In this issue of Blood, Walter et al demonstrate an association between heat shock protein 90 (HSP90) and spleen tyrosine kinase (SYK) as a critical upstream component in tonic BCR signaling of Burkitt lymphoma (BL). Integrity of this HSP90-SYK complex appears to be critical for maintenance of tonic BCR signaling, which keeps BL cells alive. Consequently, the authors propose HSP90 as a novel therapeutic target in BL.1
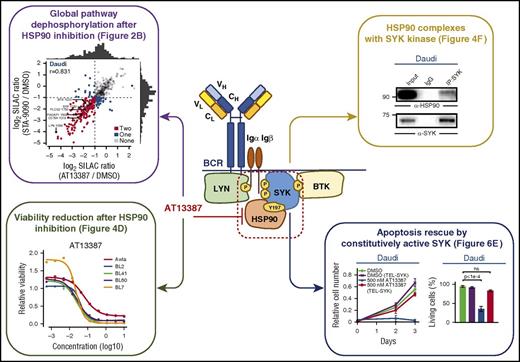
The HSP90-SYK complex regulates survival of BL cells. The HSP90 inhibitor AT13387 interferes with BL B-cell survival and signaling activity. By SILAC-based phosphoproteomic analysis, Walter et al show dramatic reduction (upper left panel) of phosphorylation of BCR-associated molecules, including LYN, SYK, and BTK, which results in reduction of BL cell viability (bottom left panel). Interestingly, HSP90 is identified as a binding partner for SYK kinase (upper right panel), and such interaction is mediated by phosphorylated tyrosine Y197 on HSP90. Apoptotic effects mediated by HSP90-SYK inhibition largely rely on HSP90-mediated destabilization of SYK kinase, as a constitutively active SYK-isoform (bottom right panel) can rescue BL cells from drug-induced apoptosis. CH, constant region of the heavy chain; CL, constant region of the light chain; DMSO, dimethyl sulfoxide; Igα, immunoglobulin α; Igβ, immunoglobulin β; IgG, immunoglobulin G; IP, immunoprecipitation; ns, not significant; P, phosphorylation ; VH, variable region of the heavy chain; VL, variable region of the light chain. See Figures 2, 4, and 6 in the article by Walter et al that begins on page 598.
The HSP90-SYK complex regulates survival of BL cells. The HSP90 inhibitor AT13387 interferes with BL B-cell survival and signaling activity. By SILAC-based phosphoproteomic analysis, Walter et al show dramatic reduction (upper left panel) of phosphorylation of BCR-associated molecules, including LYN, SYK, and BTK, which results in reduction of BL cell viability (bottom left panel). Interestingly, HSP90 is identified as a binding partner for SYK kinase (upper right panel), and such interaction is mediated by phosphorylated tyrosine Y197 on HSP90. Apoptotic effects mediated by HSP90-SYK inhibition largely rely on HSP90-mediated destabilization of SYK kinase, as a constitutively active SYK-isoform (bottom right panel) can rescue BL cells from drug-induced apoptosis. CH, constant region of the heavy chain; CL, constant region of the light chain; DMSO, dimethyl sulfoxide; Igα, immunoglobulin α; Igβ, immunoglobulin β; IgG, immunoglobulin G; IP, immunoprecipitation; ns, not significant; P, phosphorylation ; VH, variable region of the heavy chain; VL, variable region of the light chain. See Figures 2, 4, and 6 in the article by Walter et al that begins on page 598.
Activation of B-cell receptor (BCR) signaling, in the presence or absence of antigenic stimulation (“activated” or “tonic” BCR signaling), is critical for the survival and proliferation of normal B cells. It is also an important and targetable pathway in several B-cell malignancies, as documented by the clinical success of Bruton’s tyrosine kinase (BTK) and phosphatidylinositol 3-kinase δ (PI3Kδ) inhibitors.2 Walter et al demonstrate an association between heat shock protein 90 (HSP90) and spleen tyrosine kinase (SYK) as a critical upstream component in tonic BCR signaling of Burkitt lymphoma (BL). Integrity of this HSP90-SYK complex appears to be critical for maintenance of tonic BCR signaling, which keeps BL cells alive. Consequently, the authors propose HSP90 as a novel therapeutic target in BL.1
HSP90 is a member within the class of molecular chaperones, which collectively ensure the proper folding of proteins in order to prevent misfolding, protein aggregation, and their ubiquitination and proteasomal degradation. Signal transduction proteins are well-characterized HSP90 substrates, which require HSP90 for maturation and conformational maintenance. Furthermore, many oncogenic proteins, including tyrosine-kinase receptors, transcription factors, cell-cycle regulatory proteins, antiapoptotic proteins, and telomerase, are HSP90 client proteins. Consequently, the genetic or pharmacologic inhibition of HSP90 disrupts many cellular signaling networks that depend upon these molecules. Small molecule HSP90 inhibitors, such as the orally bioavailable, small molecule HSP90 inhibitor AT13387, selectively bind to HSP90, thereby inhibiting its chaperone function and promoting the degradation of proteins that may be involved in tumor cell proliferation and survival. Given the importance of HSP90 for the stability and function of various oncogenic proteins, HSP90 inhibitors have been under clinical development for a while, with mixed results, and some have been promising in selected solid (ALK-rearranged non–small cell lung cancer, HER2+ breast cancer) and hematological (multiple myeloma) malignancies.
By screening the in vitro sensitivity of BL cell lines in response to a panel of agents that included cytotoxic drugs, signaling inhibitors, and AT13387, the investigators noted striking activity of AT13387 to induce apoptosis of BL cells. Stable isotope labeling with amino acids in cell culture (SILAC)-based phosphoproteomic analyses demonstrated that HSP90 targeting downregulated BCR signaling, inhibiting the activation of the BCR-related kinases LYN, SYK, and BTK. Interestingly, SYK inhibition interfered with BL cell survival in a fashion that was similar to HSP90 inhibition, whereas neither LYN downregulation nor BTK inhibition with ibrutinib had any comparable effects. Based on these observations, the authors studied in more detail the interactions between HSP90 and SYK and report their importance for tonic BCR signaling, and the requirement for phosphorylation of tyrosine Y197 residues on HSP90 for its function. The authors conclude that HSP90 “chaperones” SYK, as a prerequisite for intact BCR signaling in BL, and on the flipside, they build the therapeutic concept that HSP90 inhibition can promote BL apoptosis through destabilization of SYK (see figure).
The study expands previous work by the same group of investigators in which they characterized the importance of tonic BCR signaling in BL through phosphoproteomic approaches.3 HSP90 inhibition has previously been shown to be effective in reducing chronic lymphocytic leukemia (CLL) cell4 and activated B-cell diffuse large B-cell lymphoma5 cell survival in vitro, and combinations of HSP90 inhibition with BCR signaling inhibitors showed promising responses in preclinical models of mantle cell lymphoma, including models of ibrutinib resistance.6 In this current study, where the authors propose HSP90 inhibition with AT13387 for targeting BCR signaling in BL, that could be a rationale for clinical trials with HSP90 inhibitors in BL, we have to consider that alternative ways for targeting BCR signaling and SYK are readily available. BTK, PI3Kδ, and SYK inhibitors all can effectively disrupt BCR signaling, and these agents generally are well tolerated and would be more selective in targeting BCR signaling when compared with AT13387, which targets a wide range of other client proteins, besides SYK. We furthermore need to take into consideration that BCR signaling inhibitors, such as ibrutinib and idelalisib, have been proven to be most effective in patients with B-cell malignancies that are driven primarily by activated, rather than tonic BCR signaling, like CLL.7 Similarly, the SYK inhibitor fostamatinib, which has been tested in patients with various B-cell malignancies, showed the highest response rates in patients with CLL.8 In patients with BL, there are, however, no reported data with this class of agents. Hence, it remains to be seen whether targeting BCR signaling in BL, using AT13387 or BCR signaling inhibitors that target, for example, SYK, will fulfill the promise raised by this current report. Besides the clinical implications, the paper by Walter et al also illustrates that proteomic and phosphoproteomic approaches can be valuable techniques for high-throughput screening of multiple signaling pathways at the same time, in the context of preclinical evaluations of drugs or as part of correlative studies, for example, in order to identify pathways associated with responsiveness or resistance to targeted therapies. Collectively, this work connects HSP90 with SYK and BCR signaling in BL and promotes therapeutic targeting of HSP90 as a way to overcome SYK-dependent growth of BL cells. This work therefore may also recharge interest in exploring HSP90 as an alternative therapeutic target in BL or other diseases in which targeting of SYK could be beneficial.
Conflict-of-interest disclosure: J.A.B. received research funding from Pharmacyclics and Gilead. E.t.H. has no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal