Key Points
Reducing therapy intensity in the ML-DS 2006 trial did not impair the excellent prognosis in ML-DS compared with the historical control.
Early treatment response and gain of chromosome 8 are independent prognostic factors.
Abstract
Children with myeloid leukemia associated with Down syndrome (ML-DS) have superior outcome compared with non-DS patients, but suffer from higher constitutional cytotoxic drug susceptibility. We analyzed the outcome of 170 pediatric patients with ML-DS enrolled in the prospective, multicenter, open-label, nonrandomized ML-DS 2006 trial by Nordic Society for Pediatric Hematology and Oncology (NOPHO), Dutch Childhood Oncology Group (DCOG), and Acute Myeloid Leukemia–Berlin-Frankfurt-Münster (AML-BFM) study group. Compared with the historical control arm (reduced-intensity protocol for ML-DS patients from the AML-BFM 98 trial), treatment intensity was reduced by lowering the cumulative dose of etoposide (950 to 450 mg/m2) and intrathecal central nervous system prophylaxis while omitting maintenance therapy. Still, 5-year overall survival (89% ± 3% vs 90% ± 4%; Plog-rank = .64), event-free survival (EFS; 87% ± 3% vs 89% ± 4%; Plog-rank = .71), and cumulative incidence of relapse/nonresponse (CIR/NR; 6% ± 3% vs 6% ± 2%; PGray = .03) did not significantly differ between the ML-DS 2006 trial and the historical control arm. Poor early treatment response (5-year EFS, 58% ± 16% vs 88% ± 3%; Plog rank = .0008) and gain of chromosome 8 (CIR/NR, 16% ± 7% vs 3% ± 2%, PGray = .02; 5-year EFS, 73% ± 8% vs 91% ± 4%, Plog rank = .018) were identified as independent prognostic factors predicting a worse EFS. Five of 7 relapsed patients (71%) with cytogenetic data had trisomy 8. Our study reveals prognostic markers for children with ML-DS and illustrates that reducing therapy did not impair excellent outcome. The trial was registered at EudraCT as #2007-006219-2.
Introduction
Children with Down syndrome (DS) have a 150-fold increased risk of myeloid leukemia (ML) before the age of 5 years.1 The majority of the reported cases with ML associated with DS (ML-DS) show a predominance of megakaryoblasts, corresponding to megakaryoblastic leukemia (AMKL) or French-American-British (FAB)-type M7 in non-DS patients.2-4 ML-DS can be preceded by a period of transient abnormal myelopoiesis (TAM).5 Both ML-DS and TAM are associated with mutations of the hematopoietic transcription factor GATA1,6 which is causative during leukemogenesis.7
Historically, outcome in children with ML-DS was thought to be poor.8 To date, excellent cure rates have been achieved for ML-DS using dose-reduced treatment protocols without hematopoietic stem cell transplantation.9-15 The reduced-intensity arm for ML-DS of the Acute Myeloid Leukemia–Berlin-Frankfurt-Münster (AML-BFM) 98 trial consisted of 4 courses of chemotherapy, containing cytarabine, idarubicin, etoposide, or mitoxantrone, and resulted in event-free survival (EFS) after 5 years of 89%.9 This is in contrast to a 5-year EFS of 54% in non-DS children with AML FAB M7 (non-DS-AMKL).16 The excellent response was attributed to the enhanced drug sensitivity of the ML-DS blasts, especially to cytarabine and anthracyclines.17,18 Still, despite reduced intensity, many patients suffer from therapy-associated toxicity.9 This determines therapy-related mortality (TRM) as the main cause of death in this cohort of patients.9-15
Different international study protocols for ML-DS vary in their treatment intensity and drug scheduling.9-15 Whereas recent North American and European studies use high doses of anthracyclines and cytarabine,9-13 Japanese studies achieved comparable survival rates using less intense treatment regimens.14,15 In contrast, the prognosis of relapsed ML-DS patients is extremely poor.12,19,20 This means that ML-DS treatment schemata have to particularly strive for the balance between appropriate chemotherapy dosage to avoid relapses and treatment-related toxicity. Despite substantial efforts, clear prognostic factors that identify those patients at high risk for relapse remain elusive precluding a risk-adapted treatment strategy.21
Here, we report the outcome of 170 pediatric patients with ML-DS enrolled in the Myeloid Leukemia Down Syndrome 2006 (ML-DS 2006) trial, conducted by the Nordic Society for Pediatric Hematology and Oncology (NOPHO), Dutch Childhood Oncology Group (DCOG), and AML-BFM study group. The protocol was based on the reduced-intensity arm for children with ML-DS in the AML-BFM 98 trial and was further intensity-reduced by excluding etoposide from the consolidation phase (reducing the cumulative dose from 950 mg/m2 to 450 mg/m2), administering 4 instead of 11 doses of intrathecal central nervous system (CNS) prophylaxis (cytarabine 20-40 mg), and excluding maintenance therapy.9 The aim of the trial was to assure the high EFS while further reducing the risk of treatment-related toxicity.
Methods
Patients
The ML-DS 2006 (EurdraCT #2007-006219-22) is a multicenter, open-label, nonrandomized trial running in Germany, Austria, Switzerland, the Czech Republic (BFM), Scandinavia (NOPHO), and The Netherlands (DCOG). It was opened on January 1, 2007 and closed on August 30, 2015. The final protocols were approved by the local ethic committees according to national laws and regulations. The trial was carried out according to the Declaration of Helsinki, the principles of Good Clinical Practice and Directive 2001/20/EC of the European Parliament and of the Council of April 4, 2001.
Patients with ML-DS with evidence of megakaryoblasts or undifferentiated blasts, confirmed by morphology and immunophenotyping (including CD34, CD117, CD7, CD13, CD33, CD15, CD36, CD56, CD41, CD42b, CD61) in the bone marrow (BM), peripheral blood, or trephine biopsy, between 6 months and 4 years were eligible for participation after legal written informed consent of the parents/custodians. Immunophenotyping was done and centrally reviewed by each collaborative study group (AML-BFM, DCOG, NOPHO). The AML-BFM and DCOG group performed central review of morphology. Patients older than 4 years of age and younger than 18 years were included if a GATA1 mutation was detected. Independent of age, DS patients with AML FAB M1-M5 were not eligible for the reduced-intensity ML-DS 2006 protocol as those cases were suspected to represent rare sporadic acute myeloid leukemia (AML) occurring in patients with DS with a different underlying biology and with a different treatment response compared with ML-DS.22 The World Health Organization (WHO) and FAB classifications were used for the initial diagnosis of AML.2,3 The diagnoses of the FAB M0 and M7 subtypes required confirmation by immunologic methods.3,23,24 In brief, for the diagnosis of FAB, M0 blasts had to be negative for myeloperoxidase activity and for B- and T-cell markers (except CD7) and show expression of myeloid antigens (CD117, CD13, or CD33). Diagnosis of FAB M7 was considered when blasts were negative for myeloperoxidase activity and had specific megakaryocytic markers (CD41, CD42b, and/or CD61) without any B- or T-cell markers (except CD7). Surface antigen expression was considered positive if at least 20% of blasts showed positive labeling.
Treatment of children with ML-DS in the AML-BFM 98 trial (historical control arm) consisted of 4 cycles of polychemotherapy followed by maintenance therapy as depicted in Figure 1. The AML-BFM 98 trial was opened from August 1998 to July 2003 and recruited 67 children with ML-DS (historical control arm) as previously described in detail.9
ML-DS 2006 protocol compared with the historical control arm (AML-BFM 98). Scheme of the different study protocols as indicated. ML-DS 2006: AIE (cytarabine 100 mg/m2 per day [days 1-2] and 100 mg/m2 per 12 hours [days 3-8], idarubicin 8 mg/m2 per day [days 3, 5, and 7], and etoposide 150 mg/m2 per day [days 6-8]); AI (cytarabine 500 mg/m2 per day [days 1-4] and idarubicin 5 mg/m2 per day [days 3 and 5]); haM (cytarabine 1 g/m2 per 12 hours [days 1-3] and mitoxantrone 7 mg/m2 per day [days 3-4]); HA (high-dose cytarabine 3 g/m2 per 12 hours [days 1-3]). The cumulative doses were 27 400 mg/m2 cytarabine, 450 mg/m2 etoposide, 34 mg/m2 idarubicin, and 14 mg/m2 mitoxantrone. Chemotherapy for children younger than 12 months of age, or weighing <12 kg, was calculated based on body weight. In addition, patients received cytarabine intrathecally at the start of each treatment block (4 doses in total, 20-40 mg per dose adapted to age) as a CNS prophylaxis. AML-BFM 98 (reduced-intensity arm for children with ML-DS; historical control arm): AIE (cytarabine 100 mg/m2 per day [days 1-2] and 100 mg/m2 per 12 hours [days 3-8], idarubicin 8 mg/m2 per day [days 3, 5, and 7] and etoposide 150 mg/m2 per day [days 6-8]); AI (cytarabine 500 mg/m2 per day [days 1-4] and idarubicin 5 mg/m2 per day [days 3 and 5]); haM (cytarabine 1 g/m2 per 12 hours [days 1-3] and mitoxantrone 7 mg/m2 per day [days 3-4]); HAE (high-dose cytarabine 3 g/m2 per 12 hours [days 1-3] and etoposide 125 mg/m2 per day [days 2-5]). Maintenance therapy until 1.5 years after start of induction therapy was thioguanine daily 40 mg/m2 per os (p.o.) and cytarabine 40 mg/m2 subcutaneously (s.c.) every 4 weeks on 4 consecutive days. The cumulative doses were ∼29 400 mg/m2 cytarabine, 950 mg/m2 etoposide, 34 mg/m2 idarubicin, and 14 mg/m2 mitoxantrone.
ML-DS 2006 protocol compared with the historical control arm (AML-BFM 98). Scheme of the different study protocols as indicated. ML-DS 2006: AIE (cytarabine 100 mg/m2 per day [days 1-2] and 100 mg/m2 per 12 hours [days 3-8], idarubicin 8 mg/m2 per day [days 3, 5, and 7], and etoposide 150 mg/m2 per day [days 6-8]); AI (cytarabine 500 mg/m2 per day [days 1-4] and idarubicin 5 mg/m2 per day [days 3 and 5]); haM (cytarabine 1 g/m2 per 12 hours [days 1-3] and mitoxantrone 7 mg/m2 per day [days 3-4]); HA (high-dose cytarabine 3 g/m2 per 12 hours [days 1-3]). The cumulative doses were 27 400 mg/m2 cytarabine, 450 mg/m2 etoposide, 34 mg/m2 idarubicin, and 14 mg/m2 mitoxantrone. Chemotherapy for children younger than 12 months of age, or weighing <12 kg, was calculated based on body weight. In addition, patients received cytarabine intrathecally at the start of each treatment block (4 doses in total, 20-40 mg per dose adapted to age) as a CNS prophylaxis. AML-BFM 98 (reduced-intensity arm for children with ML-DS; historical control arm): AIE (cytarabine 100 mg/m2 per day [days 1-2] and 100 mg/m2 per 12 hours [days 3-8], idarubicin 8 mg/m2 per day [days 3, 5, and 7] and etoposide 150 mg/m2 per day [days 6-8]); AI (cytarabine 500 mg/m2 per day [days 1-4] and idarubicin 5 mg/m2 per day [days 3 and 5]); haM (cytarabine 1 g/m2 per 12 hours [days 1-3] and mitoxantrone 7 mg/m2 per day [days 3-4]); HAE (high-dose cytarabine 3 g/m2 per 12 hours [days 1-3] and etoposide 125 mg/m2 per day [days 2-5]). Maintenance therapy until 1.5 years after start of induction therapy was thioguanine daily 40 mg/m2 per os (p.o.) and cytarabine 40 mg/m2 subcutaneously (s.c.) every 4 weeks on 4 consecutive days. The cumulative doses were ∼29 400 mg/m2 cytarabine, 950 mg/m2 etoposide, 34 mg/m2 idarubicin, and 14 mg/m2 mitoxantrone.
Treatment plan and study design
Treatment consisted of 4 cycles of polychemotherapy as depicted in Figure 1. A good early response was defined as <5% blasts in the BM at the start of the second block. After every course, toxicity was evaluated using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events v3.0 (CTCAE v3.0). The protocol required that each course should only be started if the child is in a good general condition without clinical signs of an infection, mucositis, or fever and with recovery of blood counts (neutrophil count, >1 × 109/L; and platelets, >80 × 109/L).
No recommendations for antibiotic or antifungal prophylaxis were made by the study protocol.
The primary objective of the ML-DS 2006 trial was to achieve a 5-year overall survival (OS) of 85% in 150 recruited patients with a standard error <5%. The secondary objectives were to reduce toxicity without impairment of outcome and identify prognostic factors (cytogenetics, clinical parameters at diagnosis, GATA1 status, and treatment response assessed by morphology prior to the commencement of each course of chemotherapy) concerning the risk of relapse, toxicity, and poor outcome. Therefore, the end points of the trial were response rate (complete remission [CR]), EFS, disease-free survival, OS, and TRM. Interim analysis of response rate, frequency of relapse, and severe adverse events was performed after inclusion of 50 and 100 patients by the data monitoring committee. The criteria to close the protocol were >12 of 24 relapses or 10 of 20 deaths after inclusion of the first 50 of 100 patients, respectively.
Cytogenetic and molecular genetic analyses
Cytogenetic and molecular genetic analyses (including GATA1 sequencing) were done and centrally reviewed by each study group (AML-BFM, DCOG, NOPHO). For the AML-BFM group, the reference laboratories performed and centrally reviewed cytogenetic (Hannover, Giessen, Germany) and molecular genetic analyses (Essen, Hannover, Germany) as previously described.6,24 For the DCOG, cytogenetic analyses were done by the university cytogenetic laboratories in The Netherlands and reviewed by a national cytogenetic review panel. Molecular genetic analyses were performed at the Erasmus MC (Rotterdam, The Netherlands).6 For NOPHO, cytogenetic analyses were done by the university cytogenetic laboratories in the member countries and reviewed by the NOPHO cytogenetic review panel as previously described.25 GATA1 analyses were performed by the Weatherall Institute of Molecular Medicine (Oxford, United Kingdom).6 Complete karyotypes were described according to the International System of Human Cytogenetic Nomenclature (ISCN).26
Statistics
CR was defined by fulfillment of the Cancer and Leukemia Group B (CALGB) criteria,27 early death (ED) being death before or within the first 6 weeks of treatment. EFS was defined as time from diagnosis to the first event or last follow-up. Events were death from any cause, failure to achieve remission, relapse, and secondary malignancy. Failure to achieve remission was considered as an event on day 0. Survival was defined as the time of diagnosis to death from any cause or last follow-up.
The Kaplan-Meier method was used to estimate survival rates.28 Differences were compared with the 2-sided log-rank test.29 Standard errors (SEs) were obtained using the Greenwood formula. Cumulative incidence of relapse/nonresponse (CIR/NR) and death in CR were calculated by the method of Kalbfleisch and Prentice and compared with the Gray test. The Cox proportional hazards model has been used to obtain the estimates and the 95% confidence interval (CI) of the relative risk for prognostic factors.30 Differences in the distribution of individual parameters among patient subsets were analyzed using the χ2 test or Fisher exact test for categorized variables and the Mann-Whitney U test for continuous variables.
Results
Patient characteristics
The population-based ML-DS 2006 trial enrolled 170 patients with de novo ML-DS between 2006 and 2015. Patient characteristics are summarized in Table 1 and are compared with the historical control arm (ML-DS children from the AML-BFM 98 trial; N = 67).9 The median follow-up for ML-DS 2006 was 4.0 years, and 9.6 years for the historical control arm.
Characteristics of ML-DS 2006 and AML-BFM 98 (ML-DS) patients
| . | ML-DS 2006 . | AML BFM 98 . | |||
|---|---|---|---|---|---|
| N . | % . | N . | % . | P . | |
| Total | 170 | 100 | 67 | 100 | |
| Sex | .475 | ||||
| Male | 85 | 50 | 30 | 44.8 | |
| Female | 85 | 50 | 37 | 55.2 | |
| FAB | .016* | ||||
| M0 | 4 | 2.8 | 6 | 9.1 | |
| M1/M2 | — | — | — | — | |
| M3 | — | — | — | — | |
| M4/M5 | — | — | 1 | 1.5 | |
| M6 | 1 | 0.8 | 1 | 1.5 | |
| M7 | 124 | 96.9 | 58 | 87.9 | |
| Other | — | — | — | — | |
| Age, y | .788 | ||||
| <1 | 24 | 14.1 | 12 | 17.9 | |
| 1 | 88 | 51.8 | 36 | 53.7 | |
| 2 | 43 | 25.30 | 15 | 22.4 | |
| 3 | 10 | 5.9 | 3 | 4.5 | |
| 4 | 3 | 1.8 | — | — | |
| ≥5 | 2 | 1.2 | 1 | 1.5 | |
| WBC, 109/L | .014* | ||||
| <10 | 144 | 87.3 | 47 | 70.1 | |
| 10-20 | 13 | 7.9 | 13 | 19.4 | |
| 20-50 | 4 | 2.4 | 4 | 6 | |
| ≥50 | 4 | 2.4 | 3 | 4.5 | |
| BM blasts, % | <.001* | ||||
| <20 | 74 | 47.1 | 12 | 18.2 | |
| ≥20 | 83 | 52.9 | 54 | 81.8 | |
| CNS involvement | |||||
| No | 136 | 100 | 64 | 100 | |
| Yes | — | — | — | — | |
| Organ involvement | .227 | ||||
| No | 166 | 97.6 | 63 | 94 | |
| Yes | 4 | 2.4 | 4 | 6 | |
| Case history, wk | .094 | ||||
| <3 | 49 | 52.7 | 22 | 37.9 | |
| ≥3 | 44 | 47.3 | 36 | 62.1 | |
| TAM history | .007 | ||||
| No | 80 | 65 | 56 | 83.6 | |
| Yes | 43 | 35 | 11 | 16.4 | |
| GATA1 | |||||
| WT | 17 | 14.8 | |||
| Mutated | 98 | 85.2 | |||
| Therapy response | |||||
| Early death | 2 | 1.2 | — | — | |
| NR/PR | — | — | — | — | |
| Relapse | 9 | 5.3 | 4 | 6 | |
| Death in CCR | 5 | 2.9 | 4 | 6 | |
| Secondary malignancy | 3 | 1.8 | 1 | 1.5 | |
| LTFU in CCR | 7 | 4.1 | 4 | 6 | |
| CCR | 144 | 84.7 | 54 | 80.6 | |
| Median | |||||
| Age, y | 1.6 | 1.76 | .752 | ||
| WBC, 109/L | 5.1 | 6.6 | .017* | ||
| Hemoglobin, g/dL | 9.3 | 8.6 | .033* | ||
| BM blasts, % | 20 | 29 | <.001* | ||
| Follow-up, y | 4.0 | 9.6 | |||
| . | ML-DS 2006 . | AML BFM 98 . | |||
|---|---|---|---|---|---|
| N . | % . | N . | % . | P . | |
| Total | 170 | 100 | 67 | 100 | |
| Sex | .475 | ||||
| Male | 85 | 50 | 30 | 44.8 | |
| Female | 85 | 50 | 37 | 55.2 | |
| FAB | .016* | ||||
| M0 | 4 | 2.8 | 6 | 9.1 | |
| M1/M2 | — | — | — | — | |
| M3 | — | — | — | — | |
| M4/M5 | — | — | 1 | 1.5 | |
| M6 | 1 | 0.8 | 1 | 1.5 | |
| M7 | 124 | 96.9 | 58 | 87.9 | |
| Other | — | — | — | — | |
| Age, y | .788 | ||||
| <1 | 24 | 14.1 | 12 | 17.9 | |
| 1 | 88 | 51.8 | 36 | 53.7 | |
| 2 | 43 | 25.30 | 15 | 22.4 | |
| 3 | 10 | 5.9 | 3 | 4.5 | |
| 4 | 3 | 1.8 | — | — | |
| ≥5 | 2 | 1.2 | 1 | 1.5 | |
| WBC, 109/L | .014* | ||||
| <10 | 144 | 87.3 | 47 | 70.1 | |
| 10-20 | 13 | 7.9 | 13 | 19.4 | |
| 20-50 | 4 | 2.4 | 4 | 6 | |
| ≥50 | 4 | 2.4 | 3 | 4.5 | |
| BM blasts, % | <.001* | ||||
| <20 | 74 | 47.1 | 12 | 18.2 | |
| ≥20 | 83 | 52.9 | 54 | 81.8 | |
| CNS involvement | |||||
| No | 136 | 100 | 64 | 100 | |
| Yes | — | — | — | — | |
| Organ involvement | .227 | ||||
| No | 166 | 97.6 | 63 | 94 | |
| Yes | 4 | 2.4 | 4 | 6 | |
| Case history, wk | .094 | ||||
| <3 | 49 | 52.7 | 22 | 37.9 | |
| ≥3 | 44 | 47.3 | 36 | 62.1 | |
| TAM history | .007 | ||||
| No | 80 | 65 | 56 | 83.6 | |
| Yes | 43 | 35 | 11 | 16.4 | |
| GATA1 | |||||
| WT | 17 | 14.8 | |||
| Mutated | 98 | 85.2 | |||
| Therapy response | |||||
| Early death | 2 | 1.2 | — | — | |
| NR/PR | — | — | — | — | |
| Relapse | 9 | 5.3 | 4 | 6 | |
| Death in CCR | 5 | 2.9 | 4 | 6 | |
| Secondary malignancy | 3 | 1.8 | 1 | 1.5 | |
| LTFU in CCR | 7 | 4.1 | 4 | 6 | |
| CCR | 144 | 84.7 | 54 | 80.6 | |
| Median | |||||
| Age, y | 1.6 | 1.76 | .752 | ||
| WBC, 109/L | 5.1 | 6.6 | .017* | ||
| Hemoglobin, g/dL | 9.3 | 8.6 | .033* | ||
| BM blasts, % | 20 | 29 | <.001* | ||
| Follow-up, y | 4.0 | 9.6 | |||
—, none; CCR, complete continuous remission; LTFU, lost to follow-up; PR, partial response; WT, wild type.
Bold P values are significant (<.05).
The median age at diagnosis was 1.6 years and the median white blood cell (WBC) count was 5.1 × 109/L. None of the ML-DS patients had initial CNS involvement and only 4 patients (2.4%) had organ involvement. GATA1 mutations were detected in 85.2% (n = 98) of the ML-DS patients. However, as previously described,6 low blast count or uncommon mutations outside of the genomic area, spanning the polymerase chain reaction, or a deletion inside this area, affecting the primer annealing site, may have resulted in a failure to detect the GATA1 mutation in some cases. Compared with the AML-BFM 98 trial, patients in the ML-DS 2006 trial had a significantly lower WBC count (5.1 × 109/L vs 6.6 × 109/L; P = .017) and higher hemoglobin levels (9.3 g/dL vs 8.6 g/dL; P = .033). Seventy-four patients (47.1%) had <20% of blasts in the BM at diagnosis compared with only 18.2% of the patients in the AML-BFM 98 trial (P < .001).
Treatment outcome
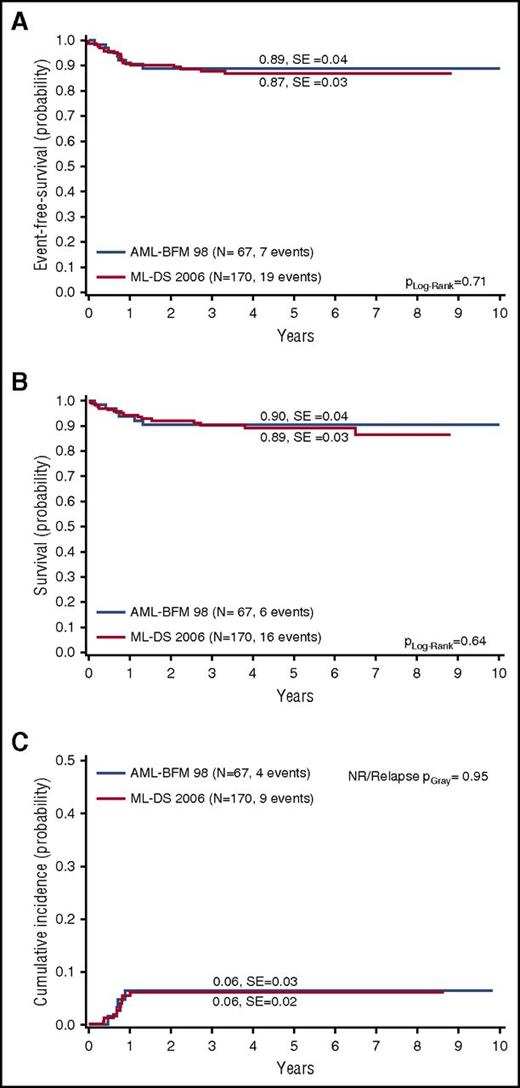
With a 5-year OS of 89% ± 3% and 5-year EFS of 87% ± 3% (Figure 2A-B), children with ML-DS have an excellent outcome. The CIR/NR was 6% ± 2% (Figure 2C). In the AML-BFM 98 trial (historical control arm; Figure 1), the 5-year OS (90% ± 4%; Plog-rank = .64) and 5-year EFS (89% ± 4%; Plog-rank = .71) were not significantly better (Figure 2A-B). The CIR/NR was 6% in both studies (PGray = .95; Figure 2C), indicating that reducing the cumulative dose of etoposide from 950 mg/m2 to 450 mg/m2 plus reducing CNS prophylaxis and excluding maintenance therapy did not increase the relapse risk.
Outcome of ML-DS 2006 patients compared with the historical control arm (AML-BFM 98). (A) EFS, (B) OS, and (C) CIR/NR for ML-DS 2006 patients in comparison with ML-DS children from the previous AML-BFM 98 trial9 (historical control arm). (A-C) Five-year probabilities are given.
Outcome of ML-DS 2006 patients compared with the historical control arm (AML-BFM 98). (A) EFS, (B) OS, and (C) CIR/NR for ML-DS 2006 patients in comparison with ML-DS children from the previous AML-BFM 98 trial9 (historical control arm). (A-C) Five-year probabilities are given.
During the whole ML-DS 2006 study period, we observed 19 events (supplemental Table 1, see supplemental Data available on the Blood Web site). Nine patients relapsed and 7 of those died. Two patients died of cardiac failure and 3 patients died of infections (Streptococcus mitis sepsis, fungal sepsis, and respiratory syncytial virus pneumonia, respectively). One patient died due to a severe macrophage activation syndrome and another one due to a transfusion incident. Three patients developed acute lymphoblastic leukemia (ALL) 1.6, 2.1, and 2.0 years after the end of chemotherapy, leading to death in 2 of those patients. We cannot conclude whether these cases represent secondary leukemias or sporadic cases of ALL in a population that is at high risk for development of ALL. Still, secondary cancer is rare in DS1 and sporadic ALL has previously been well documented after ML-DS.31
Interestingly, male patients showed superior outcome (5-year OS, 98% vs 81% ± 5%, Plog-rank = .002; 5-year EFS, 94% ± 3% vs 79% ± 5%, Plog-rank = .0075). Also, patients with a good early response (n = 123) had significantly better outcome with a superior 5-year OS (92% ± 3% vs 57% ± 16%; Plog-rank < .0001), 5-year EFS (88% ± 3% vs 58% ± 16%; Plog-rank = .0008), and a lower CIR/NR (3% ± 2% vs 27% ± 18%, PGray = .003).
Cytogenetic analysis
Cytogenetic data were available for n = 122 patients (72%), which did not significantly differ from those patients without cytogenetic data with respect to 5-year OS (89% ± 3% vs 89% ± 5%; Plog-rank = .73), 5-year EFS (86% ± 4% vs 89% ± 5%; Plog-rank = .94), and CIR/NR (7% ± 2% vs 5% ± 3%; PGray = .73). Of note, neither normal karyotype (ie, 47,XX/XY,+21c), monosomy 7/7q−, aberrations of chromosome 13, or the long arm of chromosome 1 (1q) were associated with a poor outcome (Table 2). None of the patients that relapsed carried one of these cytogenetic aberrations. In contrast, ML-DS patients with gain of chromosome 8 (+8; n = 37) had a significantly worse 5-year EFS (73% ± 8% vs 91% ± 4%; PLog-Rank = .017). The CIR/NR was greatly increased (16% ± 7% vs 3% ± 2%; PGray = .02). In fact, 5 of 7 relapsed patients with cytogenetic data had trisomy 8. The remaining 2 patients had isochromosome 7 (i[7]) or addition of material from chromosome 16 (add[16]), respectively. Hence, no relapses occurred in patients without gain of chromosome 8, aberrations of chromosome 16, or isochromosome 7 (n = 81).
Five-year EFS/OS and CIR/NR of defined subgroups in the ML-DS 2006 study
| . | N . | Events . | EFS, % . | P . | Deaths . | OS, % . | P . | Relapse/NR . | CIR/NR, % . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 170 | 19 | 87 | 16 | 89 | 9 | 6 | |||
| Sex | .008* | .002* | .330 | |||||||
| Male | 85 | 4 | 94 | 2 | 98 | 3 | 4 | |||
| Female | 85 | 15 | 79 | 14 | 81 | 6 | 8 | |||
| Age, y | .920 | .740 | .580 | |||||||
| <1 | 24 | 2 | 91 | 1 | 96 | 1 | 5 | |||
| 1 | 88 | 10 | 86 | 8 | 89 | 3 | 4 | |||
| 2 | 43 | 6 | 84 | 6 | 86 | 4 | 10 | |||
| ≥3 | 15 | 1 | 90 | 1 | 90 | 1 | 10 | |||
| WBC, ×109/L | .640 | .680 | .580 | |||||||
| <5 | 79 | 12 | 82 | 10 | 86 | 6 | 9 | |||
| 5-10 | 65 | 5 | 91 | 4 | 93 | 2 | 3 | |||
| 10-15 | 9 | 1 | 89 | 1 | 89 | — | — | |||
| ≥15 | 12 | 1 | 90 | 1 | 88 | 1 | 10 | |||
| Initial BM blasts, % | .100 | .100 | .060 | |||||||
| <20 | 74 | 12 | 81 | 11 | 83 | 7 | 11 | |||
| ≥20 | 83 | 6 | 92 | 5 | 93 | 2 | 3 | |||
| Early response, % BM blasts | <.001* | <.001* | .01* | |||||||
| ≤5 | 123 | 12 | 88 | 9 | 92 | 4 | 4 | |||
| >5 | 10 | 4 | 58 | 4 | 57 | 2 | 22 | |||
| Case history, wk | .076 | .250 | .070 | |||||||
| <3 | 49 | 3 | 92 | 3 | 95 | 1 | 2 | |||
| ≥3 | 44 | 8 | 79 | 6 | 83 | 5 | 13 | |||
| TAM history | .130 | .280 | .310 | |||||||
| No | 43 | 2 | 95 | 2 | 94 | 1 | 3 | |||
| Yes | 80 | 10 | 85 | 8 | 89 | 5 | 7 | |||
| GATA1 | .170 | .220 | .360 | |||||||
| Mutated | 98 | 11 | 87 | 9 | 90 | 5 | 6 | |||
| WT | 17 | 0 | 100 | 0 | 100 | 0 | 0 | |||
| Cytogenetics | 122 | |||||||||
| Normal (47,XY/XX +21c) | .480 | .330 | .160 | |||||||
| Yes | 29 | 2 | 91 | 1 | 97 | 0 | 0 | |||
| No | 93 | 12 | 84 | 10 | 88 | 7 | 8 | |||
| Gain of chr 8 | .018* | .071 | .020* | |||||||
| Yes | 37 | 8 | 73 | 6 | 77 | 5 | 16 | |||
| No | 85 | 6 | 91 | 5 | 95 | 2 | 3 | |||
| Monosomy 7/7q− | .350 | .460 | .500 | |||||||
| Yes | 7 | 0 | 100 | 0 | 100 | 0 | 0 | |||
| No | 115 | 14 | 85 | 11 | 89 | 7 | 3 | |||
| chr 13 aberrations | .460 | .520 | .590 | |||||||
| Yes | 4 | 0 | 100 | 0 | 100 | 0 | 0 | |||
| No | 118 | 14 | 85 | 11 | 89 | 7 | 3 | |||
| 1q aberrations | .260 | .280 | .430 | |||||||
| Yes | 10 | 0 | 100 | 0 | 100 | 0 | 0 | |||
| No | 112 | 14 | 85 | 11 | 88 | 7 | 3 | |||
| chr 16 aberrations/ i(7) | .001* | <.001* | <.001* | |||||||
| Yes | 4 | 2 | 38 | 2 | 33 | 2 | 63 | |||
| No | 118 | 12 | 87 | 9 | 91 | 5 | 5 |
| . | N . | Events . | EFS, % . | P . | Deaths . | OS, % . | P . | Relapse/NR . | CIR/NR, % . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 170 | 19 | 87 | 16 | 89 | 9 | 6 | |||
| Sex | .008* | .002* | .330 | |||||||
| Male | 85 | 4 | 94 | 2 | 98 | 3 | 4 | |||
| Female | 85 | 15 | 79 | 14 | 81 | 6 | 8 | |||
| Age, y | .920 | .740 | .580 | |||||||
| <1 | 24 | 2 | 91 | 1 | 96 | 1 | 5 | |||
| 1 | 88 | 10 | 86 | 8 | 89 | 3 | 4 | |||
| 2 | 43 | 6 | 84 | 6 | 86 | 4 | 10 | |||
| ≥3 | 15 | 1 | 90 | 1 | 90 | 1 | 10 | |||
| WBC, ×109/L | .640 | .680 | .580 | |||||||
| <5 | 79 | 12 | 82 | 10 | 86 | 6 | 9 | |||
| 5-10 | 65 | 5 | 91 | 4 | 93 | 2 | 3 | |||
| 10-15 | 9 | 1 | 89 | 1 | 89 | — | — | |||
| ≥15 | 12 | 1 | 90 | 1 | 88 | 1 | 10 | |||
| Initial BM blasts, % | .100 | .100 | .060 | |||||||
| <20 | 74 | 12 | 81 | 11 | 83 | 7 | 11 | |||
| ≥20 | 83 | 6 | 92 | 5 | 93 | 2 | 3 | |||
| Early response, % BM blasts | <.001* | <.001* | .01* | |||||||
| ≤5 | 123 | 12 | 88 | 9 | 92 | 4 | 4 | |||
| >5 | 10 | 4 | 58 | 4 | 57 | 2 | 22 | |||
| Case history, wk | .076 | .250 | .070 | |||||||
| <3 | 49 | 3 | 92 | 3 | 95 | 1 | 2 | |||
| ≥3 | 44 | 8 | 79 | 6 | 83 | 5 | 13 | |||
| TAM history | .130 | .280 | .310 | |||||||
| No | 43 | 2 | 95 | 2 | 94 | 1 | 3 | |||
| Yes | 80 | 10 | 85 | 8 | 89 | 5 | 7 | |||
| GATA1 | .170 | .220 | .360 | |||||||
| Mutated | 98 | 11 | 87 | 9 | 90 | 5 | 6 | |||
| WT | 17 | 0 | 100 | 0 | 100 | 0 | 0 | |||
| Cytogenetics | 122 | |||||||||
| Normal (47,XY/XX +21c) | .480 | .330 | .160 | |||||||
| Yes | 29 | 2 | 91 | 1 | 97 | 0 | 0 | |||
| No | 93 | 12 | 84 | 10 | 88 | 7 | 8 | |||
| Gain of chr 8 | .018* | .071 | .020* | |||||||
| Yes | 37 | 8 | 73 | 6 | 77 | 5 | 16 | |||
| No | 85 | 6 | 91 | 5 | 95 | 2 | 3 | |||
| Monosomy 7/7q− | .350 | .460 | .500 | |||||||
| Yes | 7 | 0 | 100 | 0 | 100 | 0 | 0 | |||
| No | 115 | 14 | 85 | 11 | 89 | 7 | 3 | |||
| chr 13 aberrations | .460 | .520 | .590 | |||||||
| Yes | 4 | 0 | 100 | 0 | 100 | 0 | 0 | |||
| No | 118 | 14 | 85 | 11 | 89 | 7 | 3 | |||
| 1q aberrations | .260 | .280 | .430 | |||||||
| Yes | 10 | 0 | 100 | 0 | 100 | 0 | 0 | |||
| No | 112 | 14 | 85 | 11 | 88 | 7 | 3 | |||
| chr 16 aberrations/ i(7) | .001* | <.001* | <.001* | |||||||
| Yes | 4 | 2 | 38 | 2 | 33 | 2 | 63 | |||
| No | 118 | 12 | 87 | 9 | 91 | 5 | 5 |
—, none.
Bold P values are significant (<.05).
Multivariate analysis
In the multivariate analysis for 5-year EFS, including sex, age, early response, as well as cytogenetic subgroups (gain of chromosome 8, aberrations of chromosome 16 or isochromosome 7) as risk factors, poor early response (relative risk [RR] = 8.55; 95% CI, 1.96-37.29; Px2 = .004) was of independent prognostic significance (Table 3). Also, gain of chromosome 8 was an independent prognostic factor (RR = 4.36; 95% CI, 1.24-15.39; Px2 = .022). The remaining parameters were not of independent prognostic value.
Multivariable Cox regression analysis of clinical factors and cytogenetics for EFS
| N = 101 . | RR . | 95% CI . | P . |
|---|---|---|---|
| Sex | 3.65 | 0.76-17.48 | .105 |
| Age <2 y | 2.29 | 0.57-9.21 | .244 |
| Trisomy 8 | 4.36 | 1.24-15.39 | .022* |
| chr 16 aberrations/i(7) | 3.92 | 0.38-40.82 | .253 |
| Early response | 8.55 | 1.96-37.29 | .004 |
| N = 101 . | RR . | 95% CI . | P . |
|---|---|---|---|
| Sex | 3.65 | 0.76-17.48 | .105 |
| Age <2 y | 2.29 | 0.57-9.21 | .244 |
| Trisomy 8 | 4.36 | 1.24-15.39 | .022* |
| chr 16 aberrations/i(7) | 3.92 | 0.38-40.82 | .253 |
| Early response | 8.55 | 1.96-37.29 | .004 |
Bold P value is significant (<.05).
Toxicity
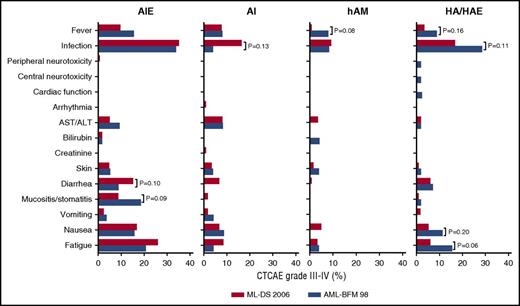
DS patients are highly susceptible to therapy-induced toxicity. To evaluate the toxicity of the ML-DS 2006 protocol, we analyzed the treatment-related adverse events after each course. Severe adverse events (CTCAE grade III or higher) were most frequently observed after induction. Thirty-five percent of the ML-DS patients presented with severe infections (Figure 3). Interestingly, the frequency of infections declined in the ML-DS patients after each block so that after the last course only 17% of the ML-DS patients presented with a grade III (or higher) infection. Other recurrent severe adverse events affected the skin or the gastrointestinal tract (vomiting/nausea, stomatitis, diarrhea) or fatigue (asthenia, lethargy, malaise).
Treatment-related toxicity of ML-DS 2006 patients. Percentage of ML-DS patients with severe adverse events (CTCAE grade III or higher) after each block of chemotherapy in comparison with the ML-DS patients, treated according to the AML-BFM 98 protocol. Only P values (Fisher exact) ≤.2 are shown. All other comparisons are PFisherExact > .2. ALT, alanine transaminase; AST, aspartate transaminase.
Treatment-related toxicity of ML-DS 2006 patients. Percentage of ML-DS patients with severe adverse events (CTCAE grade III or higher) after each block of chemotherapy in comparison with the ML-DS patients, treated according to the AML-BFM 98 protocol. Only P values (Fisher exact) ≤.2 are shown. All other comparisons are PFisherExact > .2. ALT, alanine transaminase; AST, aspartate transaminase.
To assess the impact of excluding etoposide from consolidation on toxicity, we compared the frequencies of treatment-related adverse events to the AML-BFM 98 trial (HA vs HAE; Figure 3). Fewer severe adverse events were reported after the HA block of the ML-DS 2006 trial compared with the HAE block of the AML-BFM 98 trial (RR, 0.559; 95% CI, 0.382-0.817). This was mainly attributed to a reduced frequency of severe adverse events due to infections (16.8% vs 28.6%; PFisherExact = .106), fever (3.5% vs 8.8%; PFisherExact = .164), nausea (5.5% vs 11.3%; PFisherExact = .163), and fatigue (asthenia, lethargy, malaise) (6.1% vs 15.5%; PFisherExact = .055), although this trend did not reach significance within each category. As expected, the analysis of the remaining courses (AIE, AI, haM), which were identical between the trials (except for CNS prophylaxis), did not show notable differences (Figure 3).
Five patients died due to therapy (cardiac failure, n = 2; infections, n = 3; Table 2) resulting in a TRM of 2.9% compared with 5% in the historical control arm (AML-BFM 98; PFisherExact= .276).
Discussion
ML-DS treatment schemata have to particularly strive for the balance between appropriate chemotherapy dosage and treatment-related toxicity.9-15 Here, we demonstrate that the international ML-DS 2006 study achieved an excellent outcome (5-year OS, 89% ± 3%; 5-year EFS, 87% ± 3%) for these children with tolerable toxicity. We established early response after 1 course of chemotherapy and trisomy 8 as independent prognostic markers.
The ML-DS 2006 trial was based on the reduced-intensity arm for ML-DS patients of the AML-BFM 98 trial. Due to the excellent outcome of the ML-DS patients in the AML-BFM 98 trial (n = 67; 5-year OS, 90% ± 4%; 5-year EFS, 89 ± 4), the treatment intensity was further reduced in the ML-DS 2006 trial by excluding etoposide from consolidation, administering 4 instead 11 doses of intrathecal CNS prophylaxis and excluding maintenance therapy. Despite this reduction, the outcome in both studies was in a similar range. The CIR/NR especially was identical in both studies (6%), validating that therapy reduction did not result in a higher relapse risk. The absence of CNS involvement in any of the patients might suggest that the ML-DS blasts cannot home to this niche and explain why we did not observe an increase in CNS relapses despite reduction of CNS prophylaxis. Although the TRM was not significantly reduced (2.9% vs 5%; PFisherExact = .276), excluding etoposide resulted in fewer severe adverse events after consolidation. However, the nonrandomized trial design and the comparison with a historical control is a potential weakness of the study, which was necessary due to the low number of ML-DS patients per year (expected accrual, 20 patients per year), and the expected low compliance with a more intense treatment arm. Still, the data may implicate that even further reduction of treatment intensity could be feasible based on prognostic factors.
Despite a general consent about longer treatment intervals and the discouraging role of hematopoietic stem cell transplantation, international study protocols for ML-DS differ substantially (Table 4).9-15 The role of high-dose cytarabine and the dosing of anthracyclines especially have yet to be defined. Whereas in most European and North American trials for ML-DS courses with high-dose cytarabine (3 g/m2 per day) are applied,9-12 Japanese studies (Japan Pediatric Leukemia/Lymphoma Study Group [JPLSG] AML D05) obtained excellent results (3-year OS: 88% ± 4% 3-year EFS: 83% ± 4%) and low TRM (1.4%) using standard-dose cytarabine (100 mg/m2 per day) only.15 Together with the results of the Toronto group that used a low-dose cytarabine-based regimen,13,32 which contained no anthracyclines and no etoposide, these data indicate that subgroups of patients with ML-DS can be cured even with much lower doses than in the current ML-DS 2006 trial. But the identification of clear prognostic factors that would predict which patients are at risk of relapse and need intense therapy remained elusive.21
Comparison of recent ML-DS trials
| . | Years . | N . | DNR, mg/m2 . | ARA-C, mg/m2 . | Etoposide, mg/m2 . | TRM, % . | 5-y OS, % . | 5-y EFS, % . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| ML-DS-06 | 2006-2015 | 170 | 240 | 27 400 | 450 | 2.9 | 89 | 87 | |
| AML-BFM 98 | 1998-2003 | 204 | 240 | 29 400 | 950 | 5.0 | 90 | 89 | 9 |
| COG A2971 | 1999-2003 | 132 | 320 | 27 200 | 0* | 2.3 | 84 | 79 | 12 |
| Al-Ahmari | 1990-2003 | 34 | 0 | 7 400 | 0 | 0 | 77 | 67 | 33 |
| JPLSG D05 | 2008-2010 | 72 | 250 | 3 500 | 1350 | 1.4 | 88 | 83 | 15 |
| . | Years . | N . | DNR, mg/m2 . | ARA-C, mg/m2 . | Etoposide, mg/m2 . | TRM, % . | 5-y OS, % . | 5-y EFS, % . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| ML-DS-06 | 2006-2015 | 170 | 240 | 27 400 | 450 | 2.9 | 89 | 87 | |
| AML-BFM 98 | 1998-2003 | 204 | 240 | 29 400 | 950 | 5.0 | 90 | 89 | 9 |
| COG A2971 | 1999-2003 | 132 | 320 | 27 200 | 0* | 2.3 | 84 | 79 | 12 |
| Al-Ahmari | 1990-2003 | 34 | 0 | 7 400 | 0 | 0 | 77 | 67 | 33 |
| JPLSG D05 | 2008-2010 | 72 | 250 | 3 500 | 1350 | 1.4 | 88 | 83 | 15 |
ARA-C, cytarabine; DNR, daunorubicin.
6-Thioguanine, 1600 mg/m2; l-asparaginase, 12 000 IU/m2.
Our study makes a significant step toward risk-adapted therapy in ML-DS. We show that the early therapy response, assessed by morphology at the start of the second block, is predictive of treatment outcome and relapse. Future trials will need to show whether monitoring of minimal residual disease could even increase this predictive value. Most importantly, however, our analysis showed that all relapses were in the cytogenetic groups of patients with trisomy 8, aberration of chromosome 16, or isochromosome 7. This means that none of the patients belonging to the other cytogenetic subgroups (n = 81) experienced a relapse. This includes patients with cytogenetic aberrations which were previously proposed to be associated with higher relapse risk, such as monosomy 7 or normal karyotype (ie, 47,XX/XY,+21c).14,21 Although the cytogenetic groups are small, limiting the confidence of the subgroup analysis, they are in accordance with a recent Japanese study15 that could neither confirm monosomy 7 nor normal karyotype as poor prognostic factors. Thus, reproducible and consistent data across the study groups imply that monosomy 7 and normal karyotype are not predictive for poor prognosis. Instead, our data indicate gain of chromosome 8 to predict a high relapse risk and poor EFS.
Despite the use of high-dose cytarabine, we observed a TRM of 2.9% (n = 5 of 170 patients), which is in the same range as the Children’s Oncology Group (COG) A2971 trial12 (2.3%; n = 3 of 132; PFisherExact = 1.000) that also used high-dose cytarabine, and which does not significantly differ from the JPSLG AML D05 trial15 (1.4%; n = 1/72; PFisherExact = 0.673) that used standard-dose cytarabine. Three patients died due to infections (Streptococcus mitis sepsis, fungal sepsis, and respiratory syncytial virus pneumonia, respectively). This is in contrast to a previous study which included the German ML-DS patients of the ML-DS 2006 trial and the AML-BFM 98 trial and found that all deaths due to infections were attributed to respiratory syncytial virus pneumonia.33 Two patients died due to cardiac failure, underscoring the sensitivity of children with DS to cardiotoxic agents. Thus, lowering cardiotoxicity, for example, by introducing liposomal formulations of daunorubicin,34 should be an aim for the development of future treatment protocols.
Our study demonstrates that ML-DS is not necessarily associated with high treatment-related toxicity even when compared with non-DS-AML patients (supplemental Figure 1). Knowing the high susceptibility of DS patients to cytostatic agents, this observation might be explained by 2 factors. First, ML-DS patients do not obtain 1 very intense course (high-dose cytarabine and mitoxantrone [HAM]) and the anthracycline doses are reduced in all courses. Second, the treatment intervals for ML-DS patients are longer (median interval between first [AIE] and second course [AI/HAM] for ML-DS vs non-DS-AML, 32 vs 25 days; P < .001) giving the patients more time to recover after each course; that is, a block was only started if the child showed recovery of blood counts and was in good general condition without clinical signs of an infection, mucositis, or fever.
Recently, wee1 kinase inhibitors35 or histone deacetylase inhibitors36,37 have been identified as promising classes of drugs for the future treatment of ML-DS. Those drugs can be introduced to upcoming ML-DS protocols to substitute other less tolerated chemotherapeutic agents while increasing/maintaining overall efficacy. With the dismal outcomes after relapse especially (7 of 9 relapsed patients died in the ML-DS 2006 trial), these new substances may help to further reduce therapy-related toxicity while preventing relapses.19
In summary, we show that therapy reduction could be achieved in children with ML-DS without compromising the excellent outcome. The identification of clinical and cytogenetic prognostic markers in our study offers new possibilities for risk-adapted therapy for children with ML-DS. This will help to further reduce therapy in low-risk patients and intensify treatment in high-risk patients to avoid relapses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all colleagues, data managers, and technicians of the participating hospitals for their valuable cooperation. The authors thank J. E. Müller (Hannover, Germany) for competent data management.
This work was supported by the Deutsche Krebshilfe e.V. and Beat Leukemia with Connor. J.-H.K. is a fellow of the Emmy Noether-Programme from the German Research Foundation (DFG; KL-2374/2-1).
Authorship
Contribution: U.C., H.H., C.M.Z., D.R., and J.-H.K. conceived and designed the study; H.H., C.M.Z., D.R., and J.-H.K. provided financial and administrative support; M.D., H.H., C.M.Z., D.R., and J.-H.K. provided study materials or patients; M.U., M.R., C.v.N., L.S., M.D., H.H., C.M.Z., D.R., and J.-H.K. collected and assembled data; M.U., M.R., C.v.N., M.Z., and J.-H.K. analyzed and interpreted data; M.U. and J.-H.K. wrote the manuscript; and all authors gave final approval of manuscript.
Conflict-of-interest disclosure: D.R. has consulting or advisory roles for Celgene, Pfizer, MSD, Astellas Pharma, Novartis, and Boehringer and receives research funding from Celgene. C.M.Z. has consulting or advisory roles for Pfizer, Daiichi Sankyo, Celgene, Novartis, Bristol-Myers Squibb, and Gilead Sciences and receives research funding from Karyopharm Therapeutics, Pfizer, Bristol-Myers Squibb, and GlaxoSmithKline. M.D. receives research funding from Beckman Coulter and Becton Dickinson. H.H. has a consulting or advisory role for Celgene. The remaining authors declare no competing financial interests.
Correspondence: Jan-Henning Klusmann, Department of Pediatric Hematology and Oncology, Hannover Medical School, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: klusmann.jan-henning@mh-hannover.de.
References
Author notes
H.H., C.M.Z., D.R., and J.-H.K. contributed equally to this work.

![Figure 1. ML-DS 2006 protocol compared with the historical control arm (AML-BFM 98). Scheme of the different study protocols as indicated. ML-DS 2006: AIE (cytarabine 100 mg/m2 per day [days 1-2] and 100 mg/m2 per 12 hours [days 3-8], idarubicin 8 mg/m2 per day [days 3, 5, and 7], and etoposide 150 mg/m2 per day [days 6-8]); AI (cytarabine 500 mg/m2 per day [days 1-4] and idarubicin 5 mg/m2 per day [days 3 and 5]); haM (cytarabine 1 g/m2 per 12 hours [days 1-3] and mitoxantrone 7 mg/m2 per day [days 3-4]); HA (high-dose cytarabine 3 g/m2 per 12 hours [days 1-3]). The cumulative doses were 27 400 mg/m2 cytarabine, 450 mg/m2 etoposide, 34 mg/m2 idarubicin, and 14 mg/m2 mitoxantrone. Chemotherapy for children younger than 12 months of age, or weighing <12 kg, was calculated based on body weight. In addition, patients received cytarabine intrathecally at the start of each treatment block (4 doses in total, 20-40 mg per dose adapted to age) as a CNS prophylaxis. AML-BFM 98 (reduced-intensity arm for children with ML-DS; historical control arm): AIE (cytarabine 100 mg/m2 per day [days 1-2] and 100 mg/m2 per 12 hours [days 3-8], idarubicin 8 mg/m2 per day [days 3, 5, and 7] and etoposide 150 mg/m2 per day [days 6-8]); AI (cytarabine 500 mg/m2 per day [days 1-4] and idarubicin 5 mg/m2 per day [days 3 and 5]); haM (cytarabine 1 g/m2 per 12 hours [days 1-3] and mitoxantrone 7 mg/m2 per day [days 3-4]); HAE (high-dose cytarabine 3 g/m2 per 12 hours [days 1-3] and etoposide 125 mg/m2 per day [days 2-5]). Maintenance therapy until 1.5 years after start of induction therapy was thioguanine daily 40 mg/m2 per os (p.o.) and cytarabine 40 mg/m2 subcutaneously (s.c.) every 4 weeks on 4 consecutive days. The cumulative doses were ∼29 400 mg/m2 cytarabine, 950 mg/m2 etoposide, 34 mg/m2 idarubicin, and 14 mg/m2 mitoxantrone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/25/10.1182_blood-2017-01-765057/6/m_blood765057f1.jpeg?Expires=1769096704&Signature=DhbyTzkCwQvbM7mSzalyeuobMrCEnhtSMiBdFksf-NiZYQxUvFMO8v61UeJTFDCOP~n2-8NBGeryhV~CenlE6GteVdOvZF2Pt3hpSBv7fMWSQYqEWcG684oJFmeQwqBbDUm3z9Q18Cq0lRdxKMpXlkg5xmsYTWbs8RfqeJIQXAhvtZ2YCRgMV5bJRAsIXdpjwkQgcBrKggzave3Y2un06AolP6S4LHRrSL4B~cHs~yolY35F7puxdSPwnaXZHF-rrXfSidzaW0grH4IJSGxD-KJt6QNqy8IVmw4hr8fuj8TX-XzOOwITfUYlsJh60fen7h2Ds1bANnr4Cb8vyeHFyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal