Key Points
In steroid-resistant acute GVHD 1-year survival without changing baseline therapy was not different after inolimomab vs ATG.
Using current care, the expected 1-year survival of these patients lies in the 55% range.
Abstract
Treatment of steroid-resistant acute graft-versus-host disease (GVHD) remains an unmet clinical need. Inolimomab, a monoclonal antibody to CD25, has shown encouraging results in phase 2 trials. This phase 3 randomized, open-label, multicenter trial compared inolimomab vs usual care in adult patients with steroid-refractory acute GVHD. Patients were randomly selected to receive treatment with inolimomab or usual care (the control group was treated with antithymocyte globulin [ATG]). The primary objective was to evaluate overall survival at 1 year without changing baseline allocated therapy. A total of 100 patients were randomly placed: 49 patients in the inolimomab arm and 51 patients in the ATG arm. The primary criteria were reached by 14 patients (28.5%) in the inolimomab and 11 patients (21.5%) in the ATG arms, with a hazard ratio of 0.874 (P = .28). With a minimum follow-up of 1 year, 26 (53%) and 31 (60%) patients died in the inolimomab and ATG arms, respectively. Adverse events were similar in the 2 arms, with fewer viral infections in the inolimomab arm compared with the ATG arm. The primary end point of this randomized phase 3 trial was not achieved. The lack of a statistically significant effect confirms the need for development of more effective treatments for acute GVHD. This trial is registered to https://www.clinicaltrialsregister.eu/ctr-search/search as EUDRACT 2007-005009-24.
Introduction
Despite prophylaxis with immunosuppressive therapy agents, nearly 50% of patients have acute graft-versus-host disease (aGVHD) after allogeneic hematopoietic cell transplantation.1-3 For decades, the first-line treatment of aGVHD remains the use of 1-2 mg/kg per day methylprednisolone or prednisone and, unfortunately, ∼30% of patients will not respond to initial treatment with steroids.4 However, despite many studies, no agents for treatment of glucocorticoid-resistant or refractory graft-versus-host disease (GVHD) have clearly emerged as a gold standard. Experts have recognized the lack of progress and recognized the absence of standard of care for secondary treatment of aGVHD.4 The American Society of Blood and Marrow Transplantation has developed recommendations for treatment of aGVHD based on a comprehensive and critical review of published reports.4
Most reports on the treatment of steroid-resistant (SR) aGvHD are retrospective, single-arm phase 2 studies. Comparison of results between these studies is complicated by the lack of standardized end points, and the small numbers of patients and survival rates do not support the choice of any specific agent.4 because 1-year survival has remained in the range of ∼30% for decades.1,5,6 Despite this unmet clinical need, it may be surprising that, to the best of our knowledge, only 2 randomized phase 2/3 studies have ever been performed in the setting of SR aGVHD.7,8 Both studies showed that neither early introduction of antithymocyte globulins (ATG) nor the substitution of ATG by an investigational drug improved the dismal outcome for these patients.
Inolimomab, a monoclonal antibody against α-chain of the interleukin-2 receptor (CD25) has been associated with encouraging response and short-term survival rates in the setting of SR aGVHD.5,9-14 Thus, a randomized, open-label trial comparing inolimomab with standard of care was designed. Rabbit ATG was chosen for all patients in the control arm based on previous reports4,15-17 and centers’ experiences. Here we report the results of this trial with 1-year minimum follow-up.
Patients and methods
The primary objective of this study was to demonstrate a higher therapy success rate with inolimomab treatment compared with usual care in patients presenting with SR aGVHD. Therapy success was defined as overall survival at 1 year without replacement of the baseline allocated treatment. Treatment of subsequent chronic GVHD, if any, was not considered as a treatment failure. The secondary objectives of this study are the overall response rate (complte response + partial response) at day 29, the effect of inolimomab on survival at intermediate periods, the incidence and consequence of chronic GVHD, infections and post-transplant lymphoproliferative disease and relapse of hematologic malignancy, total amount of steroids used duration of hospital stay, and the safety profile of inolimomab. The dose and schedule of inolimomab and of ATG are detailed in Figure 1. All patients in the control arm received rabbit ATG (Genzyme, a Sanofi company). The prespecified statistical plan is provided in the supplemental Appendix, available on the Blood Web site.
Study design. (A) All patients in the control arm received rabbit ATG (Genzyme, a Sanofi company) at the same dose of 2.5 mg/kg for 4 consecutive days. Inolimomab was delivered IV at a dose of 0.3 mg/kg per day for the induction phase and 0.2 mg/kg per day for maintenance. (B) Study design for the inolimomab arm.
Study design. (A) All patients in the control arm received rabbit ATG (Genzyme, a Sanofi company) at the same dose of 2.5 mg/kg for 4 consecutive days. Inolimomab was delivered IV at a dose of 0.3 mg/kg per day for the induction phase and 0.2 mg/kg per day for maintenance. (B) Study design for the inolimomab arm.
Inclusion and exclusion criteria
Adult patients (≥18 years) who underwent a first allogeneic bone marrow or peripheral stem cell transplant (SCT) from human leukocyte antigen (HLA)-matched sibling donor or 10/10 and 9/10 HLA unrelated donor for treatment of hematologic malignancy were eligible. Patients received either a myeloablative or reduced-intensity conditioning regimen. Patients had to be in complete remission or in chronic phase or at least with stable disease (concerning chronic lymphocytic leukemia [CLL], high- and low-grade non-Hodgkin lymphoma, myeloma, and myelodysplastic syndrome) at the time of the hematopoietic SCT (HSCT). GVHD prophylaxis with short methotrexate and cyclosporine or tacrolimus or mycophenolate mofetil (MMF) and cyclosporine were eligible.
The study included patients with a first episode of grades II to IV aGVHD (according to the consensus scoring system)18 developing within 100 days after HSCT (thus excluding late acute GVHD). Patients must have already received methylprednisolone (MP) at a daily dose of 2 mg/kg as GVHD treatment and must have shown a resistance as defined by one of the following: GVHD progressing after ≥3 days of MP treatment, or GVHD persisting without improvement after 7 days of MP treatment. Patients were excluded from the study if one or more of the following statements were applicable: post–donor lymphocyte infusion GVHD; HLA-mismatched unrelated donor; transplantation other than for hematologic malignancy; cord blood transfusion; patients who received prophylactic regimens of GVHD with corticosteroids; patients on mechanical ventilatory support; progression of the malignancy at the time of inclusion; serum creatinine >30 mg/L; patients with vasopressor treatment; or uncontrolled infection(s) (ie, documented bacterial, parasitic, or fungal infection) within 72 hours before study entry despite adapted treatment. Neither continuation of antibiotics for a controlled infection nor prophylactic/empiric antibiotics warranted exclusion; pregnant or lactating females; any history of hypersensitivity/allergy to murine products and any other component of study drug; positive HIV serology; ECOG >3; aspartate aminotransferase or alanine aminotransferase >10× upper limits of normal; serum albumin ≤15 g/L; or minor patient and those incapable of giving informed consent.
Statistical analyses
This was an open-label, controlled, multicenter, randomized trial comparing inolimomab with standard of care. All centers agreed to use ATG as the control (Figure 1). The randomization was based on an a priori list of 150 binary numbers, with a block size of 2, without stratification. The significance of the effect of inolimomab compared with control has been assessed through Kaplan-Meier estimates and by using a proportional hazards CPH Cox survival regression analysis adjusted for baseline severity. A prespecified list of variables measuring the severity at baseline has been provided in this statistical analysis plan. The assessment of treatment effect has been tested at the 1-sided .05 type 1 level. Assuming use of proportional hazard regression, a target hazard ratio (HR) of 1.33, expected control success rate of 20% (thus, a difference as large as 15% favoring inolimomab vs control) would be detected with at least 75% power at 95% 2-sided confidence, when the sample size is >50 patients per group, by taking a reasonable assumption of correlation between baseline conditions and outcome of at least R = 0.7. Variables considered for adjustment were HLA mismatch; use of an unrelated donor; skin, gut, and liver severe involvement (stage 3 and 4); and remission status of the malignancy. With this model being considered as reference, at a second step we completed model I by model II in assessing the effect of other potential predictors. These variables were: age, number of identified viral infections, body mass index, sex, myeloablative treatment, and Karnofsky index at randomization. Missing data were imputed by variables calibration maximizing the correlations. For secondary end points, the Fine and Gray model was used both for univariate and multivariate analyses. Statistical analyses were conducted at a 0.05 2-sided significance, unless otherwise stated. R statistical package (R, v2.12.2) was used.
The protocol was approved by the institutional ethics review boards and the study was performed per the Declaration of Helsinki guidelines. This trial has been registered as EUDRACT 2007-005009-24.
Results
Forty-nine and 51 patients were randomly selected for the inolimomab and ATG arms, respectively. All patients were analyzed for safety and in the intention-to-treat analysis. Median time to onset of aGVHD was day 23 (range, 15-52). The second-line treatment was started 6 days after initial steroid treatment (range, 3-9) without significant difference between patients allocated to inolimomab or ATG. Nineteen patients (38.8%) and 17 patients (33.3%) completed the study at 1 year. Reason of failure was mainly death before 1 year (26 and 31 in the inolimomab and ATG arms, respectively; other causes were withdrawal by the investigator in 3 patients in the inolimomab and 2 in the ATG arms, mostly because of worsening of clinical condition).
Patient, transplant, and disease characteristics are summarized in Table 1. Many of the patients were transplanted for acute leukemia using peripheral blood stem cells. More than 60% have been transplanted from an unrelated donor and the conditioning regimen pre-transplant was myeloablative in ∼40%. None of the characteristics described in Table 1 was statistically different between patients assigned to inolimomab or the control arm. The GVHD grade at randomization was III (36 patients [36%] had stage 3 or 4 skin involvement; 51 [51%] had stage 3 or 4 gut disease; and 5 [5%] had stage 3 or 4 liver involvement, without imbalance between treatment arms). There was no imbalance per disease stage or grade between the 2 arms.
Patient, disease, and transplant characteristics according to treatment arm
| Characteristic . | Inolimomab (n = 49) . | ATG (n = 51) . | P . |
|---|---|---|---|
| Sex, n (%) | .555 | ||
| Male | 22 (44.9) | 26 (51) | |
| Female | 27 (55.1) | 25 (49) | |
| Age, mean (SD) | 46.2 (12.6) | 47.1 (12.96) | .727 |
| Disease | |||
| AML/MDS/MPD | 14/4/2 | 10 / 7 / 2 | |
| ALL | 7 | 6 | NS |
| CLL/lymphoma/myeloma | 4 / 8 / 4 | 6 / 10 / 7 | |
| Other | 8 | 3 | |
| CR at HSCT, n (%) | 26 (53.1) | 28 (54.9) | .905 |
| Source of cells, n (%) | |||
| Peripheral blood | 40 (81.6) | 39 (76.5) | .698 |
| Marrow | 9 (18.4) | 12 (23.5) | |
| GVHD prophylaxis, n (%) | |||
| CSA + MTX | 23 (46.9) | 21 (41.2) | .704 |
| CSA + MMF | 22 (44.8) | 19 (37.3) | .566 |
| ATG | 13 (26.5) | 11 (21.5) | .72 |
| Donor type, n (%) | |||
| Matched sibling | 15 (30.6) | 20 (39.2) | .489 |
| Matched UD (10/10 allelic) | 31 (63.3) | 30 (58.8) | .802 |
| 9/10 UD | 3 (6.1) | 1 (2) | .581 |
| Donor characteristic | |||
| Age, mean (SD) | 39.3 (12.5) | 37.0 (12.3) | .821 |
| Sex (M/F) | 31/18 | 37/14 | .435 |
| Conditioning regimen, n (%) | |||
| Myeloablative | 21 (42.9) | 19 (37.3) | .713 |
| Reduced intensity | 28 (57.1) | 32 (62.7) | .713 |
| Irradiation-based | 18 (36.7) | 14 (27.4) | .435 |
| Characteristic . | Inolimomab (n = 49) . | ATG (n = 51) . | P . |
|---|---|---|---|
| Sex, n (%) | .555 | ||
| Male | 22 (44.9) | 26 (51) | |
| Female | 27 (55.1) | 25 (49) | |
| Age, mean (SD) | 46.2 (12.6) | 47.1 (12.96) | .727 |
| Disease | |||
| AML/MDS/MPD | 14/4/2 | 10 / 7 / 2 | |
| ALL | 7 | 6 | NS |
| CLL/lymphoma/myeloma | 4 / 8 / 4 | 6 / 10 / 7 | |
| Other | 8 | 3 | |
| CR at HSCT, n (%) | 26 (53.1) | 28 (54.9) | .905 |
| Source of cells, n (%) | |||
| Peripheral blood | 40 (81.6) | 39 (76.5) | .698 |
| Marrow | 9 (18.4) | 12 (23.5) | |
| GVHD prophylaxis, n (%) | |||
| CSA + MTX | 23 (46.9) | 21 (41.2) | .704 |
| CSA + MMF | 22 (44.8) | 19 (37.3) | .566 |
| ATG | 13 (26.5) | 11 (21.5) | .72 |
| Donor type, n (%) | |||
| Matched sibling | 15 (30.6) | 20 (39.2) | .489 |
| Matched UD (10/10 allelic) | 31 (63.3) | 30 (58.8) | .802 |
| 9/10 UD | 3 (6.1) | 1 (2) | .581 |
| Donor characteristic | |||
| Age, mean (SD) | 39.3 (12.5) | 37.0 (12.3) | .821 |
| Sex (M/F) | 31/18 | 37/14 | .435 |
| Conditioning regimen, n (%) | |||
| Myeloablative | 21 (42.9) | 19 (37.3) | .713 |
| Reduced intensity | 28 (57.1) | 32 (62.7) | .713 |
| Irradiation-based | 18 (36.7) | 14 (27.4) | .435 |
ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; CSA, cyclosporine; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorders; MTX, methotrexate; SD, standard deviation; UD; unrelated donor.
Analyses of the primary end point
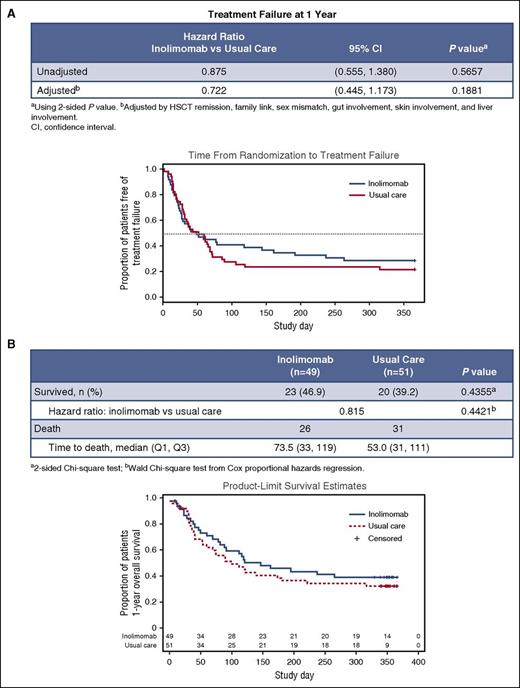
Forty-nine and 51 patients were allocated to receive inolimomab or ATG, respectively. All patients in both arms were analyzed for safety and in the intention-to-treat analyses. Premature death or withdrawals (before 1 year) were recorded in 30 and 34 patients in the inolimomab and ATG arms, respectively. This was mainly related to death before 1 year post-randomization (26 and 31 deaths after inolimomab and ATG, respectively). All surviving patients were followed for a minimum of 1 year after randomization unless death occurred. Twenty-three (46.9%) and 20 (39.2%) patients survived 1 year after being treated with either inolimomab or ATG, respectively. The time to treatment failure (defined as death or change of baseline treatment regimen) is shown in Figure 2A. No significant difference was found between the 2 treatment arms (inolimomab adjusted HR 0.7 (95% confidence interval [CI], 0.4-1.1; P = .56].
Main study endpoints. (A) Time from randomization to treatment failure (defined as death or change of baseline treatment regimen). (B) 1-year overall survival by treatment arm.
Main study endpoints. (A) Time from randomization to treatment failure (defined as death or change of baseline treatment regimen). (B) 1-year overall survival by treatment arm.
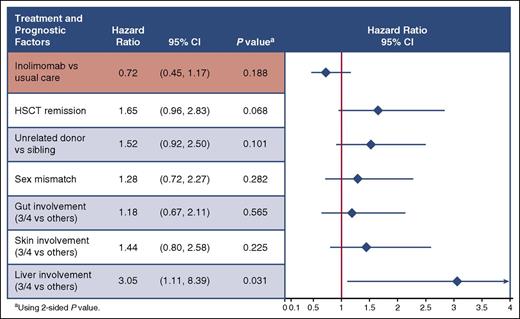
The 1-year survival estimates depicted in Figure 2B were not statistically different between the 2 treatment arms (in the 45% range 1-year survival rate). The difference in the estimate between survival and time to treatment failure (10%-15%) merely reflects treatment failure in both arms (that do not differ significantly). Finally as stated in the method section, prespecified Cox regression analyses were performed to take into account confounding factors. As depicted in Figure 3, even after adjustment, inolimomab was not associated with improved outcome, compared with ATG (HR = 0.7 [95% CI, 0.4-1.1]; P = .18). Among other tested factors (see Material and methods) only liver stage at randomization was associated with a significant worse outcome (HR = 3.0 [95% CI, 1.1-8.4]; P = .03). Thus, although associated with a slight but nonsignificant survival advantage, inolimomab failed to reach the specified composite primary end point. Finally, 80.2% and 75.4% (P > .5) of patients alive at 1 year had evidence of severe chronic GVHD after ATG and inolimomab treatment, respectively.
Primary end point: 1-year treatment failure using Cox regression analysis.
We took advantage of the randomized control setting of the trial to gain insight into prognostic factors that could influence outcome in this dismal setting. Using Cox proportional to analyze 1-year survival, patients transplanted from an unrelated donor (HR = 2.2 [95% CI, 1.2-3.8]; P = .006) did worse, as did patients older than 60 years of age (HR = 2.5 [95% CI, 1.3-4.9]; P = .006).
The analysis of cause of death revealed, as expected, that GVHD-related death and/or infection accounted for 80% of deaths, but relapse-related death remained of concern because 11 patients overall died of relapse.
Analyses of secondary end points
The cumulative incidence of relapse of the underlying malignant disease was not statistically significant in the 2 arms (13% and 6% after inolimomab and ATG, respectively; P = .06; number of relapsed patients = 6 and 3, respectively). This led to a 1-year postrandomization progression-free survival estimate rate of 28.6% and 21.6% after inolimomab and ATG, respectively. There was no statistical evidence that inolimomab did better for the primary end point in patients with skin (HR = 1.62) or gut (HR = 0.65) involvement. As expected in this setting, the rate of overall infection and adverse event was high. However, there was no difference between the 2 treatment arms, except for slightly less serious viral infections after inolimomab (Table 2).
Adverse events by treatment arm
| 1-year AE . | Inolimomab, n (%) (n = 49) . | Usual care, n (%) (n = 51) . |
|---|---|---|
| At least one serious AE | 47 (96) | 46 (90) |
| At least one grade 3, 4, or 5 AE | 49 (100) | 50 (98) |
| At least one AE related to treatment | 7 (14) | 21 (41) |
| At least one AE resulting in treatment discontinuation | 7 (14) | 2 (4) |
| At least one AE resulting in death | 26 (53) | 30 (59) |
| At least one viral infection | 38 (78) | 47 (92) |
| CMV reactivation | 21 (42.9) | 17 (33.3) |
| EBV reactivation | 24 (49.0) | 27 (52.9) |
| Other viral infection | 22 (44.9) | 34 (66.7) |
| At least one bacterial infection | 40 (82) | 43 (84) |
| At least one fungal infection | 17 (35) | 19 (37) |
| At least one parasitic infection | 3 (6) | 4 (8) |
| Sepsis | 7 (14) | 12 (24) |
| Septic shock | 2 (4) | 8 (16) |
| 1-year AE . | Inolimomab, n (%) (n = 49) . | Usual care, n (%) (n = 51) . |
|---|---|---|
| At least one serious AE | 47 (96) | 46 (90) |
| At least one grade 3, 4, or 5 AE | 49 (100) | 50 (98) |
| At least one AE related to treatment | 7 (14) | 21 (41) |
| At least one AE resulting in treatment discontinuation | 7 (14) | 2 (4) |
| At least one AE resulting in death | 26 (53) | 30 (59) |
| At least one viral infection | 38 (78) | 47 (92) |
| CMV reactivation | 21 (42.9) | 17 (33.3) |
| EBV reactivation | 24 (49.0) | 27 (52.9) |
| Other viral infection | 22 (44.9) | 34 (66.7) |
| At least one bacterial infection | 40 (82) | 43 (84) |
| At least one fungal infection | 17 (35) | 19 (37) |
| At least one parasitic infection | 3 (6) | 4 (8) |
| Sepsis | 7 (14) | 12 (24) |
| Septic shock | 2 (4) | 8 (16) |
AE, adverse event; CMV, cytomegalovirus; EBV, Epstein-Barr virus.
Discussion
Clinical trials using inolimomab or other drugs targeting the IL-2 receptor have shown promising results in phase 2 studies for treatment of SR aGVHD.5,9-14,19-24 However, none of these previous trials were randomized. This phase 3 study was developed to compare outcome between inolimomab and standard therapy. Because there were no approved second-line therapies for aGVHD, we chose the optimum standard therapy for the comparator arm. After reviewing the literature and discussion on transplant center preferences, we chose ATG because it was the most widely used agent and found no evidence that any alternatives would provide higher response rates.4 Although ATG treatment given for SR aGVHD has never been formally tested in the setting of a double-blinded, placebo-controlled randomized trial, investigators who designed this study agreed that ATG had been studied in the largest numbers of SR GVHD patients for toxicity, efficacy, and overall survival. The primary objective of this study was to evaluate overall survival at 1 year without replacement of baseline allocated therapy. Although the primary end point of this randomized phase 3 trial was not achieved, these results suggest improved 1-year overall survival with inolimomab or ATG compared with previously reported outcomes.4 The results of this prospective randomized study show that inolimomab, like ATG, is generally well tolerated with a similar incidence of adverse events when given as therapy for SR aGVHD.
Although the response rate for inolimomab was similar to that observed in other phase 2 trials,4 there was no clinically or statistically significant advantage using inolimomab compared with ATG on survival rate in this randomized trial. The clinical responses to ATG observed in this study were like previous nonrandomized studies for patients with SR GVHD. A report at a single institution in the 1990s described an overall response rate of 54% in patients receiving ATG for SR aGVHD, and durable complete responses in 20% of patients.17 The results of the only other randomized multicenter trial comparison (ABX-CBL vs ATG) also provided similar rates nearly 10 years ago.7 The current randomized trial provides evidence that, using current supportive treatment, the 1-year expected survival rate is within the 45%, which seems to be higher than the expected 30% rate usually reported. Other IL-2 and IL2-R targeted therapies have been used in the setting of SR aGVHD including denileukin diftitox or dacluzimab (alone or in combination with anti–tumor necrosis factor-α monoclonal antibodies).4 As in the case of inolimomab, these latter 2 compounds gave promising results in the setting of phase 2 trials but have never been tested in randomized trials thus far. Correlation of disease response (or failure) with lymphocytes subsets (either effector or regulatory T cells) would be useful to confirm the biological effect of any new therapy in this context. Thus, results of this randomized trial may be used as a benchmark for future randomized trials.
We also took advantage of this trial to test factors that can adversely affect the outcome if they are not well balanced in the 2 arms. Only liver stage at randomization was associated with a significant worse outcome, as previously reported.5,6,25 We also sought factors that could influence outcome in this dismal setting and found that patients transplanted from an unrelated donor did worse, as did patients older than 60 years of age. Although older age might be expected, the finding of worse outcome in patients transplanted from unrelated donors has not been described thus far. Given the paucity of data coming from randomized trials in the setting of SR aGVHD, we performed additional post hoc analyses on nonrelapse mortality and GVHD relapse rate (supplemental Appendix). Only older patients’ age was significantly associated with nonrelapse mortaility (HR, 1.054; 95% CI, 1.015-1.09; P = .006).
Exclusion of patients with major liver functional test abnormalities (that could be considered as marker of hepatic GVHD), and of patients with performance status >3 led to selection against “excessively sick” SR GVHD patients, should also be considered when reviewing our data. We cannot rule out that this study was underpowered to detect a statistical difference between the 2 arms and would have needed more patients to do so. Indeed, to detect a difference of 15%, we needed 434 patients per group. This lack of a statistically significant effect confirms the deep need for development of more effective treatments for SR aGVHD, and we urge physicians to test any promising drug in the setting of a controlled, randomized trial.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was funded by Jazz Pharmaceuticals.
Authorship
Contribution: J.-P.V., G.S., and EUSA Pharma designed the study; P.L. and G.S. designed the statistical plan; G.S. wrote the manuscript; Jazz Pharmaceuticals collected data and supervised the analysis; G.S., S.F., I.Y.-A., F.S., K.B., M.M., D.B., and J.-P.V. included patients; and all authors critically read and approved the manuscript.
Conflict-of-interest disclosure: G.S., M.M., and I.Y.-A. have received speaker fees from Jazz Pharmaceuticals. J.O.-B. and F.S. have been invited to a conference by Jazz Pharmaceuticals. P.G., Z.M., C.L., and T.C. are employees of Jazz Pharmaceuticals, and in the course of employment have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Gérard Socié, Hematology Transplantation, APHP, Hôpital St Louis, 1 Ave Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: gerard.socie@aphp.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal