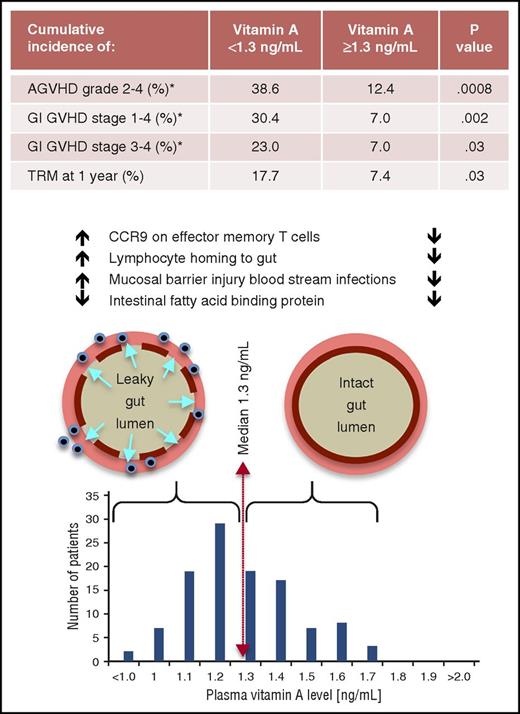
In this issue of Blood, Lounder et al report that, in children, vitamin A levels below the median at 30 days after hematopoietic stem cell transplant (HSCT) are associated with increased cumulative incidence of gastrointestinal (GI) graft-versus-host disease (GVHD).1
Vitamin A levels and posttransplant intestinal permeability, acute GVHD (AGVHD), GI GVHD, and treatment-related mortality (TRM). Vitamin A levels were normally distributed at 30 days posttransplant. Levels below the median were associated with significantly higher rates of acute GVHD (and specifically mild as well as severe acute GI GVHD) and TRM. Additional data in the article showed that lower vitamin A levels were associated with threefold higher rates of “mucosal barrier injury” blood stream infections (a surrogate marker for increased intestinal permeability) and increased CCR9 expression on effector memory T cells which promotes increased intestinal lymphocyte homing. For all variables shown, the converse was otherwise true for patients whose vitamin A levels were equal to or greater than the median. The 1 exception was that low levels of intestinal fatty acid–binding protein were not impacted by vitamin A status. *Cumulative incidence through day 100 posttransplant.
Vitamin A levels and posttransplant intestinal permeability, acute GVHD (AGVHD), GI GVHD, and treatment-related mortality (TRM). Vitamin A levels were normally distributed at 30 days posttransplant. Levels below the median were associated with significantly higher rates of acute GVHD (and specifically mild as well as severe acute GI GVHD) and TRM. Additional data in the article showed that lower vitamin A levels were associated with threefold higher rates of “mucosal barrier injury” blood stream infections (a surrogate marker for increased intestinal permeability) and increased CCR9 expression on effector memory T cells which promotes increased intestinal lymphocyte homing. For all variables shown, the converse was otherwise true for patients whose vitamin A levels were equal to or greater than the median. The 1 exception was that low levels of intestinal fatty acid–binding protein were not impacted by vitamin A status. *Cumulative incidence through day 100 posttransplant.
Vitamin A is an essential dietary nutrient, available to humans in 2 forms: preformed vitamin A (retinol and retinyl ester) and plant-based provitamin A carotenoids. Most of the body’s vitamin A is stored in the liver as hepatic retinyl esters. Vitamin A blood levels are homeostatically regulated to maintain a narrow range. Preformed and provitamin A must be metabolized intracellularly by gut mucosal cells to retinal and retinoic acid, the active forms that support biological functions. Lounder et al review vitamin A’s known immunological roles in the health of the intestinal mucosa; simply put, most of the existing literature supports its anti-inflammatory role in limiting chemical and infectious gut injuries (albeit some contradictory mouse data exist). Therefore, to understand their GVHD observation, Lounder et al explored associations between higher and lower vitamin A levels and measurements of retinol-binding protein 4, intestinal fatty acid–binding protein, interleukin-22 (IL-22), CCR9, and effector memory T cells (see figure).
In the last few decades, our understanding of GVHD pathophysiology has expanded much beyond conditioning-mediated disruption of the intestinal mucosa and the classic interaction of donor T cells with hematopoietic antigen-presenting cells. We better appreciate how perturbation of the fecal microbiota integrates with the cellular immune system to promote inflammatory disease and diarrhea. A nonexhaustive list of important players in this regard includes: intestinal stem cells and their closely aligned Paneth cells, the protective intestinal mucous layer, intestinal fatty acids like butyrate, bacterial composition of the feces and its regulation by defensins.2,3 Antibiotic therapy and diet can further disrupt the fecal microbiota and in turn affect mucosal integrity. It is worth viewing the intriguing results of Lounder et al through the lens of this broader understanding of GVHD pathophysiology and with a shift in focus to GVHD interventions that move beyond traditional systemic immunosuppressive therapies.
Recognizing the critical integration of fecal microbiota and gut immunology, our gastroenterology colleagues have also explored associations with intestinal disease. One study found that among children diagnosed with “persistent diarrhea” (>14 days) but not with inflammatory bowel disease (IBD) per se, diversity of the gut microbiota and its key bacterial phylotypes differed significantly between groups with normal or deficient vitamin A levels.4 They have also studied alternatives to systemic immunosuppressive therapy in IBD. A detailed and well-designed meta-analysis of therapy in pediatric Crohn disease examined 5 randomized clinical trials and 2 further nonrandomized trials.5 The authors concluded that exclusive enteral nutrition (EEN) was as effective as corticosteroids at inducing a remission. In adults, EEN has been less effective than corticosteroids. Greater noncompliance with 6 to 8 weeks of unpalatable EEN, and reluctance to be tube fed, at least contributes to inferior outcomes in adults.6 The use of more palatable polymeric formulae might enhance compliance. Enteral nutrition significantly modulates the microbiota in pediatric Crohn disease but, because microbiota diversity becomes even more narrow, there remains a need to better understand what exactly correlates with clinical improvement.7 These observations together with the well-known negative side effects of corticosteroids and other immunosuppressive agents should propel us to explore gentler and potentially equally efficacious alternative therapies to prevent or treat gut GVHD.
Will vitamin A become the next universal advance in HSCT supportive care? Some of us remember implementing a new standard of care in 2002 after Ruutu et al published (in Blood) their noteworthy results of a randomized prospective study showing significant survival benefit attributed to ursodeoxycholic acid prophylaxis after HSCT.8 Although the mechanisms behind this observation still lack clarity, their approach was associated with a significantly lower incidence of clinically relevant liver, intestinal, and skin GVHD. Recognizing the important role that vitamins play in preventing inflammatory diseases, others have explored vitamin D supplementation after HSCT and reported a reduction in grade 3-4 acute GVHD after allogeneic HSCT in recipients with normal baseline vitamin D levels.9 Borrowing from the management of necrotizing enterocolitis in premature infants, Lounder’s colleagues at Cincinnati Children’s Hospital also conducted a pilot study in small children of peritransplant and enterally administered donor human breast milk. They sought to investigate whether breast milk would be well tolerated and would modify the microbiome and reduce inflammation after HSCT based on the presence of human milk oligosaccharides which are noted to be anti-inflammatory.10 Control patients who received standard nutrition had significantly increased stool facultative anaerobes, Streptococcaceae, and Actinomycetaceae and elevated soluble IL-2 receptor levels (as an immunological marker of inflammation) compared with breast milk–fed children. A randomized study of this approach is in progress (registered at www.clinicaltrials.gov as #NCT02470104).
Although Lounder et al might one day advise that vitamin A supplementation be adopted as a universal standard practice for HSCT, they dutifully acknowledge that additional data are first needed. Comparative adult data could help address the generalizability of their results. It might be more feasible in adults than in children to measure intestinal permeability directly, rather than having to rely on the surrogate marker of mucosal barrier injury blood stream infections. Lounder et al wisely propose as next steps a pilot study of vitamin A supplementation in order to maintain levels in the 75th to 95th percentile for age early after HSCT, followed by the ultimate test of a randomized study to establish the clinical benefit of this approach.
Eventually, I suspect that further advancement in our understanding of how the microbiome, intestinal mucosa, and the immune system are integrated will lead to a multifaceted intestinal GVHD supportive care package which could include ursodeoxycholic acid, more judicious antibiotic choices, greater use of EEN or human breast milk, and attention to vitamin levels.
Conflict-of-interest disclosure: The author declares no competing financial interests.