Key Points
Significant improvement in outcome is a reality for newly diagnosed AL amyloidosis in the past decade.
Six-month mortality among transplant ineligible patients has declined since 2005.
Abstract
In light of major advances in immunoglobulin light chain (AL) amyloidosis, we evaluated the trends in presentation, management, and outcome among 1551 newly diagnosed AL amyloidosis patients seen in our institution from 2000 to 2014. As compared with the 2 intervals 2000-2004 and 2005-2009, patients diagnosed in 2010-2014 were less likely to have >2 involved organs. Utilization of autologous stem cell transplant (ASCT) was similar across all periods, about one-third of patients, but there was an increase in the use of pre-ASCT bortezomib induction and of unattenuated melphalan conditioning in 2010-2014 compared with earlier periods. Non-ASCT first-line regimen changed with 65% of patients in 2010-2014 received bortezomib-based therapy, 79% of patients in 2005-2009 received melphalan-dexamethasone, and 64% of patients in 2000-2004 received melphalan-prednisone. The rate of better than very good partial response (VGPR) was higher in more recent periods (66% vs 58% vs 51%; P = .001), a change largely driven by improved VGPR rates in the non-ASCT population. Overall survival (OS) has improved, with inflection points for improvement differing for the ASCT and non-ASCT groups. In the ASCT population, the greatest gains were after 2010 (4-year OS, 91% compared with 73% and 65%). In the non-ASCT group, greatest gains were after 2005 (4-year OS, 38%, 32%, and 16%). Fewer patients died within 6 months of diagnosis in the 2 later periods (24% vs 25% vs 37%; P < .001). Overall, outcomes among patients with AL amyloidosis have improved with earlier diagnosis, higher rates of VGPR, lower early mortality, and improved OS.
Introduction
The field of immunoglobulin light chain (AL) amyloidosis has witnessed significant advances in diagnosis, treatment options, and response assessment methods over the past 15 years. The introduction of serum free light chain (sFLC) assays early in the millennium enabled the detection and quantification of the amyloidogenic light chain in the serum, which may be at a level below the detection sensitivity of serum protein electrophoresis and immunofixation.1 Moreover, sFLC assays allowed the establishment of reliable and validated response criteria.2 Confirmation of the amyloid precursor protein type can be ascertained with high sensitivity and specificity using mass spectrometry sequencing,3 which was introduced into our routine practice in 2009. Treatment options have expanded, following advances in the field of multiple myeloma. High-dose melphalan followed by autologous stem cell transplant (ASCT), first reported in AL amyloidosis in 1993,4 has made a significant impact on the ability to achieve deep hematological response, organ response, and improved survival in patients eligible for this procedure.5 For patients ineligible for ASCT, melphalan in combination with dexamethasone (MDex) showed effective results.6 Bortezomib added to the alkylator/corticosteroid backbone has increased the rate of deep hematological responses,7,8 and bortezomib-based regimens are the most used induction regimens. Immunomodulatory drugs (IMiDs) have been less effective and less well tolerated in AL patients compared with myeloma,9-11 but remain an effective treatment option for some patients. The use of daratumumab, an anti-CD38 monoclonal antibody approved in 2015 for myeloma, is an appealing treatment option because of its single-agent activity and minimal toxicity.12,13 Moreover, monoclonal antibodies targeting amyloid deposits are undergoing studies with promising results.14,15
The survival of patients with AL amyloidosis has improved over time, but 6-month mortality had largely remained unchanged over multiple decades.16,17 In light of major advances in recent years, we aimed to study the trends in patients and disease characteristics, treatment use, and outcome in 1551 newly diagnosed AL amyloidosis patients seen in our institution between 2000 and 2014.
Patients and methods
A total of 1551 patients with biopsy-proven systemic AL amyloidosis who were seen in our institution within 90 days of diagnosis between 1 January 2000 and 31 December 2014 were included in this study. Patients were excluded if they had been previously treated for AL amyloidosis or multiple myeloma, had AL amyloidosis resulting from a lymphoproliferative disorder, or had incidental amyloid deposits in bone marrow (BM)/fat tissue without demonstrable visceral involvement. The study was approved by the Mayo Foundation institutional review board. All patients gave written informed consent for medical record review according to the institutional review board and Minnesota state law. Data were extracted from a prospectively maintained database and all patients' charts were reviewed to assure data completeness and accuracy.
The study cohort was divided into 3 cohorts that spanned equal time intervals based on the year of diagnosis—2000-2004 (n = 422, 27% of all patients), 2005-2009 (n = 604, 39%), and 2010-2014 (n = 525, n = 34%)—to assess trends in patients’ characteristics, management, and outcome over the years. If not otherwise specified, data by period are presented in the following order: 2010-2014 followed by 2005-2009 and 2000-2004. Treatment was categorized as ASCT (upfront or following induction treatment), melphalan-prednisone (MP), MDex, bortezomib-based regimen, IMiD-based regimen, and single-agent dexamethasone. The following patients were not included in the analyses by treatment category: patients who received no treatment (n = 120; 8%); type of treatment was unconfirmed (n = 46; 3%); and patients treated with other forms of therapy (n = 6; anthracycline-based treatment [n = 3]; heart transplant only [n = 2]; alemtuzumab [n = 1]).
For BM plasma cell (BMPC) percentage, the highest estimate of the BM aspiration and biopsy was used. The estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease equation. Serum alkaline phosphatase is presented as a relative proportion of the upper limit of normal (ULN) at the time the test was performed adjusted to sex and age. Organ involvement was defined according to consensus criteria.18 To estimate the number of organs involved, only heart, kidney, liver, and nerve were included.
Hematological response to treatment was available in 966 of the 1385 evaluable patients (70%), largely missing in patients who died within 6 months of diagnosis (78% of which lacked response evaluation). The hematological response was based on consensus criteria,2 defining 4 groups of response using the sFLC: complete response, very good partial response (VGPR), partial response (PR), and no response. Of note, the majority of patients in the 2000-2004 period had incomplete data for staging, mainly resulting from a lack of N-terminal prohormone of brain natriuretic peptide (NT-proBNP)/BNP levels at diagnosis (83% of patients in the 2000-2004 period could not be staged by the Mayo 2004 staging, whereas 69% of the patients in the 2000-2004 period could not be assigned by the Mayo 2012 staging).
The χ2 and Fisher's exact tests were used to compare differences between continuous variables, and the Wilcoxon signed-rank test was used for nonparametric group comparisons. Binary logistic regression model was built to identify independent predictors of death in the first 6 months of diagnosis (ie, early death). Results of the binary logistic regression model were reported as odds ratios and their 95% confidence intervals. Survival analysis was done using the Kaplan-Meier method. Progression-free survival (PFS) was defined as time from diagnosis to disease progression or death (available for 1314 patients, 85% of study population). For the definition of progression, we used both hematological progression (n = 338) and organ progression (n = 226), as defined by consensus guidelines.19 In patients who died before response was available, we considered an event in those who died within 6 months of their diagnosis. Overall survival (OS) was calculated from time of diagnosis to death or last follow-up. Variables associated with OS on univariate analysis at P < .1 were entered into a Cox proportional hazards regression models for multivariate analysis. The univariate and multivariate analyses for OS were performed separately for ASCT and non-ASCT patients because of the major differences between groups, which may affect the variables associated with survival in each set of patients. Statistical analysis was performed on JMP software (SAS, Cary, NC).
Results
The baseline characteristics of the entire cohort (n = 1551) and by diagnosis periods are presented in Table 1. Patients from the 2010-2014 period were slightly older, more likely to be seen within 30 days of their diagnosis (67% vs 58% vs 64%; P = .004), reside a little closer to the Mayo Clinic (median, 321 vs 379 vs 368 miles; P = .01), and were less likely to have extensive organ involvement (defined as more than 2 involved organs) compared with the 2005-2009 and 2000-2004 periods (15% vs 24% vs 20%, respectively; P < .001). Moreover, the rates of cardiac and liver involvement were significantly lower in the 2010-2014 period compared with the 2005-2009 and 2000-2004 periods (heart, 71% vs 79% vs 76%: P = .01; liver, 16% vs 22% vs 27%; P < .001). Levels of NT-proBNP did not change over time, but patients in the most recent period had lower levels of troponin T (P = .008). Patients with hepatic involvement had similar levels of serum alkaline phosphatase between periods (median, 2.5-fold ULN vs 2.4-fold ULN vs 2.5-fold ULN; P = .91).
Baseline and treatment characteristics of the entire cohort and by time period
| Characteristic . | Entire cohort (N = 1551) . | 2010-2014 (N = 525) . | 2005-2009 (N = 604) . | 2000-2004 (n = 422) . | P, entire cohort . | P, 2005-2009 vs 2010-2014 . |
|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 63 (55-70) | 63 (56-71) | 62 (55-69) | 62 (54-69) | .08 | .03 |
| Age ≥65 y, n (%) | 688 (44) | 253 (48) | 248 (41) | 187 (44) | .055 | .01 |
| Male, n (%) | 976 (63) | 337 (64) | 376 (62) | 263 (62) | .76 | .5 |
| Organ involved, n (%) | ||||||
| >2 organs | 307 (20) | 79 (15) | 145 (24) | 83 (20) | <.001 | <.001 |
| Cardiac | 1173 (76) | 375 (71) | 478 (79) | 320 (76) | .01 | .002 |
| Renal | 947 (61) | 307 (58) | 396 (66) | 244 (58) | .01 | .01 |
| Hepatic | 329 (21) | 84 (16) | 131 (22) | 114 (27) | <.001 | .01 |
| Neurological | 318 (21) | 120 (23) | 127 (21) | 71 (17%) | .06 | .45 |
| λ restricted, n (%) | 1166 (75) | 397 (76) | 460 (76) | 309 (73) | .54 | .83 |
| BMPCs, median (IQR) | 10 (7-20) | 10 (6-20) | 10 (6-20) | 12 (8-22) | .06 | .76 |
| dFLC | n = 1356 | n = 523 | n = 594 | n = 239 | ||
| Median, mg/dL (IQR) | 23.7 (10.4-62.2) | 24.5 (10.1-62.1) | 22.3 (9.5-61.6) | 24.8 (12.8-64.5) | .17 | .33 |
| ≥18 mg/dL, n (%) | 803 (59) | 305 (58) | 344 (58) | 154 (64) | .18 | .89 |
| NT-proBNP | n = 1132 | n = 501 | n = 556 | n = 75 | ||
| Median, pg/mL (IQR) | 2438 (470-7025) | 2389 (424-6669) | 2475 (561-7310) | 1767 (406-8286) | .4 | .2 |
| ≥8500 pg/mL, n (%) | 224 (21) | 102 (20) | 122 (22) | 17 (23) | .78 | .52 |
| Troponin T | n = 1321 | n = 509 | n = 584 | n = 228 | ||
| Median, ng/mL (IQR) | 0.03 (<0.01-0.08) | 0.02 (<0.01-0.07) | 0.03 (<0.01-0.09) | 0.03 (<0.01-0.09) | .008 | .003 |
| ≥0.035 ng/mL, n (%) | 610 (46) | 216 (42) | 281 (48) | 113 (5) | .09 | .06 |
| ≥0.06 ng/mL, n (%) | 463 (35) | 151 (30) | 224 (38) | 88 (39) | .004 | .002 |
| eGFR, mL/min per 1.73 m2, median (IQR) | 64 (45-79) | 66 (47-86) | 64 (43-79) | 59 (43-71) | <.001 | .010 |
| eGFR <30 mL/min per 1.73 m2, n (%) | 198 (13) | 52 (10) | 87 (14) | 59 (14) | .05 | .02 |
| Urinary proteinuria, g per 24 h, median (IQR) | 1.4 (0.2-5.7) | 1.2 (0.2-5.3) | 1.6 (0.2-5.9) | 1.4 (0.2-5.8) | .39 | .18 |
| Mayo AL amyloidosis 2004 stage, % | n = 1123 | n = 498 | n = 552 | n = 73 | ||
| I/II/III | 19/38/43 | 21/38/41 | 16/39/45 | 23/30/47 | .16 | .11 |
| Mayo AL amyloidosis 2012 stage, % | n = 1206 | n = 503 | n = 572 | n = 131 | ||
| I/II/III/IV | 21/22/25/32 | 23/23/23/31 | 19/22/27/32 | 24/24/20/32 | .64 | .43 |
| Treatment categories, n (%)* | <.001 | <.001 | ||||
| ASCT | 482 (31) | 172 (33) | 177 (29) | 133 (32) | ||
| MP | 155 (10) | 2 (<1) | 12 (2) | 141 (34) | ||
| MDex | 420 (27) | 97 (18) | 288 (48) | 35 (8) | ||
| Bortezomib-based | 222 (14) | 201 (38) | 20 (3) | 1 (<1) | ||
| IMiD-based | 58 (4) | 10 (2) | 30 (5) | 18 (4) | ||
| Single-agent dexamethasone | 42 (3) | 0 | 16 (3) | 26 (6) | ||
| Unknown | 46 (3) | 10 (2) | 20 (3) | 16 (4) | ||
| No treatment | 120 (8) | 33 (6) | 40 (7) | 47 (11) | ||
| Hematological response to first-line treatment (n = 966) (%) | <.001 | .008 | ||||
| CR | 335 (35) | 137 (36) | 145 (36) | 53 (29) | ||
| VGPR | 241 (25) | 113 (30) | 88 (22) | 40 (22) | ||
| PR | 208 (21) | 81 (21.5) | 92 (22) | 35 (19) | ||
| NR | 182 (19) | 47 (12.5) | 80 (20) | 55 (30) |
| Characteristic . | Entire cohort (N = 1551) . | 2010-2014 (N = 525) . | 2005-2009 (N = 604) . | 2000-2004 (n = 422) . | P, entire cohort . | P, 2005-2009 vs 2010-2014 . |
|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 63 (55-70) | 63 (56-71) | 62 (55-69) | 62 (54-69) | .08 | .03 |
| Age ≥65 y, n (%) | 688 (44) | 253 (48) | 248 (41) | 187 (44) | .055 | .01 |
| Male, n (%) | 976 (63) | 337 (64) | 376 (62) | 263 (62) | .76 | .5 |
| Organ involved, n (%) | ||||||
| >2 organs | 307 (20) | 79 (15) | 145 (24) | 83 (20) | <.001 | <.001 |
| Cardiac | 1173 (76) | 375 (71) | 478 (79) | 320 (76) | .01 | .002 |
| Renal | 947 (61) | 307 (58) | 396 (66) | 244 (58) | .01 | .01 |
| Hepatic | 329 (21) | 84 (16) | 131 (22) | 114 (27) | <.001 | .01 |
| Neurological | 318 (21) | 120 (23) | 127 (21) | 71 (17%) | .06 | .45 |
| λ restricted, n (%) | 1166 (75) | 397 (76) | 460 (76) | 309 (73) | .54 | .83 |
| BMPCs, median (IQR) | 10 (7-20) | 10 (6-20) | 10 (6-20) | 12 (8-22) | .06 | .76 |
| dFLC | n = 1356 | n = 523 | n = 594 | n = 239 | ||
| Median, mg/dL (IQR) | 23.7 (10.4-62.2) | 24.5 (10.1-62.1) | 22.3 (9.5-61.6) | 24.8 (12.8-64.5) | .17 | .33 |
| ≥18 mg/dL, n (%) | 803 (59) | 305 (58) | 344 (58) | 154 (64) | .18 | .89 |
| NT-proBNP | n = 1132 | n = 501 | n = 556 | n = 75 | ||
| Median, pg/mL (IQR) | 2438 (470-7025) | 2389 (424-6669) | 2475 (561-7310) | 1767 (406-8286) | .4 | .2 |
| ≥8500 pg/mL, n (%) | 224 (21) | 102 (20) | 122 (22) | 17 (23) | .78 | .52 |
| Troponin T | n = 1321 | n = 509 | n = 584 | n = 228 | ||
| Median, ng/mL (IQR) | 0.03 (<0.01-0.08) | 0.02 (<0.01-0.07) | 0.03 (<0.01-0.09) | 0.03 (<0.01-0.09) | .008 | .003 |
| ≥0.035 ng/mL, n (%) | 610 (46) | 216 (42) | 281 (48) | 113 (5) | .09 | .06 |
| ≥0.06 ng/mL, n (%) | 463 (35) | 151 (30) | 224 (38) | 88 (39) | .004 | .002 |
| eGFR, mL/min per 1.73 m2, median (IQR) | 64 (45-79) | 66 (47-86) | 64 (43-79) | 59 (43-71) | <.001 | .010 |
| eGFR <30 mL/min per 1.73 m2, n (%) | 198 (13) | 52 (10) | 87 (14) | 59 (14) | .05 | .02 |
| Urinary proteinuria, g per 24 h, median (IQR) | 1.4 (0.2-5.7) | 1.2 (0.2-5.3) | 1.6 (0.2-5.9) | 1.4 (0.2-5.8) | .39 | .18 |
| Mayo AL amyloidosis 2004 stage, % | n = 1123 | n = 498 | n = 552 | n = 73 | ||
| I/II/III | 19/38/43 | 21/38/41 | 16/39/45 | 23/30/47 | .16 | .11 |
| Mayo AL amyloidosis 2012 stage, % | n = 1206 | n = 503 | n = 572 | n = 131 | ||
| I/II/III/IV | 21/22/25/32 | 23/23/23/31 | 19/22/27/32 | 24/24/20/32 | .64 | .43 |
| Treatment categories, n (%)* | <.001 | <.001 | ||||
| ASCT | 482 (31) | 172 (33) | 177 (29) | 133 (32) | ||
| MP | 155 (10) | 2 (<1) | 12 (2) | 141 (34) | ||
| MDex | 420 (27) | 97 (18) | 288 (48) | 35 (8) | ||
| Bortezomib-based | 222 (14) | 201 (38) | 20 (3) | 1 (<1) | ||
| IMiD-based | 58 (4) | 10 (2) | 30 (5) | 18 (4) | ||
| Single-agent dexamethasone | 42 (3) | 0 | 16 (3) | 26 (6) | ||
| Unknown | 46 (3) | 10 (2) | 20 (3) | 16 (4) | ||
| No treatment | 120 (8) | 33 (6) | 40 (7) | 47 (11) | ||
| Hematological response to first-line treatment (n = 966) (%) | <.001 | .008 | ||||
| CR | 335 (35) | 137 (36) | 145 (36) | 53 (29) | ||
| VGPR | 241 (25) | 113 (30) | 88 (22) | 40 (22) | ||
| PR | 208 (21) | 81 (21.5) | 92 (22) | 35 (19) | ||
| NR | 182 (19) | 47 (12.5) | 80 (20) | 55 (30) |
Boldface indicates significance at P < .05.
CR, complete response; NR, no response.
6 patients received other forms of therapy.
Renal involvement was less common in 2010-2014 in comparison with 2005-2009 only (58% vs 66% vs 58%, respectively; P = .01). Overall, the eGFR varied over time, but 24-hour urine protein did not (Table 1). For those patients with renal involvement, the eGFR was higher in 2010-2014 compared with the other respective periods (64 vs 59 vs 58 mL/min per 1.73 m2; P = .004).
There was no significant difference in tumor burden between periods as measured by difference between involved and uninvolved light chains (dFLC). BMPC percentage by aspiration/biopsy was marginally lower in 2010-2014 and 2005-2009 compared with 2000-2004 (median, 10% vs 10% vs 12%; P = .06). No differences in the major fluorescent in situ hybridization abnormalities were seen between periods (available since 2005; t(11;14) 48% in 2010-2014 vs 51% in 2005-2009, P = .18; chromosome 13 abnormalities 38% vs 34%, P = .22; trisomies 29% vs 23%, P = .11).
Baseline characteristics among the ASCT and non-ASCT cohorts by time period and by treatment category can be viewed in supplemental Tables 1, 2, and 3, respectively, available on the Blood Web site.
Trends in first-line treatment
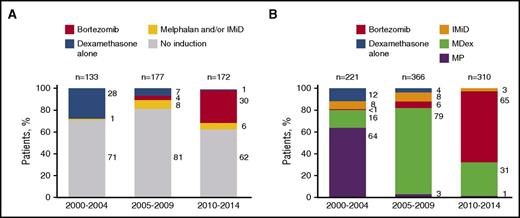
Treatment categories by period can be viewed in Table 1. The proportion of patients who did not receive any form of therapy was lower in 2010-2014 and 2005-2009 compared with 2000-2004 (6% vs 7% vs 11%, respectively; P = .05). Approximately one-third of patients received ASCT over the 3 periods, although patients transplanted in 2010-2014 were less likely to have >1 involved organs (40% vs 49% vs 53%; P = .05), were more likely to receive induction before ASCT (38% vs 19% vs 29%; P < .0001), and were more likely to have ASCT conditioning with melphalan 200 mg/m2 (82% vs 60% vs 61%; P < .001). In addition, bortezomib was more frequently used as a pre-ASCT induction in the most recent period (Figure 1A). Duration of pre-ASCT induction changed over time; the median number of cycles was 3 in the 2010-2014 period compared with 2 in the 2000-2004 and 2005-2009 periods (P < .001).
Treatment distribution by period. (A) Induction among ASCT patients. (B) Regimens used among non-ASCT patients.
Treatment distribution by period. (A) Induction among ASCT patients. (B) Regimens used among non-ASCT patients.
For patients not receiving ASCT, the first-line regimens used changed over the 3 periods (Figure 1B). MP was the most commonly used regimen in the 2000-2004 period (64% of all non-ASCT regimens at that time). Its use fell to 3% by 2005-2009 as MDex use increased to 79%. By 2010-2014, MDex use receded to 31% with bortezomib-based regimens being used in 65% of the non-ASCT patients. IMiD use as first-line treatment was never very high, decreasing from 8% to 3% in the most recent period. The number of treatment cycles was available for 644 of 897 (72%) patients treated with nontransplant regimens. The median number of cycles was 5 (interquartile range [IQR], 2-7) in 2010-2014, 5 (IQR 2-8) in 2005-2009, and 4 (IQR, 1-7) in 2000-2004 (P = .03).
Trends in response to first-line treatment
For the entire cohort, the proportion of patients who achieved VGPR or better to first-line treatment was higher in more recent periods (66% vs 58% vs 51%; P = .001). However, no difference in the complete response rate was seen over time, 36% in both 2010-2014 and 2005-2009 and 29% in 2000-2004 (P = .22). Fewer patients failed to achieve at least PR in 2010-2014 (12%) compared with 2005-2009 (20%) and 2000-2004 (30%; P < .001). Among patients who received ASCT, no significant change was noted in the rates of ≥VGPR over time (77% vs 70% vs 73%, respectively; P = .42). There was no difference in rates of ≥VGPR between those who received prior induction or underwent upfront ASCT [76% vs 73%; P = .47]). In contrast, among patients treated with nontransplant regimens, the rate of ≥VGPR improved significantly over time (58% vs 49% vs 24%, respectively; P < .001). Rates of ≥VGPR by regimen type are presented in supplemental Figure 1.
The rate of organ response also improved in 2010-2014 compared with prior periods (56% vs 49% vs 44%, respectively; P = .02). This improvement in organ response was more significant among patients treated with nontransplant therapies (43% vs 37% vs 21%, respectively; P < .001) than among patients who underwent ASCT (74% vs 66% vs 65%, respectively; P = .2).
Trends in early mortality
Fewer patients died within 6 months of diagnosis in the more recent periods (24% vs 25% vs 37% of all patients, respectively; P < .001). This observation held true for both the ASCT-treated patients (2% vs 7% vs 11%; P = .007) and non-ASCT patients (35% vs 33% vs 49%; P < .001), with a notable inflection point for the non-ASCT patients after 2004. By regimen type, death rates within 6 months of diagnosis were as follows: ASCT, 6%; MP, 43%; MDex, 26%; bortezomib-based, 31%; IMiD-based, 31%; and dexamethasone alone, 57%. The difference in early death was no different between MDex and bortezomib-based therapy (P = .2). By response, early death rates were marginally higher in those patients who were treated with bortezomib and attained VGPR or better compared with those treated with MDex and achieved a similar degree of response (6% vs 2%, P = .06), but not among those attaining PR or less (22% vs 31%; P = .21). Rates of early death in MDex- and bortezomib-treated patients across different subgroups are presented in supplemental Table 4. The results of a binary logistic regression to identify independent predictors for early death can be viewed in Table 2. Predictors for early death were >2 involved organs, dFLC ≥18 mg/dL, and troponin ≥0.035 ng/mL. Compared with MDex, the use of a bortezomib-based regimen had an odds ratio for early death of 1.5 (95% confidence interval, 1.02-2.3) in the non-ASCT cohort (P = .03).
Univariate and multivariate analysis for predictor of early death (<6 mo) in the non-ASCT population
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| . | OR . | 95% CI . | P . | OR . | 95% CI . | P . |
| Age ≥65, y | 1.0 | 0.7-1.3 | .83 | NI | ||
| Male | 1.2 | 0.9-1.6 | .23 | NI | ||
| >2 organs involved | 1.5 | 1.1-2.1 | <.001 | 1.6 | 1.1-2.5 | .02 |
| dFLC ≥18 mg/dL | 3.0 | 2.1-4.3 | <.001 | 2.1 | 1.4-3.3 | <.001 |
| BMPCs ≥10% | 1.4 | 1.03-1.8 | .02 | NS | ||
| eGFR <30 mL/min per 1.73 m2 | 0.7 | 0.5-1.1 | .13 | NI | ||
| Mayo 2004 stage | ||||||
| I | Reference | Reference | ||||
| II | 3.1 | 1.1-13.3 | .03 | 2.0 | 0.7-8.9 | .22 |
| III | 11.7 | 4.2-48.8 | <.001 | 6.1 | 2.1-26.4 | <.001 |
| NT-proBNP > 8500 pg/mL | 3.4 | 2.4-4.9 | <.001 | 2.0 | 1.3-3.0 | <.001 |
| Treatment category | ||||||
| MDex | Reference | Reference | ||||
| MP | 2.1 | 1.4-3.1 | <.001 | 2.1 | 0.8-5.6 | .13 |
| Bortezomib-based | 1.3 | 0.9-1.8 | .21 | 1.5 | 1.02-2.3 | .03 |
| IMiD-based | 1.3 | 0.7-2.2 | .46 | 1.5 | 0.7-3.3 | .28 |
| Dexamethasone alone | 3.7 | 1.9-7.2 | <.001 | 3.4 | 1.01-11 | .047 |
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| . | OR . | 95% CI . | P . | OR . | 95% CI . | P . |
| Age ≥65, y | 1.0 | 0.7-1.3 | .83 | NI | ||
| Male | 1.2 | 0.9-1.6 | .23 | NI | ||
| >2 organs involved | 1.5 | 1.1-2.1 | <.001 | 1.6 | 1.1-2.5 | .02 |
| dFLC ≥18 mg/dL | 3.0 | 2.1-4.3 | <.001 | 2.1 | 1.4-3.3 | <.001 |
| BMPCs ≥10% | 1.4 | 1.03-1.8 | .02 | NS | ||
| eGFR <30 mL/min per 1.73 m2 | 0.7 | 0.5-1.1 | .13 | NI | ||
| Mayo 2004 stage | ||||||
| I | Reference | Reference | ||||
| II | 3.1 | 1.1-13.3 | .03 | 2.0 | 0.7-8.9 | .22 |
| III | 11.7 | 4.2-48.8 | <.001 | 6.1 | 2.1-26.4 | <.001 |
| NT-proBNP > 8500 pg/mL | 3.4 | 2.4-4.9 | <.001 | 2.0 | 1.3-3.0 | <.001 |
| Treatment category | ||||||
| MDex | Reference | Reference | ||||
| MP | 2.1 | 1.4-3.1 | <.001 | 2.1 | 0.8-5.6 | .13 |
| Bortezomib-based | 1.3 | 0.9-1.8 | .21 | 1.5 | 1.02-2.3 | .03 |
| IMiD-based | 1.3 | 0.7-2.2 | .46 | 1.5 | 0.7-3.3 | .28 |
| Dexamethasone alone | 3.7 | 1.9-7.2 | <.001 | 3.4 | 1.01-11 | .047 |
Boldface indicates significance at P < .05.
CI, confidence interval; OR, odds ratio; NI, not included; NS, not significant.
Trends in PFS and OS
Median PFS was better in the more recent study periods (Figure 2A): 16 months in 2010-2014; 11 months in 2005-2009; and 6 months in 2000-2004 (P < .001). The PFS by period among the ASCT-treated patients was not significantly different over time (Figure 2B): 53 vs 44 vs 40 months; P = .2. In contrast, patients in the non-ASCT group enjoyed a significant improvement in PFS (Figure 2C): 8 vs 7 vs 4 months, respectively; P < .001, and in a landmark analysis for non-ASCT patients who remained progression-free and alive at 6 months, the difference in PFS in favor of the most recent periods remained statistically significant (median, 26 vs 27 vs 18 months; P = .03) (Figure 2D). Finally, in a subgroup analysis of all patients who achieved ≥VGPR, no difference in PFS was noted between periods (P = .14), including in a separate analysis for ASCT (P = .39) and non-ASCT treatments (P = .43).
PFS by time periods and study cohorts. (A) All patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .13). (B) ASCT-treated patients (2000-2004 vs 2010-2014, P = .23; 2000-2004 vs 2005-2009, P = .2; 2005-2009 vs 2010-2014, P = .5). (C) Standard treatment patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .53). (D) Landmark analysis at 6 months among standard treatment patients (2000-2004 vs 2010-2014, P = .02; 2000-2004 vs 2005-2009, P = .01; 2005-2009 vs 2010-2014, P = .92).
PFS by time periods and study cohorts. (A) All patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .13). (B) ASCT-treated patients (2000-2004 vs 2010-2014, P = .23; 2000-2004 vs 2005-2009, P = .2; 2005-2009 vs 2010-2014, P = .5). (C) Standard treatment patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .53). (D) Landmark analysis at 6 months among standard treatment patients (2000-2004 vs 2010-2014, P = .02; 2000-2004 vs 2005-2009, P = .01; 2005-2009 vs 2010-2014, P = .92).
Median follow-up for surviving patients was 5.6 years (IQR, 3.1-8.8 years). OS rates improved over time (Figure 3A): 2-year OS was 60% in 2010-2014, 54% in 2005-2009, and 42% in 2000-2004 and 4-year OS was 54% vs 42% vs 31% (P < .001). Notably, for those patients receiving ASCT, the greatest gains in OS were seen in the 2010-2014 period: 4-year OS 91% vs 73% vs 65%; P < .0001 (Figure 3B). In contrast, for those patients not undergoing ASCT, OS gains were made beginning in 2005 without substantial incremental improvement in the 2010-2014 period (4-year OS, 38% vs 32% vs 16%; P < .001) (Figure 3C). In a landmark analysis for non-ASCT patients who were alive at 6 months, OS advantage remained in favor of the 2 recent periods (2-year OS, 65% vs 63% vs 44%; P < .001) (Figure 3D). Last, in a subgroup analysis of all patients who achieved ≥VGPR, no difference in OS was noted between periods (P = .57), including in a separate analysis for ASCT (P = .16) and non-ASCT treatments (P = .41).
OS by time periods and study cohorts. (A) All patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .001). (B) ASCT-treated patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P = .07; 2005-2009 vs 2010-2014, P < .001). (C) Standard treatment patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .33). (D) Landmark analysis at 6 months among standard treatment patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .11).
OS by time periods and study cohorts. (A) All patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .001). (B) ASCT-treated patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P = .07; 2005-2009 vs 2010-2014, P < .001). (C) Standard treatment patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .33). (D) Landmark analysis at 6 months among standard treatment patients (2000-2004 vs 2010-2014, P < .001; 2000-2004 vs 2005-2009, P < .001; 2005-2009 vs 2010-2014, P = .11).
For the 1123 patients with cardiac biomarkers available for staging (72% of the study population), changes in survival over time were stratified by Mayo 2004 staging. Patients in stage I had survival improvement over time, reaching a statistical significance (P = .03; supplemental Figure 2). Patients in stage II had marginal improvement in survival over the study span (P = .08), whereas patients in stage IIIa/IIIb had a poor survival that remained unchanged across the study periods. When using the 2012 revised Mayo staging, an improvement in survival over time was seen only among patients at stage III (data not shown).
Univariate analysis and multivariate models for OS
Univariate analysis for factors influencing OS including treatment period is shown in Table 3. Age was not significant in the ASCT population, whereas it was for the non-ASCT population. Separate multivariate models were constructed for ASCT and non-ASCT patients (Table 3). Multivariate analyses suffered from the fact that in the 2000-2004 period 43%, 46%, and 82% of patients were missing dFLC, troponin T, and NT-proBNP measurements, respectively. For this reason, the multivariate analysis was done both without and with troponin and dFLC.
Univariate and multivariate analysis for OS
| Model 1: ASCT patients . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Univariate analysis . | Multivariate analysis A . | Multivariate analysis B . | ||||||
| . | RR . | 95% CI . | P . | RR . | 95% CI . | P . | RR . | 95% CI . | P . |
| Period | |||||||||
| 2010-2014 | Reference | Reference | Reference | ||||||
| 2005-2009 | 2.9 | 1.7-5.3 | <.001 | 2.4 | 1.4-4.5 | .001 | 2.6 | 1.5-5.0 | <.001 |
| 2000-2004 | 3.9 | 2.3-7.0 | <.001 | 3.3 | 1.9-6.2 | <.001 | 2.8 | 1.5-5.6 | <.001 |
| Age ≥ 65 | 1.3 | 0.9-1.8 | .11 | Not included | Not included | ||||
| >1 organs involved | 2.2 | 1.7-3.0 | <.001 | 1.7 | 1.2-2.4 | .001 | 1.8 | 1.3-2.7 | .001 |
| dFLC ≥ 18 mg/dl | 2.3 | 1.6-3.2 | <.001 | Not included | 1.9 | 1.3-2.9 | <.001 | ||
| BMPCs≥10% | 2.0 | 1.5-2.7 | <.001 | 2.1 | 1.5-2.8 | <.001 | 2.2 | 1.5-3.2 | <.001 |
| Full-dose melphalan conditioning | 0.3 | 0.2-0.4 | <.001 | 0.4 | 0.3-0.6 | <.001 | 0.5 | 0.3-0.7 | <.001 |
| Troponin T≥0.035 ng/mL | 2.7 | 1.9-3.8 | <.001 | Not included | 1.6 | 1.01-2.4 | .04 | ||
| eGFR<30 mL/min/1.73 m2 | 1.7 | 0.9-2.7 | .08 | Not significant | Not significant | ||||
| Model 1: ASCT patients . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Univariate analysis . | Multivariate analysis A . | Multivariate analysis B . | ||||||
| . | RR . | 95% CI . | P . | RR . | 95% CI . | P . | RR . | 95% CI . | P . |
| Period | |||||||||
| 2010-2014 | Reference | Reference | Reference | ||||||
| 2005-2009 | 2.9 | 1.7-5.3 | <.001 | 2.4 | 1.4-4.5 | .001 | 2.6 | 1.5-5.0 | <.001 |
| 2000-2004 | 3.9 | 2.3-7.0 | <.001 | 3.3 | 1.9-6.2 | <.001 | 2.8 | 1.5-5.6 | <.001 |
| Age ≥ 65 | 1.3 | 0.9-1.8 | .11 | Not included | Not included | ||||
| >1 organs involved | 2.2 | 1.7-3.0 | <.001 | 1.7 | 1.2-2.4 | .001 | 1.8 | 1.3-2.7 | .001 |
| dFLC ≥ 18 mg/dl | 2.3 | 1.6-3.2 | <.001 | Not included | 1.9 | 1.3-2.9 | <.001 | ||
| BMPCs≥10% | 2.0 | 1.5-2.7 | <.001 | 2.1 | 1.5-2.8 | <.001 | 2.2 | 1.5-3.2 | <.001 |
| Full-dose melphalan conditioning | 0.3 | 0.2-0.4 | <.001 | 0.4 | 0.3-0.6 | <.001 | 0.5 | 0.3-0.7 | <.001 |
| Troponin T≥0.035 ng/mL | 2.7 | 1.9-3.8 | <.001 | Not included | 1.6 | 1.01-2.4 | .04 | ||
| eGFR<30 mL/min/1.73 m2 | 1.7 | 0.9-2.7 | .08 | Not significant | Not significant | ||||
| Model 2: Non-ASCT patients . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Univariate analysis . | Multivariate analysis A . | Multivariate analysis B . | ||||||
| . | RR . | 95% CI . | P . | RR . | 95% CI . | P . | RR . | 95% CI . | P . |
| Period | |||||||||
| 2010-2014 | Reference | Not significant | Not significant | ||||||
| 2005-2009 | 1.1 | 0.9-1.3 | .3 | ||||||
| 2000-2004 | 1.8 | 1.5-2.2 | <.001 | ||||||
| Age ≥ 65 y | 1.2 | 1. 1-1.4 | .005 | 1.2 | 1.1-1.4 | .009 | 1.3 | 1.04-1.5 | .01 |
| >2 organs involved | 1.4 | 1.1-1.6 | <.001 | 1.4 | 1.2-1.7 | <.001 | 1.3 | 1.04-1.5 | .01 |
| BMPCs ≥10% | 1.3 | 1.1-1.5 | .002 | 1.2 | 1.1-1.4 | .008 | NS | ||
| dFLC ≥18 mg/dL | 1.6 | 1.3-1.8 | <.001 | NI | 1.4 | 1.1-1.6 | .001 | ||
| Treatment category | |||||||||
| MDex | Reference | Reference | Reference | ||||||
| MP | 1.9 | 1.6-2.4 | <.001 | 1.6 | 1.2-2.1 | <.001 | 2.0 | 1.4-2.8 | <.001 |
| Bortezomib-based | 1.0 | 0.8-1.2 | .98 | 1.1 | 0.8-1.4 | .58 | 1.0 | 0.8-1.4 | .77 |
| IMiD-based | 1.0 | 0.7-1.3 | .87 | 1 | 0.7-1.4 | .92 | 1.2 | 0.8-1.7 | .34 |
| Dexamethasone alone | 2.1 | 1.5-2.9 | <.001 | 2.0 | 1.4-2.9 | <.001 | 1.9 | 1.1-3.0 | .02 |
| Troponin T ≥0.035 ng/mL | 2.2 | 1.9-2.7 | <.001 | NI | 2.2 | 1.8-2.6 | <.001 | ||
| eGFR <30 mL/min per 1.73 m2 | 0.9 | 0.8-1.1 | .53 | NI | NI | ||||
| Model 2: Non-ASCT patients . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Univariate analysis . | Multivariate analysis A . | Multivariate analysis B . | ||||||
| . | RR . | 95% CI . | P . | RR . | 95% CI . | P . | RR . | 95% CI . | P . |
| Period | |||||||||
| 2010-2014 | Reference | Not significant | Not significant | ||||||
| 2005-2009 | 1.1 | 0.9-1.3 | .3 | ||||||
| 2000-2004 | 1.8 | 1.5-2.2 | <.001 | ||||||
| Age ≥ 65 y | 1.2 | 1. 1-1.4 | .005 | 1.2 | 1.1-1.4 | .009 | 1.3 | 1.04-1.5 | .01 |
| >2 organs involved | 1.4 | 1.1-1.6 | <.001 | 1.4 | 1.2-1.7 | <.001 | 1.3 | 1.04-1.5 | .01 |
| BMPCs ≥10% | 1.3 | 1.1-1.5 | .002 | 1.2 | 1.1-1.4 | .008 | NS | ||
| dFLC ≥18 mg/dL | 1.6 | 1.3-1.8 | <.001 | NI | 1.4 | 1.1-1.6 | .001 | ||
| Treatment category | |||||||||
| MDex | Reference | Reference | Reference | ||||||
| MP | 1.9 | 1.6-2.4 | <.001 | 1.6 | 1.2-2.1 | <.001 | 2.0 | 1.4-2.8 | <.001 |
| Bortezomib-based | 1.0 | 0.8-1.2 | .98 | 1.1 | 0.8-1.4 | .58 | 1.0 | 0.8-1.4 | .77 |
| IMiD-based | 1.0 | 0.7-1.3 | .87 | 1 | 0.7-1.4 | .92 | 1.2 | 0.8-1.7 | .34 |
| Dexamethasone alone | 2.1 | 1.5-2.9 | <.001 | 2.0 | 1.4-2.9 | <.001 | 1.9 | 1.1-3.0 | .02 |
| Troponin T ≥0.035 ng/mL | 2.2 | 1.9-2.7 | <.001 | NI | 2.2 | 1.8-2.6 | <.001 | ||
| eGFR <30 mL/min per 1.73 m2 | 0.9 | 0.8-1.1 | .53 | NI | NI | ||||
Boldface indicates significance at P < .05.
RR, risk ratio.
For those patients who had ASCT, multivariate analysis without troponin and dFLC in the model (Table 3, multivariate model 1A), variables that were independently predictive of OS were the period of diagnosis, amyloid involvement of >1 organ, use of full-dose melphalan conditioning, and BMPCs ≥10%. The relative risk of death compared with the 2010-2014 period was 2.4 for patients in 2005-2009 and 3.3 for those in 2000-2004. When troponin T and dFLC were added into the model (at 0.035 ng/mL and 18 mg/dL cutoffs, respectively), the results were similar, with troponin and dFLC also being independent predictors of OS (Table 3, multivariate model 1B).
For those patients who did not undergo ASCT, the variables that were independently predictive of OS in the multivariate analysis without troponin and dFLC included (Table 3, multivariate 2A) were age ≥65 years, >2 organs involved, BMPCs ≥10%, and treatment category; MP-treated and dexamethasone alone treated patients fared the worst compared to MDex with similar benefit (relative risk, 0.6-0.7) observed by any of the other regimens. When the modeling included troponin T and dFLC, they were both associated with a poorer OS, whereas BMPCs were no longer significant (Table 3, multivariate 2B). The period of diagnosis was no longer an independent risk factor for survival in the non-ASCT cohort, presumably because treatment was period-dependent.
Discussion
In this study, we describe the characteristics of newly diagnosed AL patients seen in our institution from 2000 through 2014 and outline the trends in patient management and outcomes. Because major advances have been seen in AL amyloidosis in the past decade, assessing the impact of these advances on response and survival to maximize the use of these changes and defining the new challenges ahead for further outcome enhancement is worthwhile. The 3 most salient findings of this study are quite encouraging. First, more patients presented to our institution with less advanced disease, both in terms of numbers and function of the involved organs. The second is that a reduction in 6-month mortality is finally a reality, with the most significant advances occurring in Mayo 2004 stages I and II. The third observation is that over the past 15 years, more patients respond to therapy and enjoy prolonged survival.
Disease recognition and early diagnosis are major obstacles for improving patient outcome. A recent Web-based survey among patients with amyloidosis or their family members outlined the delay in diagnosis and the exhaustive diagnostic pathway that patients often experience.20 Diagnosis within 6 months of initial symptoms occurred in only 37% of patients, whereas another 37% had a significant delay in diagnosis of more than 12 months from onset of symptoms. Moreover, almost half of patients had seen 4 or more different physicians within various specialties before the diagnosis was made. This patient-reported survey emphasized that recognizing amyloidosis remains a challenge for physicians. A comparison of patients seen between 1988 and 1998 at the Mayo Clinic or by an Italian multicenter network revealed that there was a lower prevalence of cardiac involvement in patients within the Italian Network that translated into a better OS.21 One of the explanations for the population disparities was the education offered to physicians in Italy to increase awareness of AL amyloidosis, thus leading to earlier diagnosis. Our current dataset demonstrates that over the past 15 years, a higher proportion of patients present to the Mayo Clinic with fewer organs involved and less organ dysfunction, which in turn has contributed to improved outcomes.
For 40 years, 6-month mortality was fixed at approximately 40% at our institution, and comparably poor fixed early mortality rates have been seen at another major center.16,17 For the first time, however, we have observed a reduction in 6-month mortality from 37% to 24%. Our data would suggest that these improvements relate to a growing availability of effective treatments and potentially better baseline disease characteristics resulting from earlier diagnosis. It is notable that the rate of early death is not improved by bortezomib or IMiD-based therapies as compared with MDex. A multivariate analysis for early death demonstrated that bortezomib was associated with a higher risk for early death than MDex, and further subanalysis suggested that this effect was more pronounced in Mayo 2004 stage II patients. This finding should be interpreted with care given the retrospective nature of this analysis. A case-control study did not find a difference in the death rate at 6 months between MDex and bortezomib in combination with MDex.8 Cardiac toxicity in bortezomib-treated patients exists.22,23
There was a dramatic improvement in 4-year OS in those patients who received ASCT in the 2010-2014 period compared with earlier periods (91% vs 73% and 65%). In the most recent periods, there were fewer patients with cardiac involvement (supplemental Table 1), and the most contemporary patients were less likely to have more than 1 organ involved. That a higher percent of these patients received full-dose melphalan as their conditioning also speaks to the possibility that they were more fit. The other potential driving forces for the improved outcomes in this most contemporary ASCT population could be that a higher percentage of these fit patients received bortezomib-based induction and unattenuated doses of melphalan conditioning. One can assume that part of the improved OS observed in the most recent period may be due to improved salvage therapies, including better access to bortezomib and IMiDs and other clinical trial opportunities using ixazomib, daratumumab, carfilzomib, and NEOD001, although our study did not address this point specifically.
Among the non-ASCT patients, the inflection point for PFS and OS improvement seems to have occurred around 2005, with no major incremental PFS or OS benefit observed in the most recent era, with 4-year OS rates of 38%, 32%, and 16% for each of the periods in descending order. The most striking change that occurred at this timeframe was the introduction of MDex as a substitute for MP6 in 2005. This change appears to have had a profound impact, with no regimen driving OS better than MDex, surprisingly not even bortezomib-based regimens, despite the fact that bortezomib-based regimens increase VGPR rates. The conundrum of why the higher VGPR rates induced by bortezomib-based regimens have not clearly translated into substantially better OS in this high-risk non-ASCT eligible population can be partially explained by a higher risk of early death with bortezomib compared with MDex, suggesting a subtle cumulative toxicity of bortezomib. Another potential explanation is the inferior outcomes seen in patients with t(11;14) treated with bortezomib.24,25 This aspect of therapy requires more study.
The limitations of this study are its retrospective design. Although our database is prospectively maintained with efforts to maintain follow-up on all patients, we were unable to ascertain detailed treatment and response in a fraction of patients, mainly those who died early in their disease course. In addition, no testing for cardiac biomarkers and sFLC was available in the earliest period. Response among the poor-prognosis patients may be underrepresented because early death precluded our ability to assess response in some. For that same reason, PFS data may not be as robust as in clinical trials and should be interpreted in caution. Finally, as a referral center, we cannot control for changes in patient referral, which may alter the patient population arriving at our institution. Indeed, more patients seen were from nearer locations and were more often seen within 30 days of their diagnosis, but the impact of this change on the baseline characteristics of the patients is uncertain.
In conclusion, this study provides a general view on our experience in the management of 1551 patients with newly diagnosed AL amyloidosis. For the first time, we have seen a reduction in death within the first 6 months of diagnosis, something that had not been achieved in the previous 40 years.16 This reduction in early mortality, especially among those patients who were ineligible for ASCT, along with earlier diagnosis and better treatment options—notably the abandonment of melphalan and prednisone and the introduction of MDex- and bortezomib-based regimens as first-line therapy—has contributed to better PFS and OS. For all patients, the greater availability of more drugs targeting plasma cells has improved the tail end of the survival curves in the past 5 to 10 years. With greater awareness of the disease and additional new drugs to target BMPCs and potentially the amyloid deposits, we anticipate that comparable advances will be made in successive decades as well.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study was supported in part by the Jabbs Foundation (Birmingham, United Kingdom), the Henry J. Predolin Foundation (USA), and National Institutes of Health National Cancer Institute grant P50 CA186781.
Authorship
Contribution: E.M. designed the study, analyzed the data, wrote the first draft, and approved the final version of the manuscript. M.A.G., S.K.K., M.Q.L., D.D., F.K.B., M.G., S.R.H., P.K., N.L., A.F., M.H., Y.L.H., W.G., R.W., T.V.K., S.R., J.A.L., Y.L., R.S.G., S.Z., and S.V.R. performed patient management, revised the manuscript critically, and approved the final version of the manuscript; R.A.K. performed patients’ follow-up, revised the manuscript critically, and participated in final data analysis and approval of the final version of the manuscript; and A.D. designed the study, analyzed the data, wrote the first draft, approved the final version of the manuscript, and performed patient management.
Conflict-of-interest disclosure: M.A.G. received consultancy from Millenium and honoraria from Celgene, Millenium, Onyx, Novartis, Smith Kline, Prothena, Ionis, and Amgen. S.K.K. received consultancy from Celgene, Millennium, Onyx, Janssen, and BMS and research funding from Celgene, Millennium, Novartis, Onyx AbbVie, Janssen, and BMS. M.Q.L. received research funding from Celgene. D.D. received research funding from Karyopharm Therapeutics, Amgen, and Millenium Pharmaceuticals. P.K. received research funding from Takeda, Celgene, and Amgen. A.D. received research funding from Celgene, Millennium, Pfizer, and Janssen and travel grant from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Angela Dispenzieri, Division of Hematology, Mayo Clinic, 200 First St, SW, Rochester, MN 55905; e-mail: dispenzieri.angela@mayo.edu.