To the editor:
Somatic, gain-of-function mutations in STAT3 and STAT5 have been described in leukemias and lymphomas1-6 and, more recently, in CD3-CD4+ T cells from a patient with lymphocytic variant hypereosinophilic syndrome7 ; whereas germ line gain-of-function mutations in STAT1 and STAT3 are associated with early onset lymphoproliferative disease, autoimmunity, and increased susceptibility to infection.8-11 The difference in presentation between somatic and germ line STAT3 mutations likely reflects the cell types affected and the period in development from which the mutation was present. Study of these rare syndromes and associated molecular lesions in STAT proteins provides valuable insight into the pathogenesis of common immunological symptoms. We report 2 patients with a novel syndrome of nonclonal eosinophilia, atopic dermatitis, urticarial rash, and diarrhea associated with the identical, somatic, gain-of-function STAT5b mutation in a hematopoietic progenitor.
Patient A is a 3-year-old girl who presented at 4 months of age with annular migratory erythema and persistent urticaria (Figure 1A). Additional clinical manifestations included (1) atopic dermatitis with alopecia totalis by 18 months; (2) angioedema to avocado, almonds, and wasp sting; and (3) 1- to 2-day episodes of abdominal distension and diarrhea every 6 to 8 weeks beginning at age 18 months with development of dysphagia and odynophagia despite normal upper and lower gastrointestinal endoscopies. Infections were limited to pneumonia at age 1 year and a measles-like illness 10 days after receiving the measles, mumps, and rubella vaccine.
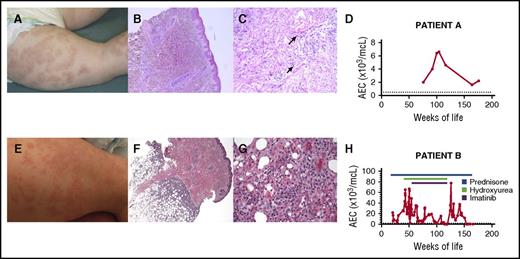
Clinical and laboratory findings in 2 children with a somatic gain-of-function mutation in STAT5b. The urticarial skin rash (A and E), underlying histological features (B, C, F, and G), and absolute eosinophil count (AEC) over time (D and H) are shown for both children. Photomicrographs show a perivascular lymphocytic infiltrate (B; original magnification ×5, hematoxylin and eosin stain) with edema and scattered eosinophils (C; original magnification ×20, hematoxylin and eosin stain) and a deep subcutaneous lymphohistiocytic infiltrate involving the lobules consistent with a lobular panniculitis (F; original magnification ×4, hematoxylin and eosin stain) with no evidence of rimming of the lymphocytes and scattered eosinophils at high power (G; original magnification ×40, hematoxylin and eosin stain). Arrows indicate representative eosinophils. The upper limit of the normal range for AEC is indicated by a dashed line.

Clinical and laboratory findings in 2 children with a somatic gain-of-function mutation in STAT5b. The urticarial skin rash (A and E), underlying histological features (B, C, F, and G), and absolute eosinophil count (AEC) over time (D and H) are shown for both children. Photomicrographs show a perivascular lymphocytic infiltrate (B; original magnification ×5, hematoxylin and eosin stain) with edema and scattered eosinophils (C; original magnification ×20, hematoxylin and eosin stain) and a deep subcutaneous lymphohistiocytic infiltrate involving the lobules consistent with a lobular panniculitis (F; original magnification ×4, hematoxylin and eosin stain) with no evidence of rimming of the lymphocytes and scattered eosinophils at high power (G; original magnification ×40, hematoxylin and eosin stain). Arrows indicate representative eosinophils. The upper limit of the normal range for AEC is indicated by a dashed line.
Her family history was significant for an older sibling with celiac disease, and a strong autoimmune predilection in multiple paternal relatives including thyroiditis, hyperparathyroidism, hypoparathyroidism, vitiligo, arthritis, systemic lupus erythematosis, and inflammatory bowel disease. The mother had 5 miscarriages in early pregnancy. There was no history of consanguinity.
Aside from the dermatological findings, the physical examination was normal. She was thriving and developmentally appropriate. Biopsy of the annular migratory rash demonstrated superficial and deep perivascular lymphohistiocytic infiltrate with edema, eosinophils, and karyorrhexis consistent with urticarial vasculitis (Figure 1B-C).
Immunological investigations demonstrated normal serum immunoglobulin levels (including immunoglobulin E [IgE]), antibody responses to childhood immunizations, and lymphocyte immunophenotyping with normal numbers of naïve and regulatory T cells. An autoantibody panel was unrevealing. Antibodies to C1q were not detected. The only consistent laboratory abnormality was peripheral eosinophilia, which peaked at 6600/μL at the age of 26 months (Figure 1C).
Patient B, a 2-year-old girl, was more severely affected. She developed a rash and feeding difficulties shortly after birth that improved transiently with elemental diet. At ∼5 months of age, she developed a generalized urticarial rash sparing her face with marked eosinophilia (21 000/μL), requiring high-dose steroid therapy (Figure 1D). At age 2, she developed subcutaneous nodules. Biopsy revealed lobular panniculitis with a perivascular infiltrate of atypical T cells (Figures 1E-F). Additional clinical manifestations included (1) atopic dermatitis with markedly elevated serum IgE levels; (2) severe bouts of bronchiolitis, occurring approximately every 2 months beginning at age 1.5 months, often associated with worsening eosinophilia; (3) episodic loose, nonbloody stools with endoscopic biopsies showing marked eosinophilic infiltration in all segments of the gastrointestinal tract; (4) failure to thrive (less than first percentile for height, 15.4th for weight); and (5) delayed speech. She had respiratory syncytial virus at age 4 months, but no other infectious manifestations.
There was no family history of autoimmunity, immunodeficiency, hematologic malignancy, miscarriages, unexplained deaths, or consanguinity.
Physical examination was notable only for cushingoid appearance and dozens of small (2-3 mm), mobile, hard subcutaneous nodules in a generalized distribution. Routine laboratory testing revealed eosinophilia (39 190/μL) despite 0.5 mg/kg prednisolone daily, lymphocytosis (13 290/μL), and markedly elevated serum IgE (>6000 mg/mL). Testing for FIP1L1-PDGFRA, JAK2 V617F, and other mutations known to be associated with myeloid neoplasms was negative. There was no evidence of clonal IG or TRG gene rearrangement. Several bone marrow biopsies showed a 90% to 95% cellular bone marrow with myeloid hyperplasia and increased eosinophil precursors. Cytogenetics were normal. Peripheral eosinophilia was a constant feature, peaking as high as 77 000/µL despite multiple therapies (Figure 1F).
Because of severe, worsening symptoms unresponsive to standard therapies, she underwent umbilical cord stem cell transplant. Her course was complicated by mucositis, rhinovirus, human herpesvirus 6 reactivation, and persistent cough. At 31 days posttransplant, she died of respiratory insufficiency. Autopsy revealed diffuse alveolar hemorrhage.
The patients were enrolled on an institutional review board–approved protocol and provided informed consent. Because of the unique, but remarkably similar, presentations in the 2 children, next-generation sequencing was performed on genomic DNA from peripheral blood mononuclear cells (PBMCs) using a panel of >300 immune-related genes. The patients shared only 1 rare or novel variant, STAT5B N642H, a single nucleotide variant described to date only as a somatic and clearly gain-of-function mutation in patients with T-cell neoplasms.1,2,4-6 Neither set of parents carried the mutation, and nail clippings from patient A were negative, suggesting somatic, rather than germ line, origin. Cell lineages were sorted from peripheral blood by flow cytometry, and high-throughput sequencing was performed to determine the relative mosaicism in several cell types. In both patients, T cells had nearly 50% variant sequence, as did patient B’s eosinophils (eosinophils were not available for patient A), and B cells and CD11c+ cells showed lower allelic frequencies of 10% to 20% (Figure 2A). The lower level of mosaicism in these lineages could be because of fewer progenitor cells bearing the mutation or a lack of competitive advantage afforded by the mutation, as is the case for B cells and natural killer cells in a mouse model of STAT5b gain of function.12
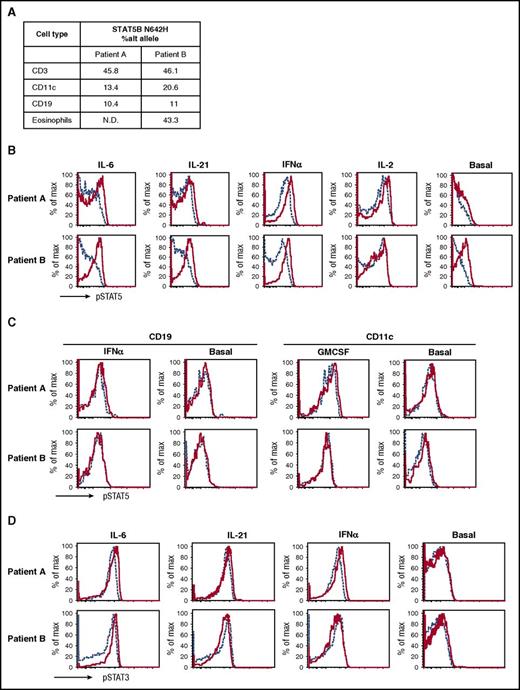
Chimerism and gain of function of the N642H mutation. (A) The allelic frequencies of the N642H mutation in the different cell types tested. The individual fluorescence-activated cell sorter–sorted cell population was sequenced with digital droplet polymerase chain reaction, and allelic frequencies were calculated as described in Milner et al.10 (B) Elevated basal and activated phosphorylated STAT5 (pSTAT5) expression in patients’ CD4+ T cells. pSTAT5 basal and stimulated by indicated cytokines in patients (solid) compared with different healthy controls (dashed) (top). (C) Normal basal and activated pSTAT5 in CD11c+ dendritic cells and CD19+ B cells. Patients (solid red) and healthy control (dashed blue) PBMCs were stimulated by interferon α (IFN-α) and granulocyte-macrophage colony-stimulating factor (GM-CSF), and pSTAT5 activation was examined in CD19+ and CD11c+ cells, respectively. (D) Normal pSTAT3 activation in CD4+ T cells stimulated with indicated cytokines in patients (solid) compared with healthy control (dashed). Patient A (top); patient B (bottom).

Chimerism and gain of function of the N642H mutation. (A) The allelic frequencies of the N642H mutation in the different cell types tested. The individual fluorescence-activated cell sorter–sorted cell population was sequenced with digital droplet polymerase chain reaction, and allelic frequencies were calculated as described in Milner et al.10 (B) Elevated basal and activated phosphorylated STAT5 (pSTAT5) expression in patients’ CD4+ T cells. pSTAT5 basal and stimulated by indicated cytokines in patients (solid) compared with different healthy controls (dashed) (top). (C) Normal basal and activated pSTAT5 in CD11c+ dendritic cells and CD19+ B cells. Patients (solid red) and healthy control (dashed blue) PBMCs were stimulated by interferon α (IFN-α) and granulocyte-macrophage colony-stimulating factor (GM-CSF), and pSTAT5 activation was examined in CD19+ and CD11c+ cells, respectively. (D) Normal pSTAT3 activation in CD4+ T cells stimulated with indicated cytokines in patients (solid) compared with healthy control (dashed). Patient A (top); patient B (bottom).
Functional studies of the patients’ T cells confirmed a marked increase in STAT5B responsiveness to an array of cytokines and minimally increased baseline activity compared with cells from their parents and unrelated controls (Figure 2B). B-cell and CD11c responses were not increased, consistent with the lower frequency of mutant-expressing cells in those populations (Figure 2C). STAT3 responses were unaffected (Figure 2D). Intracellular cytokine staining of stimulated CD4+ T cells revealed increased Th2 cytokines and a paucity of the Th1 cytokine IFN-γ (supplemental Figure 1A, available on the Blood Web site). Interleukin 17 (IL-17) production and CXCR5+ T follicular helper cells, whose differentiation is potently inhibited by IL-2/STAT5B, were reduced in both patients (supplemental Figure 1B-C). Foxp3+ regulatory T cells, whose development and activation are dependent on IL-2, were normal in patient A, but increased, accompanied by abnormal Th2 cytokine coproduction, in patient B (supplemental Figure 1D).
In mouse models of increased STAT5B activity, enhanced IL-3 responsiveness and thymic stromal lymphopoietin production lead to severe mast cell–driven, atopic dermatitis-like inflammation.13 Whereas the presence of the identical gain-of-function STAT5b mutation in multiple hematopoietic cell types likely explains many of the striking common symptoms observed in these 2 patients, some of their clinical manifestations may be attributable to early environmental exposures, the precise developmental state of the original progenitor cell with the somatic mutation, and/or germ line modifiers. The alopecia in patient A, for instance, could reflect a paternally inherited genetic predilection to autoimmunity that was not identified in our gene resequencing panel. The diverse lineages in which the mutation was found suggest an early hematopoietic progenitor that underwent somatic mutation, creating a phenotype distinct from the previously reported neoplastic phenotype. These patients presented with what appeared to be a congenital syndrome of significant immune dysregulation, the type of which is typically because of germ-line defects, raising the possibility that patients such as these could be found in a variety of ages and settings. Furthermore, identification of the molecular cause of such presentations suggests the potential for targeted therapeutic intervention.
The online version of this article contains a data supplement.
Authorship
Acknowledgment: This work was supported by the intramural program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Contribution: C.A.M. performed research and analyzed data; L.X., B.C., A.D., and A.F.F. performed research; S.H. performed research and analyzed data; G.K. and T.R.L. performed research; M.O. analyzed data; M.M. and G.O. performed research; S.P. analyzed data; S.D.R., J.N., and J.S. performed research and analyzed data; M.R. analyzed data; and A.D.K. and J.D.M. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amy D. Klion, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MD 20892; e-mail: aklion@niaid.nih.gov; and Joshua D. Milner, National Institute of Allergy and Infectious Diseases, 9000 Rockville Pike, NIH 10-CRC, Room 11N240, Bethesda, MD 20892; e-mail: jdmilner@niaid.nih.gov.
References
Author notes
C.A.M. and L.X. contributed equally to this study.
A.D.K. and J.D.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal