAIM found extended thromboprophylaxis beneficial in certain high-risk subsets, it also noted a significant increase in major bleeding.11 At this time, professional guidelines recommend against extended prophylaxis for hospitalized medical patients.12
Recently, multiple direct oral anticoagulants (DOACs) have been approved and are now recommended as first-line therapy for several indications because of their favorable efficacy and bleeding rates.13 Several randomized trials have compared extended DOAC thromboprophylaxis vs standard-course LMWH prophylaxis in acutely ill medical patients. Although DOACs offer a convenient means to prevent posthospitalization VTE, it remains unclear whether this intervention has a positive risk–benefit ratio in the overall population of acutely hospitalized medical patients. To better answer this, we performed the following systematic review and meta-analysis.
We performed a systematic literature search in Medline, using PUBMED, in an attempt to identify all randomized clinical trials comparing extended prophylaxis in hospitalized medical (nonsurgical) patients with any DOAC compared with standard-course prophylaxis. Papers were independently reviewed by 2 authors for relevance and inclusion. Exact search criteria are reported in supplemental Table 1, available on the Blood Web site. Data were extracted on study design and characteristics, total VTE, symptomatic VTE, total bleeding, and major bleeding. Relative risk (RR) was calculated with a corresponding 95% confidence interval (CI), using a Mantel-Haenszel random-effects model. Absolute risk differences and the number needed to treat or number needed to harm were generated, along with forest plots. All analysis was performed with Review Manager (version 5.3 The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Our search identified 338 publications, of which 313 were excluded on initial screening by title and abstract. Full texts were obtained of the remaining 25 studies, of which 22 were excluded for the following reasons: commentary articles (12), study protocol (1), review article (8), data unavailable (1). Three trials, enrolling 22 142 patients, were included.14-16 Apixaban, rivaroxaban, and betrixaban were each evaluated by 1 study. Using the overall populations from each study, 11 064 patients were randomly assigned to extended DOAC prophylaxis, whereas 11 078 were randomly assigned to standard-course LMWH prophylaxis.
Pooled analysis identified a significant decrease in the rates of total and symptomatic VTE in those who received extended DOAC prophylaxis. VTE (including screened asymptomatic VTE) occurred in 4.30% of the patients receiving extended-course DOACs and 5.61% of patients receiving standard-course LMWH (RR, 0.76; 95% CI, 0.67-0.87; P < .0001; I2 = 0%). The number of patients needed to treat to prevent 1 VTE was 76. Symptomatic VTE occurred in 1.11% of patients treated with extended-course DOACs and 1.68% of patients treated with standard-course LMWH. DOAC extended prophylaxis was associated with a significant reduction in symptomatic VTE (RR, 0.66; 95% CI, 0.51-0.86; P = .002; I2 = 0%). The absolute risk reduction was 0.57%, with a number needed to treat of 176 (Table 1).
Total and symptomatic venous thromboembolic events with extended DOAC prophylaxis vs standard enoxaparin prophylaxis

The pooled analysis also identified a significant increase in total and major bleeding with extended-course DOACs. Bleeding occurred in 4.82% of patients receiving extended-course DOACs compared with 3.16% of patients receiving standard-course LMWH. The analysis demonstrates a statistically relevant increase in bleeding (RR, 1.74; 95% CI, 1.05-2.90; P < .001; I2 = 92%), with an absolute risk difference of 1.67% and a number needed to harm of 60. Major bleeding occurred in 0.59% of patients treated with extended-course DOACs and 0.35% of patients treated with standard-course LMWH. DOAC extended prophylaxis was associated with a significantly increased rate of major bleeding (RR, 1.71; 95% CI, 1.07-2.75; P = .03; I2 = 23%). The absolute risk difference is 0.24%, with a number needed to harm of 417 (Table 2).
Total and major bleeding events with extended DOAC prophylaxis vs standard enoxaparin prophylaxis
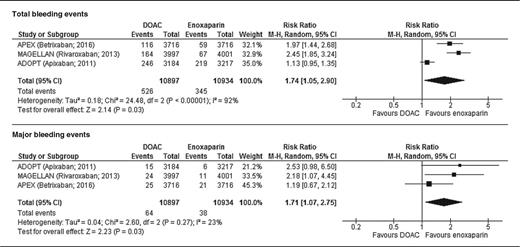
This analysis suggests that extended-course DOAC thromboprophylaxis in medical patients is associated with a significant decrease in total and symptomatic VTE compared with standard-course LMWH. The rate of symptomatic VTE in this population was low, however, as was the absolute risk reduction with extended prophylaxis. The benefit of VTE reduction found in those who received extended prophylaxis with a DOAC must be weighed against the significant increase in both total and major bleeding found in this analysis, suggesting against routine use of extended prophylaxis in the general population of hospitalized medical patients.
This analysis drew similar conclusions to the previously published study of standard vs extended enoxaparin, EXCLAIM, which suggested a VTE risk-reduction benefit does exist, but at the cost of significant bleeding.11 EXCLAIM found certain subsets of medical patients were more likely to benefit, including those with decreased mobility and older age.11 Future studies of extended thromboprophylaxis are likely to find a more favorable risk–benefit ratio by evaluating higher-risk populations, similar to analyses that have been performed in certain postsurgical populations.9,10 Indeed, an ongoing study evaluating extended thromboprophylaxis with rivaroxaban, MARINER, is selecting patients at high risk for VTE based on D-dimer and International Medical Prevention Registry on Venous Thromboembolism (IMPROVE VTE) risk score.17
A limitation of this study and its conclusions regarding extended prophylaxis is heterogeneity among the included DOACs.14-16 Efficacy and safety profiles likely vary between the drugs. Further, 1 agent, betrixaban, is not currently approved by the US Food and Drug Administration.14 Another barrier to interpretation of these results is the underlying heterogeneity of the included studies, most notably indicated by the high I2 for total clinically relevant bleeding. This may be a result of the heterogeneity of total clinically relevant bleeding among the standard-course enoxaparin control arms (ADOPT rate of 6.81%; MAGELLAN, 1.67%; APEX, 1.59%). Last, a major issue with the interpretation of this study and real-world application of these results is a poor understanding of the natural history of asymptomatic VTE and how its detection alters rates of symptomatic VTE and bleeding. Although APEX performed compression ultrasonography at thromboprophylaxis completion, ADOPT and MAGELLAN’s routine screening ultrasounds may have affected their outcome measures.14-16 Patients who developed asymptomatic VTE were omitted from further safety analysis and may have received further intervention.
Despite the former limitations, it is reassuring that results were generally uniform across the included studies.14-16 The overall data suggest that despite the relative convenience of extended prophylaxis with DOACs, they provide only a small risk reduction in VTE, at the cost of significantly increased bleeding. They therefore offer a less-than-ideal risk–benefit ratio and reconfirm the American College of Chest Physicians recommendations against extended-course thromboprophylaxis.12 Future studies looking at the utility of this intervention in high-risk populations of medical patients are underway and may find a more favorable prophylactic benefit in these enriched populations. The current evidence remains against the routine use of extended prophylaxis in unselected medical patients.
The online version of this article contains a data supplement.
Authorship
Contribution: D.L.T. contributed to study design, performed data extraction, analyzed the data, and critically revised the manuscript; J.Y.B. performed study selection, assessed study quality, and reviewed the manuscript; T.G.D. supervised the overall research and reviewed the manuscript; and J.J.S. designed the methods, performed study selection, assessed study quality, supervised the overall research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph J. Shatzel, Oregon Health and Science University Division of Hematology and Medical Oncology, Mail Code: L586, 3181 S.W. Sam Jackson Park Rd, Portland, OR 97239-3098; e-mail: shatzel@OHSU.edu.
