Key Points
CD4+CD25+FoxP3+ T regulatory cells and CD11c+ dendritic cells protect against antibody-mediated murine TRALI.
Murine TRALI is associated with reduced IL-10 levels, and IL-10 administration prevents and rescues TRALI development.
Abstract
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-related fatalities and is characterized by acute respiratory distress following blood transfusion. Donor antibodies are frequently involved; however, the pathogenesis and protective mechanisms in the recipient are poorly understood, and specific therapies are lacking. Using newly developed murine TRALI models based on injection of anti–major histocompatibility complex class I antibodies, we found CD4+CD25+FoxP3+ T regulatory cells (Tregs) and CD11c+ dendritic cells (DCs) to be critical effectors that protect against TRALI. Treg or DC depletion in vivo resulted in aggravated antibody-mediated acute lung injury within 90 minutes with 60% mortality upon DC depletion. In addition, resistance to antibody-mediated TRALI was associated with increased interleukin-10 (IL-10) levels, and IL-10 levels were found to be decreased in mice suffering from TRALI. Importantly, IL-10 injection completely prevented and rescued the development of TRALI in mice and may prove to be a promising new therapeutic approach for alleviating lung injury in this serious complication of transfusion.
Introduction
Transfusion-related acute lung injury (TRALI) is a syndrome characterized by acute respiratory distress within 6 hours following blood transfusions and is presently the leading cause of transfusion-related mortality.1,2 Apart from supportive measures (oxygen and ventilation), no therapy currently exists for TRALI. A significant number of TRALI cases are related to the presence of HLA- or human neutrophil antigen–specific antibodies in the donor blood.3,4 A 2-hit model has been hypothesized to underlie antibody-mediated TRALI, where the first hit comprises patient-predisposing factors such as inflammation and the second hit is due to donor antibodies in the transfused blood.1 Other first-hit patient risk factors for TRALI include chronic alcohol abuse, liver surgery, shock, higher peak airway pressure while undergoing mechanical ventilation, smoking, and positive intravascular fluid balance.5 Systemic inflammation is characterized by elevated recipient interleukin-6 (IL-6)6,7 and IL-8 levels5-7 and appears to be a major risk factor for TRALI induction. More specifically, the acute phase protein C-reactive protein, a general biomarker for acute infection and inflammation, was recently shown to synergistically act with 34-1-2s to enhance antibody-mediated TRALI in otherwise TRALI-resistant mice.8 The lung injury of TRALI is considered part of the spectrum of acute respiratory distress syndrome, although the mechanisms responsible for TRALI are distinct from other lung injury states such as those associated with sepsis or aspiration-induced lung injury.1 The pathogenesis of antibody-mediated TRALI, however, is still poorly understood, although animal models have added to our understanding of some of the recipient mechanisms of TRALI induction, for example using a mouse-model based on injection of the anti–major histocompatibility complex (anti-MHC) class I antibody 34-1-2s.9 Polymorphonuclear neutrophils (PMNs), for instance, were shown to be directly stimulated by 34-1-2s enabling reactive oxygen species (ROS) production and subsequent damage to the pulmonary endothelium.9-17
Of interest, wild-type (WT) BALB/c mice are generally resistant to 34-1-2s antibody-mediated TRALI, whereas mice with severe combined immunodeficiency (SCID) are hypersensitive to the antibody’s effects.18 The relative protection of BALB/c against antibody-mediated TRALI was shown to be primarily mediated by T cells,18 although the T lymphocyte subsets responsible were not thoroughly analyzed. We demonstrate here that recipient resistance to the effects of TRALI-inducing antibodies is mediated via the Treg–DC axis related to an IL-10 response to the transfusion. More importantly, IL-10 administration completely protected against antibody-mediated TRALI induction, and this suggests that stimulating the IL-10 anti-inflammatory pathway may be a potent therapeutic strategy for alleviating the lung injury.
Methods
The study design is described in supplemental Methods (available on the Blood Web site).
Mice
BALB/c (H-2d, BALB/cAnNCrl) and C57BL/6 (H-2b, C57BL/6NCrl) mice were obtained from Charles River Laboratories (Montreal, QC, Canada). Gp91phox knockout (KO) mice (B6.129S6-Cybbtm1din/J background strain 129xC57BL/6), CD11c-DTR mice (B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J, background strain C57BL/6), and IL-10 KO mice (B6.129P2-Il10tm1Cgn/J, background strain C57BL/6) were obtained from The Jackson Laboratory (Bar Harbor, ME). All gp91phox KO mice were examined for ROS production and compared with WT C57BL/6 mice, as described in supplemental Methods. All mice were males and were housed for at least 1 week in our animal facility before initiating experiments at an age of 8 to 10 weeks. All animal studies were approved by the St. Michael’s Hospital Animal Care Committee, Toronto.
Antibodies and reagents, in vivo cell depletions, tissue processing, and bone marrow collection are described in supplemental Methods.
Antibody-mediated murine TRALI models
Mice were always weighed to determine the dose of the antibody. Depleting antibodies or isotype antibodies were administered prior to initiating TRALI. On the day of experiment, TRALI-inducing antibodies or isotype control antibodies were injected, which marked the initiation of the experiment. All matching isotype control antibodies were injected with the same dose of antibody as the TRALI antibody. For the BALB/c TRALI model, 34-1-2s (4.5. mg/kg) was injected IV in 100 µL injection volume. For the C57BL/6 TRALI model, a cocktail of 34-1-2s (45 mg/kg) and AF6-88.5.5.3 (4.5 mg/kg) was administered IV as a single injection in a 600 µL injection volume. TRALI-inducing antibody dosages were selected upon pre-experimentation and matching isotype control antibodies at the same dosages and volumes were used in each experiment to exclude any effects of protein- and volume-related hydrostatic lung injury. Upon injection of TRALI-inducing antibodies or isotype control antibodies, body temperatures indicative of systemic shock were recorded every 30 minutes (up to 90 minutes) using an RET-3 rectal probe for mice (VWR International, Mississauga, ON, Canada) connected to a traceable digital thermometer (model 77776-726; Physitemp Instruments, Inc., Clifton, NJ). Recombinant mouse IL-10 (carrier-free; BioLegend, San Diego, CA) was injected at 45 µg/kg IV either together with the TRALI antibodies (prophylactic) or 15 minutes after injection of TRALI antibodies (therapeutic). Therapeutic IL-10 injection was performed only after confirmation of at least a 2-degree drop in rectal temperatures 10 minutes after TRALI-antibody injection, indicative of the onset of murine TRALI. Ninety minutes after antibody injection, mice were anesthetized with Avertin (2% final in phosphate-buffered saline [PBS], intraperitoneal), and blood was collected by cardiac puncture for plasma cytokine analysis (described in supplemental Methods). The lungs were harvested for macroscopic observation (imaging), determination of lung wet-to-dry (W/D) weight ratios, pulmonary PMN counts, pulmonary myeloperoxidase (MPO) activity, and lung tissue histology as described in supplemental Methods. Notably, all lung tissue histology slides were blindly assessed by a board-certified and senior staff pathologist at St. Michael’s Hospital in Toronto (G.Y.). Lung function measurements were also performed as described in supplemental Methods.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.07 software for Windows (GraphPad Software, San Diego, CA) with statistical significance set at P < .05. The specific statistical tests used are listed in each of the figure legends. All error bars in the manuscript represent standard deviation (SD).
Results
CD4+ Tregs protect against antibody-mediated TRALI in BALB/c mice
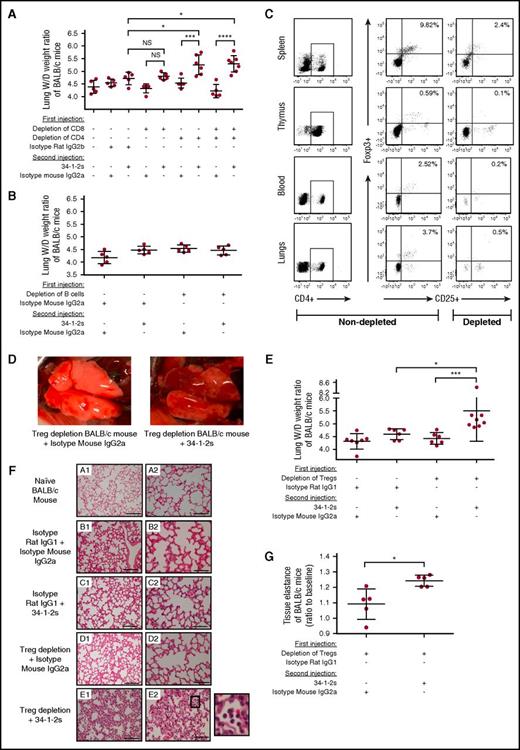
To determine the lymphocyte subset capable of protecting against murine antibody-mediated TRALI, in vivo depletion of BALB/c mice using antibodies specifically targeting either CD4+ and/or CD8+ T cells or B cells (supplemental Figures 1 and 2) was performed prior to inducing TRALI by injection with 34-1-2s. By 90 minutes after antibody infusion, only mice depleted of CD4+ T cells demonstrated a significant increase in lung W/D weight ratios (Figure 1A-B). To determine which subset of CD4+ T cells was responsible for the TRALI protection, CD4+CD25+ FoxP3+ Tregs were depleted in vivo by injection of an anti-CD25 depleting antibody, and depletion efficiency was analyzed in spleen, thymus, blood, and lungs (Figure 1C). This antibody did not affect the conventional CD4+ and CD8+ T cells (supplemental Figure 3). Treg depletion enabled the 34-1-2s TRALI-inducing antibody to cause significant acute lung injury as macroscopically observed with patchy atelectasis and hemorrhages (Figure 1D) and additionally measured by increased lung W/D weight ratios (Figure 1E). Lung histological analysis also demonstrated increased alveolar thickening, acute inflammatory infiltrates (mainly neutrophils in the airspaces), and alveolar exudates indicating pulmonary edema19 (Figure 1F), and increased tissue elastance was also observed (Figure 1G).
CD4+CD25+FoxP3+Tregs protect against antibody-mediated acute lung injury. (A-B) Lung W/D weight ratios of untreated (−) BALB/c mice or mice that first underwent in vivo depletions of T cells (A) or B cells (B) by the indicated antibody injections (+) and were then injected with the TRALI-inducing antibody 34-1-2s or indicated control antibodies. (C) Representative flow cytometric dot plot analysis of spleen, thymus, peripheral blood, and lung cells showing the extent of a typical in vivo depletion of CD4+CD25+FoxP3+ Tregs (splenic cells were depleted from 9.82% to 2.4%, thymic cells were depleted from 0.59% to 0.1%, peripheral blood cells were depleted from 2.52% to 0.2%, and lung cells were depleted from 3.7% to 0.5% of total CD4+ T cells). (D) Representative macroscopic images of lungs from a Treg-depleted mouse 90 minutes after injection of the indicated control antibody (left) or 34-1-2s (right). (E) Lung W/D weight ratios of CD4+CD25+FoxP3+ Treg-depleted BALB/c mice injected with the indicated isotype control antibodies and/or 34-1-2s. (F) Lung histology from BALB/c mice receiving the indicated antibody injections: subpanels A1-E1 and A2-E2 represent lung tissue images taken at magnification ×20 and ×40, respectively. Representative images of each indicated group are shown. A zoom of indicated square in E2 is depicted alongside and shows alveolar PMN infiltration. Scale bars represent 100 µM in subpanels A1-E1 and 50 µM in subpanels A2-E2. (G) Measurement of tissue elastance in Treg-depleted BALB/c mice that received either 34-1-2s injection or indicated isotype control. All mice were analyzed 90 minutes after the second injections. For the statistical analyses, only significant comparisons of interest are shown. Panel A was analyzed with a 1-way ANOVA with a Tukey’s post hoc test, panel E was analyzed with a 1-way ANOVA with Dunn’s post hoc test, and panel G was analyzed by a 1-tailed Mann-Whitney test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, ***P < .001, ****P < .0001. IgG, immunoglobulin G; NS, nonsignificant.
CD4+CD25+FoxP3+Tregs protect against antibody-mediated acute lung injury. (A-B) Lung W/D weight ratios of untreated (−) BALB/c mice or mice that first underwent in vivo depletions of T cells (A) or B cells (B) by the indicated antibody injections (+) and were then injected with the TRALI-inducing antibody 34-1-2s or indicated control antibodies. (C) Representative flow cytometric dot plot analysis of spleen, thymus, peripheral blood, and lung cells showing the extent of a typical in vivo depletion of CD4+CD25+FoxP3+ Tregs (splenic cells were depleted from 9.82% to 2.4%, thymic cells were depleted from 0.59% to 0.1%, peripheral blood cells were depleted from 2.52% to 0.2%, and lung cells were depleted from 3.7% to 0.5% of total CD4+ T cells). (D) Representative macroscopic images of lungs from a Treg-depleted mouse 90 minutes after injection of the indicated control antibody (left) or 34-1-2s (right). (E) Lung W/D weight ratios of CD4+CD25+FoxP3+ Treg-depleted BALB/c mice injected with the indicated isotype control antibodies and/or 34-1-2s. (F) Lung histology from BALB/c mice receiving the indicated antibody injections: subpanels A1-E1 and A2-E2 represent lung tissue images taken at magnification ×20 and ×40, respectively. Representative images of each indicated group are shown. A zoom of indicated square in E2 is depicted alongside and shows alveolar PMN infiltration. Scale bars represent 100 µM in subpanels A1-E1 and 50 µM in subpanels A2-E2. (G) Measurement of tissue elastance in Treg-depleted BALB/c mice that received either 34-1-2s injection or indicated isotype control. All mice were analyzed 90 minutes after the second injections. For the statistical analyses, only significant comparisons of interest are shown. Panel A was analyzed with a 1-way ANOVA with a Tukey’s post hoc test, panel E was analyzed with a 1-way ANOVA with Dunn’s post hoc test, and panel G was analyzed by a 1-tailed Mann-Whitney test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, ***P < .001, ****P < .0001. IgG, immunoglobulin G; NS, nonsignificant.
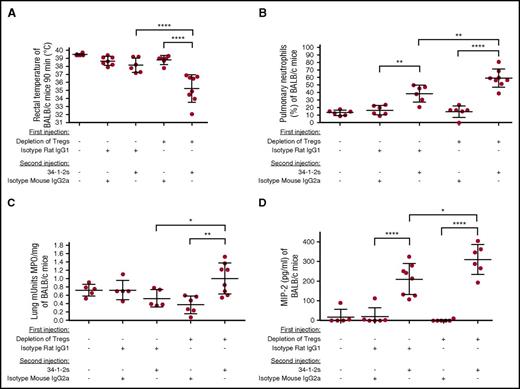
In vivo depletion of Tregs also caused several other physiological changes associated with the acute lung injury at 90 minutes after 34-1-2s injection. All Treg-depleted mice had a significant drop in rectal temperatures (Figure 2A), and pulmonary PMN accumulation was significantly increased compared with controls as determined by microscopic examination of lung tissue cytospins (Figure 2B) as well as biochemically by measuring lung tissue MPO activity (Figure 2C). Furthermore, compared with either Treg-depleted mice administered an isotype control antibody or nondepleted mice, Treg-depleted mice administered 34-1-2s showed significantly elevated serum macrophage inflammatory protein 2 (MIP-2; the murine equivalent of human IL-8) levels after injection of 34-1-2s (Figure 2D).
Tregs inhibit TRALI-associated physiological responses. (A) Rectal temperatures of naive BALB/c mice or mice first treated (+) or not (−) with the indicated antibodies for Treg depletion and then injected with the TRALI-inducing antibody 34-1-2s or indicated control antibodies. (B) Pulmonary PMN percentages in the BALB/c mice treated in panel A. (C) MPO activity in the lung tissue of Treg-depleted BALB/c mice treated in panel A. (D) Plasma MIP-2 levels of BALB/c mice treated in panel A. All mice were analyzed 90 minutes after the second injections. For the statistical analyses, only significant comparisons of interest are shown. Panels A-D were analyzed with a 1-way ANOVA with Tukey’s post hoc test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, **P < .01, ****P < .0001.
Tregs inhibit TRALI-associated physiological responses. (A) Rectal temperatures of naive BALB/c mice or mice first treated (+) or not (−) with the indicated antibodies for Treg depletion and then injected with the TRALI-inducing antibody 34-1-2s or indicated control antibodies. (B) Pulmonary PMN percentages in the BALB/c mice treated in panel A. (C) MPO activity in the lung tissue of Treg-depleted BALB/c mice treated in panel A. (D) Plasma MIP-2 levels of BALB/c mice treated in panel A. All mice were analyzed 90 minutes after the second injections. For the statistical analyses, only significant comparisons of interest are shown. Panels A-D were analyzed with a 1-way ANOVA with Tukey’s post hoc test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, **P < .01, ****P < .0001.
Antibody-mediated TRALI requires PMNs and ROS in C57BL/6 mice
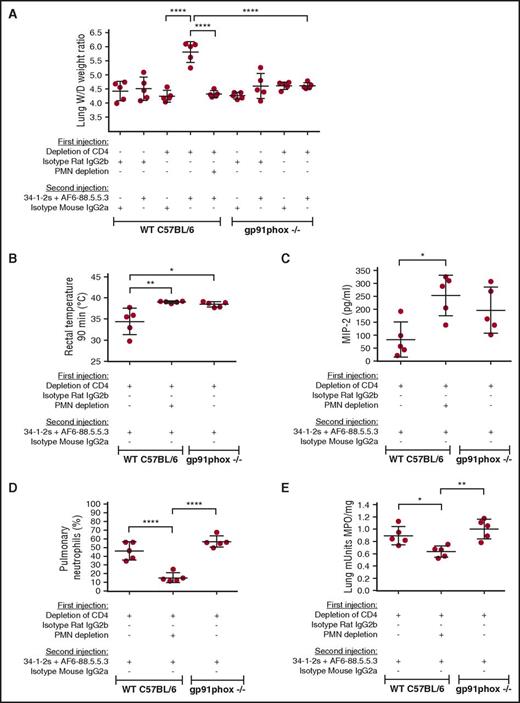
We developed an antibody-mediated TRALI model on the C57BL/6 background to enable us to conduct specific gene KO studies. C57BL/6 mice were injected with a cocktail of 2 MHC class I monoclonal antibodies, 34-1-2s and AF6-88.5.5.3. AF6-88.5.5.3 reacts specifically with the H-2Kb haplotype,20 whereas 34-1-2s is specific for the H-2K/Dd MHC antigens but also cross-reacts with MHC class I molecules of the H-2Kb haplotype.21 Similar to the BALB/c TRALI model, when C57BL/6 mice were CD4+ T-cell depleted and administered the cocktail of monoclonal antibodies, TRALI occurred and was characterized by significantly elevated lung W/D weight ratios (Figure 3A). The lung injury could be prevented by prior in vivo PMN depletion (supplemental Figure 4), suggesting that PMNs were the effector cells responsible for the acute lung damage (Figure 3A). PMN depletion did not exert a decrease in rectal temperatures in CD4+ T-cell–depleted WT mice upon TRALI-inducing antibody injection (Figure 3B) but did cause an increase in MIP-2 levels (Figure 3C). Further support for the role of PMNs as effector cells in TRALI was shown by demonstrating that the TRALI-inducing antibody cocktail did not stimulate lung injury in CD4+ T-cell–depleted gp91phox KO mice lacking ROS production (supplemental Figure 5; Figure 3A). Additionally, compared with CD4+ T-cell–depleted WT mice, the CD4+ T-cell–depleted gp91phox KO mice did not display a reduction in rectal temperatures (Figure 3B) and displayed statistically comparable levels of MIP-2 (Figure 3C), pulmonary PMN infiltration (Figure 3D), and lung MPO activity (Figure 3E).
PMNs and ROS are required for antibody-mediated TRALI induction. (A) Lung W/D weight ratios of WT C57BL/6 mice or gp91phox KO mice which first underwent in vivo depletions of CD4+ T cells and/or PMN by the indicated antibody injections (+) or not (−) and were then injected with the TRALI-inducing antibody cocktail 34-1-2s + AF6-88.5.5.3 or indicated control antibodies. (B-E) Rectal temperatures (B), plasma MIP-2 levels (C), pulmonary PMN percentages (D), and lung MPO enzyme activity (E) in CD4+ T cell-depleted WT C57BL/6 mice with or without PMN depletion or gp91phox KO mice injected (+) or not (−) with the indicated antibodies. All mice were analyzed 90 minutes after the second injections. For the statistical analyses, only significant comparisons of interest are shown. All panels were analyzed by a 1-way ANOVA with Tukey’s post hoc test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, **P < .01, ****P < .0001.
PMNs and ROS are required for antibody-mediated TRALI induction. (A) Lung W/D weight ratios of WT C57BL/6 mice or gp91phox KO mice which first underwent in vivo depletions of CD4+ T cells and/or PMN by the indicated antibody injections (+) or not (−) and were then injected with the TRALI-inducing antibody cocktail 34-1-2s + AF6-88.5.5.3 or indicated control antibodies. (B-E) Rectal temperatures (B), plasma MIP-2 levels (C), pulmonary PMN percentages (D), and lung MPO enzyme activity (E) in CD4+ T cell-depleted WT C57BL/6 mice with or without PMN depletion or gp91phox KO mice injected (+) or not (−) with the indicated antibodies. All mice were analyzed 90 minutes after the second injections. For the statistical analyses, only significant comparisons of interest are shown. All panels were analyzed by a 1-way ANOVA with Tukey’s post hoc test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, **P < .01, ****P < .0001.
DCs potently protect against antibody-mediated TRALI
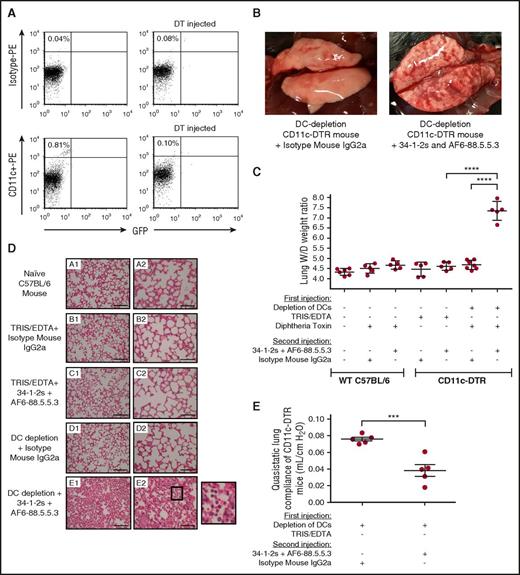
As Treg function is intimately associated with DCs, we determined whether DCs could also suppress TRALI. We depleted DCs in vivo by administering diphtheria toxin (DT) to CD11c-diphteria toxin receptor (DTR) transgenic mice,22 which caused significant depletion of CD11c+high cells 24 hours after DT injection (Figure 4A). Importantly, DC-depleted mice injected with the TRALI-inducing antibody cocktail displayed significant lung injury within 90 minutes, characterized by diffuse macroscopic pulmonary hemorrhage (Figure 4B), high lung W/D weight ratios (Figure 4C), and clear histologic signs of lung damage, including increased alveolar thickening, acute inflammatory infiltrates (mainly neutrophils in the alveolar space), and alveolar exudates indicating pulmonary edema19 (Figure 4D). Lung function was also heavily impaired in these mice, as evident from decreased quasistatic lung compliance (the slope of the pressure–volume loop and, as such, reflects the static elastic recoil pressure of the lungs at a given lung volume), which indicates the lungs are become “stiffer” (Figure 4E). To negate the effects of volume overload, none of these events occurred in control mice given identical volumes of isotype control antibodies (Figures 3-6).
DCs convey strong protection against antibody-mediated TRALI. (A) Representative flow cytometric dot plot analysis of splenocytes showing in vivo depletion of CD11chigh+ DCs (from 0.81% to 0.10% of total spleen cells) compared with isotype staining. (B) Representative macroscopic images of lungs from an in vivo DC-depleted mouse 90 minutes after injection of either a mouse immunoglobulin G2a (IgG2a) isotype control antibody (left) or 34-1-2s + AF6-88.8.8.3 (right). (C) Lung W/D weight ratios of WT C57BL/6 mice or CD11c-DTR mice that first underwent in vivo depletions of DC by the indicated injections (+) or not (−) of DT and were then injected with the TRALI-inducing antibody cocktail 34-1-2s + AF6-88.5.5.3 or indicated control antibodies. (D) Lung histology from naive WT C57BL/6 mice (subpanel A1-A2) and CD11c-DTR mice (subpanels B-E) receiving the indicated antibody injections. Subpanels A1-E1 and A2-E2 represent lung tissue images taken at original magnification ×20 and ×40, respectively. Representative images of each indicated group are shown. A zoom of indicated square in subpanel E2 (lung section from mice depleted of DCs and injected with 34-1-2s + AF6-88.5.5.3) is depicted and shows alveolar PMN infiltration. Scale bars represent 100 µM in subpanels A1-E1 and 50 µM in subpanels A2-E2. (E) Quasistatic lung compliance in DC-depleted CD11c-DTR mice that were injected (+) or not (−) with the indicated antibodies. All mice were analyzed 90 minutes after the second injection. For the statistical analyses, only significant comparisons of interest are shown. Panel C was analyzed with a 1-way ANOVA with a Tukey’s post hoc test, panel E was analyzed by a 1-tailed unpaired t test. Each dot represents 1 mouse, and error bars represent SD. ***P < .001, ****P < .0001.
DCs convey strong protection against antibody-mediated TRALI. (A) Representative flow cytometric dot plot analysis of splenocytes showing in vivo depletion of CD11chigh+ DCs (from 0.81% to 0.10% of total spleen cells) compared with isotype staining. (B) Representative macroscopic images of lungs from an in vivo DC-depleted mouse 90 minutes after injection of either a mouse immunoglobulin G2a (IgG2a) isotype control antibody (left) or 34-1-2s + AF6-88.8.8.3 (right). (C) Lung W/D weight ratios of WT C57BL/6 mice or CD11c-DTR mice that first underwent in vivo depletions of DC by the indicated injections (+) or not (−) of DT and were then injected with the TRALI-inducing antibody cocktail 34-1-2s + AF6-88.5.5.3 or indicated control antibodies. (D) Lung histology from naive WT C57BL/6 mice (subpanel A1-A2) and CD11c-DTR mice (subpanels B-E) receiving the indicated antibody injections. Subpanels A1-E1 and A2-E2 represent lung tissue images taken at original magnification ×20 and ×40, respectively. Representative images of each indicated group are shown. A zoom of indicated square in subpanel E2 (lung section from mice depleted of DCs and injected with 34-1-2s + AF6-88.5.5.3) is depicted and shows alveolar PMN infiltration. Scale bars represent 100 µM in subpanels A1-E1 and 50 µM in subpanels A2-E2. (E) Quasistatic lung compliance in DC-depleted CD11c-DTR mice that were injected (+) or not (−) with the indicated antibodies. All mice were analyzed 90 minutes after the second injection. For the statistical analyses, only significant comparisons of interest are shown. Panel C was analyzed with a 1-way ANOVA with a Tukey’s post hoc test, panel E was analyzed by a 1-tailed unpaired t test. Each dot represents 1 mouse, and error bars represent SD. ***P < .001, ****P < .0001.
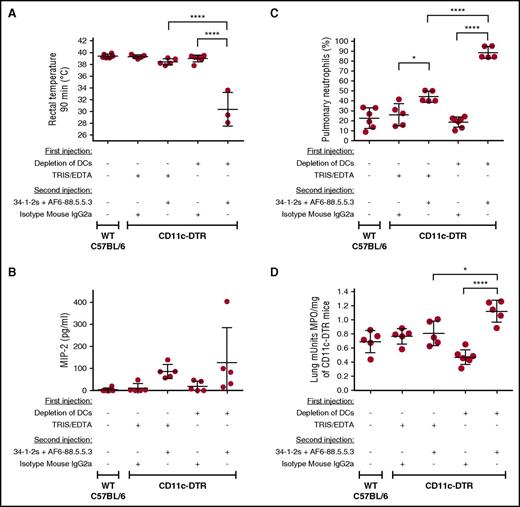
DCs inhibit TRALI-associated physiological responses. (A-D) Rectal temperatures (A), MIP-2 levels (B), pulmonary PMN percentages (C), and lung MPO enzyme activity (D) of either naive WT C57BL/6 mice or CD11c-DTR mice that were injected (+) or not (−) with the indicated reagents or antibodies. All mice were analyzed 90 minutes after the second injection. For the statistical analyses, only significant comparisons of interest are shown. The comparisons shown in panels A,C-D were analyzed by a 1-way ANOVA with a Tukey’s post hoc test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, ****P < .0001.
DCs inhibit TRALI-associated physiological responses. (A-D) Rectal temperatures (A), MIP-2 levels (B), pulmonary PMN percentages (C), and lung MPO enzyme activity (D) of either naive WT C57BL/6 mice or CD11c-DTR mice that were injected (+) or not (−) with the indicated reagents or antibodies. All mice were analyzed 90 minutes after the second injection. For the statistical analyses, only significant comparisons of interest are shown. The comparisons shown in panels A,C-D were analyzed by a 1-way ANOVA with a Tukey’s post hoc test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, ****P < .0001.
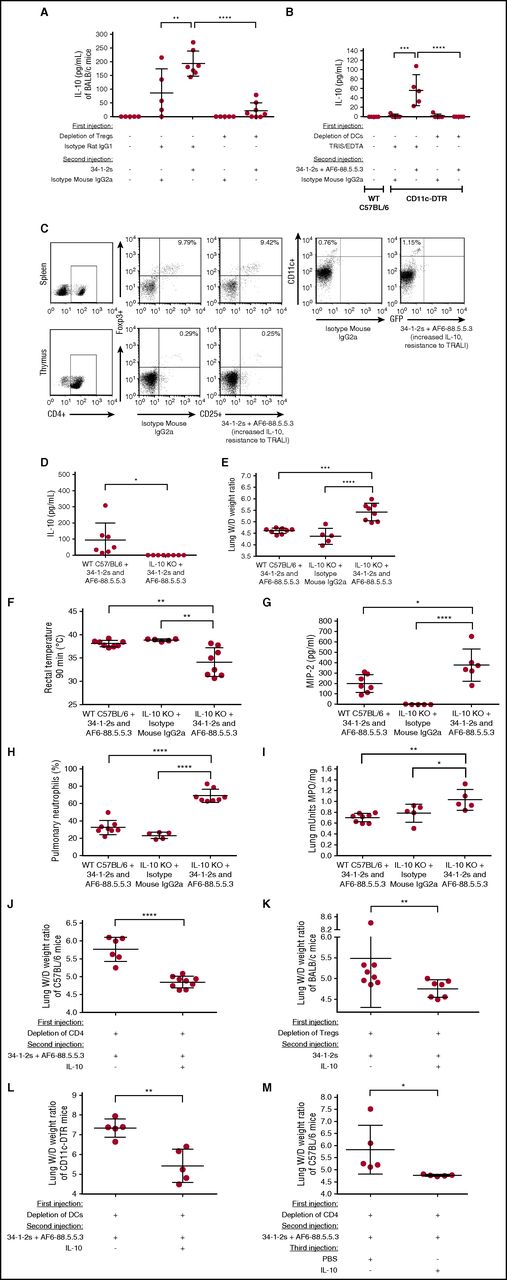
Tregs and DCs suppress TRALI by stimulating secretion of IL-10, a novel therapeutic agent. (A) Plasma IL-10 levels in naive BALB/c mice or mice first treated (+) or not (−) with the indicated antibodies for Treg depletion and then injected with the TRALI-inducing antibody 34-1-2s or indicated control antibodies. (B) Plasma IL-10 levels of naive C57BL/6 mice or CD11c-DTR mice injected (+) or not (−) with the indicated reagents or antibodies. (C) CD4+CD25+FoxP3+ Treg levels in spleen and thymus and CD11c+ DCs in spleen of the same C57BL/6 mouse injected with isotype mouse IgG2a or 34-1-2s + AF6-88.5.5.3 (TRALI resistant). (D) Plasma IL-10 levels in WT C57BL/6 mice or IL-10 KO mice treated 34-1-2s + AF6-88.5.5.3. (E-I) Lung W/D weight ratios (E), body temperature (F), MIP-2 levels (G), pulmonary PMN percentages (H), and lung MPO enzyme activity (I) of WT C57BL/6 mice treated with 34-1-2s + AF6-88.5.5.3 or IL-10 KO mice treated with either isotype mouse immunoglobulin G2a (IgG2a) or 34-1-2s + AF6-88.5.5.3. (I) Lung W/D weight ratios of CD4+ T-cell–depleted C57BL/6 mice (J), Treg-depleted BALB/c mice (K), and DC-depleted CD11c-DTR mice (L) infused with 34-1-2s + AF6-88.5.5.3 and treated prophylactically with (+) or not (−) with murine IL-10 administration (45 µg/kg IV). (M) Lung W/D weight ratios of CD4+ T cell depleted C57BL/6 mice, infused with 34-1-2s + AF6-88.5.5.3, and treated therapeutically 15 minutes later with (+) or without (−) murine IL-10 administration (45 µg/kg IV) after onset of TRALI (at least 2-degree drop in rectal temperature 10 minutes after TRALI-antibody injection). All mice were analyzed 90 minutes the second injection. For the statistical analysis, only significant comparisons of interest are shown. The comparisons shown in panels A-B,E-I were analyzed a with 1-way ANOVA with Tukey’s post hoc test; panels D,J,L-M were analyzed with 1-tailed unpaired t test; and panel K was analyzed with a 1-tailed Mann-Whitney test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Tregs and DCs suppress TRALI by stimulating secretion of IL-10, a novel therapeutic agent. (A) Plasma IL-10 levels in naive BALB/c mice or mice first treated (+) or not (−) with the indicated antibodies for Treg depletion and then injected with the TRALI-inducing antibody 34-1-2s or indicated control antibodies. (B) Plasma IL-10 levels of naive C57BL/6 mice or CD11c-DTR mice injected (+) or not (−) with the indicated reagents or antibodies. (C) CD4+CD25+FoxP3+ Treg levels in spleen and thymus and CD11c+ DCs in spleen of the same C57BL/6 mouse injected with isotype mouse IgG2a or 34-1-2s + AF6-88.5.5.3 (TRALI resistant). (D) Plasma IL-10 levels in WT C57BL/6 mice or IL-10 KO mice treated 34-1-2s + AF6-88.5.5.3. (E-I) Lung W/D weight ratios (E), body temperature (F), MIP-2 levels (G), pulmonary PMN percentages (H), and lung MPO enzyme activity (I) of WT C57BL/6 mice treated with 34-1-2s + AF6-88.5.5.3 or IL-10 KO mice treated with either isotype mouse immunoglobulin G2a (IgG2a) or 34-1-2s + AF6-88.5.5.3. (I) Lung W/D weight ratios of CD4+ T-cell–depleted C57BL/6 mice (J), Treg-depleted BALB/c mice (K), and DC-depleted CD11c-DTR mice (L) infused with 34-1-2s + AF6-88.5.5.3 and treated prophylactically with (+) or not (−) with murine IL-10 administration (45 µg/kg IV). (M) Lung W/D weight ratios of CD4+ T cell depleted C57BL/6 mice, infused with 34-1-2s + AF6-88.5.5.3, and treated therapeutically 15 minutes later with (+) or without (−) murine IL-10 administration (45 µg/kg IV) after onset of TRALI (at least 2-degree drop in rectal temperature 10 minutes after TRALI-antibody injection). All mice were analyzed 90 minutes the second injection. For the statistical analysis, only significant comparisons of interest are shown. The comparisons shown in panels A-B,E-I were analyzed a with 1-way ANOVA with Tukey’s post hoc test; panels D,J,L-M were analyzed with 1-tailed unpaired t test; and panel K was analyzed with a 1-tailed Mann-Whitney test. Each dot represents 1 mouse, and error bars represent SD. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Similar to the Treg-depleted mice, DC depletion followed by injection of the TRALI-inducing antibodies also resulted in a significant drop in rectal temperature (Figure 5A) and similar MIP-2 levels (Figure 5B) but a very high percentage of pulmonary PMN accumulation (Figure 5C) and lung MPO activity (Figure 5D). Moreover, 60% of the DC-depleted TRALI mice died within 90 minutes after antibody injection, with death being accompanied by excessive oral frothing, which may also have prevented any further increase in MIP-2 levels. Sodium dodecyl sulfate gel electrophoresis of the froth revealed the presence of a large amount of albumin and other proteins (supplemental Figure 6).
Murine TRALI is associated with reduced IL-10 levels, and IL-10 administration fully prevents and rescues TRALI development
We next tested if Tregs and DCs may be conveying their TRALI protective effects via secretion of IL-10, a cytokine with potent anti-inflammatory properties.23 When TRALI-resistant WT BALB/c mice were injected with 34-1-2s, there was a significant increase in plasma IL-10 production (Figure 6A); however, if the mice were first depleted of Tregs and then infused with 34-1-2s, IL-10 production was significantly diminished (Figure 6A). To address the role of DCs in IL-10 production, CD11c-DTR mice either depleted of DCs or not were injected with the TRALI-inducing antibody cocktail. Nondepleted CD11c-DTR mice infused with the TRALI-inducing antibody cocktail demonstrated a significant increase in IL-10 production, whereas DC depletion abolished IL-10 production (Figure 6B). TRALI-resistant mice, which were injected with anti-MHC class I antibodies and displayed increased IL-10 levels, demonstrated a 1.5-fold increase in DCs, whereas the Treg numbers remained relatively unaltered (Figure 6C).
When IL-10 KO mice were treated with the TRALI-inducing antibodies, as expected, there was no effect on IL-10 production (Figure 6D), but the infused mice showed significantly increased lung W/D weight ratios (Figure 6E), decreased rectal temperatures (Figure 6F), increased MIP-2 levels (Figure 6G), and increased pulmonary PMN accumulation (Figure 6H) and lung MPO activity (Figure 6I). To directly test the prophylactic and TRALI-preventing potential of IL-10, TRALI-susceptible CD4+ T-cell–depleted C57BL/6 mice were coinjected with IL-10 or PBS together with the TRALI-inducing antibodies. Compared with depleted mice receiving PBS, administration of IL-10 resulted in the complete prevention of TRALI development (Figure 6J). Similarly, and more specifically, IL-10 coinjection with the TRALI-inducing antibodies also prevented severe acute injury upon Treg depletion (Figure 6K) or DC depletion (Figure 6L). To test the therapeutic potential of IL-10 in rescuing TRALI, IL-10 was injected 15 minutes after onset of TRALI (after at least a 2-degree drop in rectal temperatures after 10 minutes) and resulted in normalization of rectal temperatures (supplemental Figure 8) and complete rescue of TRALI compared with mice injected with PBS (Figure 6M). All the IL-10–injected mice (Figure 6J-M) also had significantly higher plasma IL-10 levels compared with the PBS-injected control mice (data not shown).
Discussion
In the current study, we investigated the pathogenesis of antibody-mediated TRALI using newly developed immunocompetent murine models. The 34-1-2s antibody used in this study was initially described as a TRALI-inducing antibody in BALB/c mice in 2006 and approximates human TRALI well.9 Our current murine models, which are novel expansions of the original model, also approximate human anti-HLA class I antibody–mediated TRALI closely based on several parameters. First, in human TRALI, anti-HLA class I antibodies are frequently implicated in pulmonary reactions in ∼19% to 50% of cases,24-26 and our murine models are based on anti-MHC class I antibody injection. Toy et al did not find a significant role for anti-HLA class I antibodies as a risk factor for human TRALI in their study5 ; however, not all anti-HLA class I antibodies are pathogenic, and the cohort by Toy and colleagues may contain a significant degree of nonpathogenic anti-HLA antibodies. In support, several studies have described transfused recipients in whom TRALI did not develop despite receiving cognate antibodies in the transfused product.27-29 In addition, anti-HLA class I antibodies have been implicated in severe as well as fatal cases of TRALI,26 which coincides with the 60% mortality we observed in the case of murine DC-depleted TRALI induction (Figures 4 and 5). Secondly, human TRALI occurs very rapidly, mostly occurring during transfusion or within the first 1 or 2 hours after transfusion,30-33 and these temporal kinetics are very similar to what we observed in our murine models (severe TRALI reactions within 90 minutes). Thirdly, pulmonary edema is one the hallmarks of acute lung injury and has been described in TRALI patients.34-36 The murine models also show similar pulmonary edema assessed by increased lung W/D weight ratios as well as high levels of protein content in the pulmonary froth (Figures 1A,E, 3A, 4C, and 6E,J-M; supplemental Figure 5). Fourthly, we recently confirmed that injection of 34-1-2s into mice results in significant thrombocytopenia,8 a finding that has also been reported in human TRALI patients.37-40 Lastly, we can clearly demonstrate pulmonary PMN accumulation in murine TRALI by histology as well as biochemically and this has also been observed in the lungs of TRALI patients upon autopsy.41 Taken together, these novel murine models, on a BALB/c and importantly on a C57BL/6 background, will provide a means to further advance our knowledge of TRALI pathogenesis by allowing us to use genetically altered mice to interrogate in vivo physiological responses.
The group that originally described 34-1-2s as a TRALI-inducing antibody in 20069 subsequently could not replicate the antibody-mediated TRALI response.42 They attributed this to a change in animal housing from standard vivarium housing to a pathogen-free housing.42 This is in accordance with our previous data demonstrating that BALB/c mice are resistant to 34-1-2s-TRALI induction.8,18 We demonstrated that infusion of the acute phase protein C-reactive protein together with 34-1-2s could break that resistance and induce TRALI in BALB/c mice.8 Moreover, in the current paper, we uncovered a novel TRALI-protective axis consisting of the classical immunosuppressive CD4+CD25+FoxP3+ Tregs, DCs, and their ability to stimulate secretion of IL-10. Using a murine SCID model, we previously suggested that T cells are protective in TRALI18 ; however, the protective effect was also found for CD8+ T cells. In that study, CD8+ T cells were positively selected in vitro via an anti-CD8 antibody, and the selected CD8+ T cells with bound anti-CD8 antibodies were subsequently injected into SCID mice. We believe the protective effect of that infusion may not have been due to the CD8+ T cells themselves but more likely due to the anti-CD8 antibody, which remained bound to the CD8+ T cells. Those antibodies may have induced immune complex formation or blockade of Fc receptors in vivo, prior to the infusion of the 34-1-2s antibody. As Fc receptors have been suggested to be crucial in MHC class I–mediated TRALI,9 blockade of Fc receptors by those anti-CD8 antibodies most likely prevented 34-1-2s–mediated TRALI in the SCID model. Using our immunocompetent murine models in the current study, without adoptive transfer of positively selected cells, we demonstrate that only removal of CD4+ Tregs, DCs, or IL-10 resulted in severe TRALI reactions upon MHC class I antibody injection (Figures 1E, 4C, and 6E). DC depletion in our murine model involved CD11c+ DCs, of which at least a fraction appeared to be CD8+ (25%) and CD4+ (15%), as determined by anti-CD4 and anti-CD8 treatment (supplemental Figure 7). We believe that likely there is hierarchy of effects in which CD11c+ DCs play a more prominent role in antibody-mediated TRALI than Tregs. Firstly, when depleting CD11c+ DCs in vivo, the severity of antibody-mediated acute lung injury was significantly worse than when eliminating Tregs in vivo (lung W/D ratios of 7.33 on average plus 60% mortality upon DC depletion vs lung W/D ratios of 5.50 on average plus no deaths upon Treg depletion; Figures 4C and 1E). These effects also corresponded to IL-10 levels, as IL-10 dropped to nearly undetectable levels upon DC depletion (Figure 6B), while upon Treg depletion, there was still some detectible IL-10 present despite being significantly lower (Figure 6A). Moreover, we have shown that within the same TRALI-resistant mice (no cellular depletion in vivo) in which IL-10 levels were significantly increased upon TRALI-inducing antibody injection, the CD11c+ DC numbers were increased while the Treg numbers stayed relatively constant (Figure 6C).
Secretion of the anti-inflammatory cytokine IL-10 is known to require stimulation by agents such as microbial products or specific antibodies.23 Accordingly, WT mice (BALB/c or C57BL/6) stimulated with the anti-MHC class I antibodies had significantly elevated levels of plasma IL-10 and were resistant to TRALI induction (Figure 6A-B). We investigated if this resistance was caused by a direct hit by the TRALI antibodies on DCs or Tregs by isolating CD11C+ DCs and CD4+CD25+FoxP3+ Tregs, culturing them with the TRALI antibodies, and analyzing the supernatants for IL-10. We could not detect any IL-10 production in vitro (data not shown), indicating that the TRALI antibodies may be working indirectly and required in vivo factors are missing in vitro. As mentioned, we could demonstrate that in this TRALI-resistant setting, CD11c+ DCs were 1.5-fold increased while Treg numbers remained relatively constant (Figure 6C), suggesting that the DCs are perhaps higher in the hierarchy of protecting against TRALI, although further studies are needed to more thoroughly test this hypothesis. In contrast to the TRALI-resistant setting, depletion of Tregs or DCs reversed the parameters; depleted mice had significantly lower levels of plasma IL-10 along with severe TRALI reactions (Figure 6A-B). Furthermore, IL-10 KO mice succumbed to TRALI upon anti-MHC class I antibody injection (Figure 6E). Furthermore, our murine findings are in full agreement with a previous study where IL-10 levels were increased in patients with transfusion-associated circulatory overload (n = 29) but low in patients with TRALI (n = 70).7 In apparent contrast, however, the same group found IL-10 levels to be increased in TRALI patients in an earlier study (n = 38).43 We believe this discrepancy could be explained by the fact that they originally investigated fold-changes of paired samples prior to and following transfusion43 in contrast to the comparison of posttransfusion TRALI samples vs transfused controls.7 In addition, Looney et al originally demonstrated that an injection of 34-1-2s into BALB/c mice stimulated increased plasma levels of IL-10 while inducing TRALI9 ; however, that response was subsequently lost,42 and our current data do not support increased IL-10 levels in antibody-mediated TRALI (Figure 6A-B). Taken together, in contrast to other acute transfusion-induced lung disorders such as transfusion-associated circulatory overload, TRALI may perhaps be uniquely characterized by lack of IL-10 secretion.
In addition to demonstrating that removal of the Treg–DC–IL-10 axis in vivo resulted in severe lung injury after anti-MHC class I antibody infusion, we validated an important role for PMNs and ROS in mediating the lung damage (Figure 3). We show that neutrophil ROS production was critically involved in exerting TRALI damage and that increased MIP-2 levels were associated with increased neutrophil influx into the lungs. While the cellular source of MIP-2 was not evaluated, our previous work in SCID mice suggests that monocytes are the most likely source of the chemokine.44 In the current study, however, mice depleted of Tregs and treated with 34-1-2s had significantly higher plasma MIP-2 levels than non-depleted mice, suggesting that Tregs also may suppress monocyte chemokine production.45
Apart from IL-8 being a known risk factor for human TRALI, one of the other major risk factors for TRALI is chronic alcohol abuse.1,5 We believe our current data may explain this risk factor for TRALI as peripheral CD4+CD25+ Tregs are significantly decreased in patients with alcoholic hepatitis, a condition generally resulting from chronic alcohol abuse.46 Moreover, our data suggest that patients with deficiencies in Treg numbers such as autoimmune diseases like immune thrombocytopenia47 may be at increased risk for TRALI induction. Interestingly, CD4+CD25+Foxp3+ Tregs have also been shown to resolve acute lung injury in an lipopolysaccharide lung injury model without involvement of pathogenic antibodies.48 This report, however, implicated Tregs to be involved after the onset of the acute lung injury insult in the recovery phase, while to our knowledge, our current study is the first to show that in antibody-mediated lung injury, Tregs actively contribute to the protection against the TRALI reaction. In addition, an antibody-independent Th17–Treg imbalance with associated decreased IL-10 levels was reported in smoke inhalation-induced acute lung injury in rats, as well as in a mouse model of smoke-exposed chronic obstructive pulmonary disease,49,50 and other studies have found that IL-10 protects against non-antibody mediated acute lung injury in murine models.51-53 Taken together, the results indicate that Tregs and IL-10 are 2 of the critical factors which can protect a host against various forms of lung injury and the current paper demonstrates that this is also applicable to TRALI. Although there are no current therapies for TRALI apart from oxygen/ventilator support, aspirin,42 and IV immunoglobulin54 were suggested to prevent TRALI in a lipopolysaccharide-infused BALB/c and SCID mouse model, respectively. The effect of aspirin, however, was found to be irreproducible by another group.55 We have not tested the effects of aspirin or IV immunoglobulin in our current TRALI mouse models. Our data show that both prophylactic (Figure 6J-L) as well as therapeutic IL-10 administration (Figure 6M) can prevent and rescue the development of antibody-mediated TRALI in mice. IL-10-therapy could be very feasible in humans for several reasons. Only short-acting IL-10 effects are needed (sufficient IL-10 levels for up to 6 hours posttransfusion). In addition, IL-10 administration to healthy volunteers has previously been shown to be safe and well tolerated without serious side effects at doses of up to 25 µg/kg and with only mild to moderate flu-like symptoms occurring in a fraction of the volunteers at doses up to 100 µg/kg.56 In the current paper, we tested a dose of 45 µg/kg and, consistent with the published human studies, we also did not observe any side effects in the mice. It will be important to validate the findings of this paper on the human level, both mechanistically and therapeutically. Future studies will investigate the role of human Tregs and DCs and their association with IL-10 in protecting against TRALI in a human setting. The cross-talk between Tregs and DCs will be further evaluated, including a possible signaling during TRALI-protection through the dendritic cell c-type lectin receptor DC-specific ICAM-3–grabbing nonintegrin, as for instance DC-specific ICAM-3–grabbing nonintegrin was shown to be essential for IL-10 production by DCs in a setting of human leprosy.57 Interestingly, Tregs have also been suggested to inhibit allergic and asthmatic immune responses via IL-10 in experimental models.58 While we have not shown a direct proof that DCs or Tregs are the source of the IL-10, we do show that they are involved in the protection against TRALI via IL-10. Alternatively, other cells may be responsible for IL-10 production, and we are currently studying this (also using human cells). Therapeutically, we suggest that IL-10 administration may be a highly promising and feasible translational strategy to explore in targeting human TRALI. As our TRALI models are specifically antibody mediated, IL-10 therapy may not be effective in treating other forms of acute lung injury.
In conclusion, a novel first-hit factor we have identified in murine TRALI is the lack of an inhibitory response released by Tregs and DCs. This lack of inhibitory response was shown to be associated with low IL-10 levels, and the lack of this inhibitory signal could be compensated by administration of IL-10, both prophylactically and therapeutically, thereby preventing and rescuing TRALI development in mice. IL-10 therapy may potentially rescue transfused patients from antibody-mediated severe acute lung injury and possibly mortality.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Health Canada and Canadian Blood Services (J.W.S. and W.M.K.) and the National Institutes of Health (NIH) (M.T.R. and A.S.W.). R.K. is the recipient of a postdoctoral fellowship from Canadian Blood Services. M.J.M. is supported by a Canadian Blood Services and Health Canada Graduate Research Fellowship. S.S. was the recipient of a Canadian Blood Services Summer Studentship. A.Z. is the recipient of a postdoctoral fellowship from the Swiss National Science Foundation (P300PB-164760). M.T.R. is supported by the NIH, National Heart, Lung, and Blood Institute (grants HL112311 and HL126547) and the NIH, National Institute on Aging (grant AG048022). A.S.W. is supported by the NIH, National Heart, Lung, and Blood Institute (grants HL112311 and HL126547).
Authorship
Contribution: R.K. designed all research, performed experiments, collected data, analyzed and interpreted data, performed statistical analyses, made the figures, and wrote and edited the paper; M.K., R.A., M.J.M., J.L., Y.L., and S.S. performed experiments and collected data; A.T. and A.L. conducted murine lung function measurements; G.Y. assessed lung tissue histology slides; M.T.R., W.M.K., L.P., and A.S.S. edited the manuscript; all authors, including E.R.S., A.S.W., A.Z., and H.Z., analyzed and interpreted data; and J.W.S. provided financial resources and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.Z. is Centre de Recherche du CHU de Québec, CHUL-UL, Québec, QC, Canada.
Correspondence: John W. Semple, Lund University, BMC C14, Klinikgatan 26, 221 84 Lund, Sweden; e-mail: john_w.semple@med.lu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal