Neutrophils are immune defenders. In this issue of Blood, Kawano et al show that, by producing prostaglandin E2 (PGE2), they also regulate hematopoietic stem cell mobilization and cause fever.1
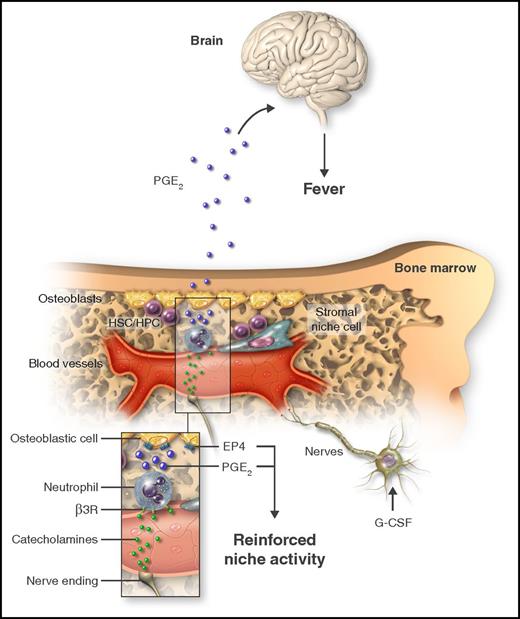
Treatment with G-CSF causes mobilization of HSCs/HPCs and triggers fever. By acting on catecholaminergic cells (nerve cells or other), G-CSF increases the adrenergic tone in the marrow, thereby generating catecholamines that in turn activate neutrophils through the β3-adrenergic receptor (β3R; inset). As a consequence of adrenergic stimulation, neutrophils produce PGE2, which can target osteoblastic cells through the EP4 receptor to promote HSC/HPC retention. Additionally, neutrophil-derived PGE2 causes fever by systemically acting on the brain. Professional illustration by Somersault18:24.
Treatment with G-CSF causes mobilization of HSCs/HPCs and triggers fever. By acting on catecholaminergic cells (nerve cells or other), G-CSF increases the adrenergic tone in the marrow, thereby generating catecholamines that in turn activate neutrophils through the β3-adrenergic receptor (β3R; inset). As a consequence of adrenergic stimulation, neutrophils produce PGE2, which can target osteoblastic cells through the EP4 receptor to promote HSC/HPC retention. Additionally, neutrophil-derived PGE2 causes fever by systemically acting on the brain. Professional illustration by Somersault18:24.
The bone marrow (BM) is home to hematopoietic stem/progenitor cells (HSCs/HPCs) and their descendants. Continued production of all blood lineages requires a structured organization and complex interactions among the many cell types that reside in the BM. Early studies identified osteoblastic cells lining the endosteal surface as important components of the hematopoietic niche, whereas macrophages and other cells of hematopoietic origin have only been recognized as regulators of the BM niche in recent years.2 Sympathetic nerves and mesenchymal progenitors are also regulators of the HSC/HPC niche. Current efforts focus on understanding how all of these very different cellular elements coordinate to preserve BM homeostasis.
Transient reorganization of the cellular niche network may be important when the BM needs to increase the supply of immune cells in response to a stress. For example, during acute infections endothelial cells respond by producing the cytokine granulocyte colony-stimulating factor (G-CSF).3 On one hand, this cytokine accelerates production of neutrophils (ie, granulopoiesis), the genuine antimicrobial defenders; but G-CSF also initiates a cascade of events within the BM that disrupts niche activity and leads to the release of HSCs/HPCs into the bloodstream. Hematologists appreciated the therapeutic potential of using G-CSF to expand and push HSCs/HPCs out of the BM for their efficient collection from blood. Indeed, blood obtained from G-CSF-treated donors is now the preferred source of HSCs/HPCs for transplantation therapy. A surprising side effect of G-CSF treatment is the appearance of low-grade fever and bone pain that responds to nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit the prostaglandin synthesis pathway. How G-CSF caused fever was unknown, until now.
While studying the mechanism of fever in experimental models of G-CSF mobilization, Kawano et al identified a previously unknown molecular and cellular circuitry in the BM that induces the production of PGE2. This lipid not only caused fever, but also limited the inhibitory effect of G-CSF on the hematopoietic niche.
The authors reasoned that if G-CSF treatment caused a rise in temperature that could be prevented by NSAIDs, activation of the arachidonic acid cascade and production of pyrogenic PGE2 might be involved in the mobilizing effects of the cytokine. They identified the microsomal PGE-1 synthase as a critical mediator of fever in G-CSF-treated mice. Surprisingly, although deficiency in this catalytic enzyme prevented changes in body temperature, it enhanced, rather than prevented, mobilization of HSCs/HPCs into blood, and these effects required its expression in the hematopoietic compartment. The authors found, however, that G-CSF did not trigger PGE2 production by acting directly on hematopoietic cells. How, then, did G-CSF control prostaglandin production?
To search for possible mechanisms, the authors revisited their own seminal work from a decade earlier demonstrating that G-CSF stimulates the catecholaminergic tone in the BM.4 They therefore predicted that catecholamines might be intermediaries needed for PGE2 production. Indeed, treatment with a β-adrenergic agonist stimulated prostaglandin release by neutrophils, and to a lesser extent by macrophages, in a process that depended on the β3-adrenergic receptor. The authors went on to demonstrate that if neutrophils were depleted before G-CSF treatment, or if the cells that produce catecholamines were eliminated, then fever disappeared from treated mice.
These findings, however, were at odds with the exaggerated mobilization of HSCs/HPCs in mice unable to synthesize PGE2, and suggested that generation of fever and mobilization were antagonistic effects. The response to this puzzle came, again, after revisiting earlier work showing that PGE2 improves HSC/HPC retention by stimulating osteoblastic cell function through production of osteopontin, an HSC/HPC retention molecule.5 In agreement with these observations, Kawano et al found that, indeed, PGE2 acts locally in the BM by dramatically increasing osteopontin on preosteoblastic cells, through the prostaglandin receptor EP4. Altogether, the data presented in this article uncover a signaling network elicited by G-CSF that stimulates catecholamine production, which acts on β3-adrenergic receptors on neutrophils to induce production of PGE2. This lipid acts locally on niche cells to limit HSC/HPC release, and systemically on the hypothalamus to induce fever (see figure).
The findings by Kawano et al are important because they provide formal demonstration that neutrophils, which are the most abundant cells in the BM, are bona fide regulators of the HSC/HPC niche. Previous studies had demonstrated that neutrophils can induce local inhibition of niches or control granulopoiesis from extramedullary tissues.6,7 The present study, however, is the first to identify a neutrophil-derived product that positively regulates niche cells. Although less abundant, monocytes and macrophages respond in a similar way, thereby reinforcing the notion that innate immune cells form an important regulatory network within the marrow. Different from other niche cells, however, neutrophils and other myeloid cells appear to provide a system that better integrates environmental signals for finer regulation of HSCs/HPCs during stress. The study thus raises the question of whether (and if so how) innate immunity regulates stem cell niches in the context of pathological stress (eg, infection or leukemia).
Finally, it is intriguing that experimental depletion of neutrophils caused mild but significant reductions in body temperature after G-CSF treatment. Because neutrophils are distributed in many more organs than the BM, and can themselves produce not only PGE2 but also catecholamines,8 it would seem that neutrophils are superbly positioned to regulate core processes, as illustrated here for body temperature and stem cell niches. Clearly, neutrophils are much more than immune defenders.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal